Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Trond Løvdal and Version 3 by Peter Tang.
The use of seaweeds in the human diet has a long history in Asia and has now been increasing also in the western world. Concurrent with this trend, there is a corresponding increase in cultivation and harvesting for commercial production. Edible seaweed is a heterogenous product category including species within the green, red, and brown macroalgae. Moreover, the species are utilized on their own or in combinatorial food products, eaten fresh or processed by a variety of technologies.
- seaweed
- macroalgae
- food safety
- microbiology
- bacteria
- viruses
1. Introduction
The global seaweed industry is worth more than USD 6 billion per year, corresponding to approx. 12 million tons/year in volume, of which about 85% comprises food products for human consumption [1]. Owing to the fact that there will be an increasing need for protein food sources to accommodate the anticipated growth in the world’s population, the seaweed industry (both aquaculture and wild-harvested) is expected to grow since seaweed is a sustainable food source. This assumed increase, together with consumers’ demands for tasty, nutritious, safe, and convenient seaweed food products, and changes in market trends, leads to a growing need to ensure microbially safe seaweed food products.
Several studies have focused on the bacterial diversity in brown (Phaeophyceae), green (Chlorophyta), and red (Rhodophyta) macroalgae (henceforward: seaweed). Bacteria inhabiting seaweed include the Proteobacteria, Actinobacteria, Bacteroidetes (CFB group), Cyanobacteria, Firmicutes, Planctomycetes, Verrumicrobia, Chloroflexi, Deinococcus-Thermus, Fusobacteria, and Tenericutes, with the Gammaproteobacteria as the most common bacterial clade [2][3][4][5][2,3,4,5]. However, there are only a few studies that specifically clarify the prevalence of human pathogens in edible seaweeds [6][7][8][6,7,8].
2. Pathogenic Microorganisms in Seaweed
2.1. Bacillus sp.
More than 140 species are at present included in the genus Bacillus [9][32], and they are commonly described as Gram-positive, rod-shaped, straight, or slightly curved cells, that appear singly, in pairs, chains, or as long filaments. They are further referred to as possessing the ability to form resistant endospores, one per cell, although sporulation remains to be documented in some of the recently described species. Bacillus spp. are commonly aerobic, but some species are facultatively anaerobic, and at least two strictly anaerobes have been described. Although the majority of the species belonging to the genus Bacillus have little or no pathogenic potential, some species are known to be associated with food-borne diseases in humans, by means of the production of heat-stable toxins. B. cereus may cause food poisoning and opportunistic infections, while some other species, including B. subtilis, B. pumilus, and B. licheniformis, have also been associated with food poisoning and human/animal infections [9][10][11][12][13][32,33,34,35,36]. Bacillus spp., among others, are efficient producers of compounds with antibacterial, antifouling, and quorum sensing inhibiting features, which make them highly successful colonizers of seaweed surfaces, and may live in an endosymbiotic relationship with seaweed [2]. Growth promoting and nutritional effects beneficial to the seaweed have been attributed to endophytic Bacillus spp., including B. cereus, B. pumilus, and B. licheniformis, and these species are associated with seaweed of the brown, green and red algae [14][15][16][37,38,39]. Spores of Bacillus spp., as exemplified in Figure 1, are very resistant to most external factors and can tolerate temperatures over 100 °C combined with pH < 3 for several minutes [17][46], but will not be able to reproduce under such conditions. Spores present in the product may on the other hand be able to germinate when the conditions allow and reproduce and eventually produce toxins that may lead to food poisoning and in the worst-case death. Table 12 summarizes limits for growth in relation to temperature, pH, water activity (aW), and water phase NaCl for some human pathogen spore formers in their vegetative form, in addition to some other potentially harmful bacteria associated with seaweed. The growth rate will decrease with lower temperatures and pH until their minimum limit is reached. A seaweed product may be considered safe to eat as long as pH is below 4.3 when stored at ≤4 °C (cf. B. cereus). If the product is to be stored at an elevated temperature, pH needs to be lowered to ≤3.7 (cf. Salmonella). B. licheniformis, B. pumilus, and B. amyloliquefaciens/subtilis are not able to grow or produce toxins at refrigerated temperatures (Table 12).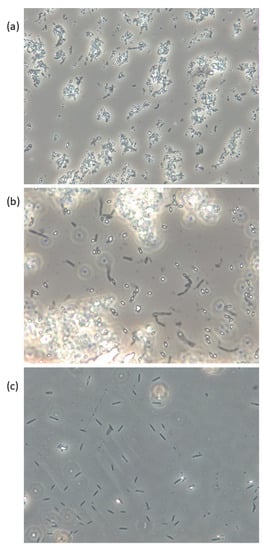
Figure 1. Live phase-contrast microscopy images of (a) B. licheniformis, (b) B. pumilus, and (c) B. subtilis isolated from Saccharina latissima and cultivated on Marine Agar. Spores appear white/bright and vegetative cells are dark. Magnification: 400×.
Table 12. Limits for growth under otherwise optimal conditions for some pathogenic bacteria of relevance for seaweeds. The data are relevant for safety and shelf life for traditional and novel seaweed food products under different processing and storage conditions.
| Pathogen | Temperature | pH | a | w | (min) | Max. Water Phase NaCl (%) | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Min. | Max. | ||||||||||
| B. cereus | 4 | 55 | 4.3 | 9.3 | 0.92 | 10 | [18] | [47] | |||||
| B. licheniformis | 11–15 | 50–55 | 4.6 | 9.8 | 0.91 | 7 | [9][19] | [32,48] | |||||
| B. pumilus | >5–15 | 40–50 | ≤6 (Some strains grow at 4.5) |
≥9.5 | <0.96 | >10 | [12][20] | [35,49] | |||||
| B. subtilis | 5.5 | 55.7 | 4.8 | 9.2 | 0.93 | >5–10 | [9][21] | [32,50] | |||||
| C. botulinum | (growth only, proteolytic) | 10 | 48 | 4.6 | 9 | 0.93 | 10 | [18] | [47] | ||||
| C. botulinum | (growth only, non-proteolytic) | 3.3 | 45 | 5.0 | 9 | 0.97 | 5 | ||||||
| C. perfringens | 10 | 52 | 5 | 9 | 0.93 | 7 | |||||||
| Pathogenic | E. coli | 6.5 | 49.4 | 4 | 9 | 0.95 | 6.5 | [18][[24] | [47 | 22][23] | ,51,52,53] | ||
| L. monocytogenes | −0.4 | 45 | 4.4 | 9.4 | 0.92 | 10 | [18] | [47] | |||||
| S. aureus | aerobe | 7 | 50 | 4 | 10 | 0.83 | 20 | ||||||
| anaerobe | 5.0 | 0.90 | [22] | [51] | |||||||||
| Salmonella | 5.2 | 42.6 | 3.7 | 9.5 | 0.94 | 8 | [18] | [47] | |||||
| V. cholerae | 10 | ~44 | 5.0 | ~10 | 0.97 | 3 | [18][25][26][27] | [47,54,55,56] | |||||
| V. parahaemolyticus | 5 | ~44 | 4.8 | ~11 | 0.94 | 8 | [18][25][26][27][28] | [47,54,55,56,57] | |||||
| V. vulnificus | 10 | ~44 | 4.4 | ~9 | 0.96 | 6 | [18][28][29] | [47,57,58] | |||||
| Aeromonas hydrophila | 0 | 42 | 6 | 7.2 (optimum) | 0.97 | 5 | [18][30] | [47,59] | |||||
2.2. Pathogenic Vibrios
Bacteria in the genus Vibrio are Gram-negative, curved rod-formed, and facultative anaerobes [31][60]. Members of the genus have the sea, brackish and fresh water as their natural habitat and are among the most common bacteria found in surface waters worldwide [32][61]. Considering the widespread prevalence of vibrios in aquatic environments, it is not surprising that seaweeds are frequently colonized by members of this genus [33][62]. There are currently over 140 Vibrio species, of which 12 are reported to be associated with infections among humans [34][35][36][63,64,65]. The most important human pathogenic species are V. cholerae, V. parahaemolyticus, and V. vulnificus [36][37][65,66], but also several other Vibrio species as V. alginolyticus, V. metschnikovii, V. fluvialis, and V. mimicus may cause infection but with less severe symptoms in humans [36][65]. The prevalence of human pathogenic vibrios and especially those possessing genes for increased pathogenicity are highly correlated with high water temperatures [38][67], and global warming is expected to favor their distribution [32][61]. As the vibrios are indigenous to the aquatic environment, there is no documented correlation between the occurrence of Vibrio and commonly applied indicator bacteria of fecal contamination. Thus, indicator organisms as coliforms do not give information on the presence of potentially pathogenic Vibrio spp.
Water and various foods have been implicated as vehicles for the highly pathogenic V. cholerae O1 and O139 as demonstrated by epidemiologic studies [39][68]. A very rare case was reported in which a woman acquired infection after eating raw, fresh seaweed transported from the Philippines as hand luggage to her home in California and eaten a month later [40][69]. However, V. cholera cannot be considered a likely pathogen associated with seaweeds.
Food poisoning caused by V. parahemolyticus and V. vulnificus associated with edible seaweeds also appears to be rare, but several documented examples from other kinds of seafood are known, e.g., prawns and oysters [41][42][70,71], the latter usually in immunocompromised individuals. Findings of V. parahemolyticus [43][72] and V. vulnificus [44][73] in seaweeds collected along the coast of Japan, prompted the authors to encourage proper hygiene practice during postharvest handling of seaweeds, especially in summer when the concentrations peaked. Vibrio spp. counts as high as log 8.2 cfu/g have been reported on raw cultivated Gracilaria changii harvested in Malaysia, indicating the potential presence of human pathogens possibly compromising food safety if consumed raw [45][20].
The vibrios are considered particularly sensitive to food processing, especially thermal treatment. However, in samples of sundried Ulva lactuca cultivated in Turkey, Vibrio spp. were reported in a number of <10 cfu/g [46][17]. Using sensitive qPCR assays combined with microbial pre-enrichment, Barberi et al., 2020 [47][74] detected pathogenic V. parahemolyticus in 78% of cultivated seaweed samples from North-East USA. Kudaka et al., 2008 [48][19] identified V. parahemolyticus in 18.8% of samples of Caulerpa lentillifera (Sea grape) cultivated in tanks. Although the thermostable hemolysin gene was not detected in any of the isolates, these findings led the authors to highlight the importance of a suitable sterilization process for C. lentillifera to ensure food safety [48][19]. V. alginolyticus was isolated from cultivated A. esculenta in Scotland, but not V. vulnificus, V. parahemolyticus, or V. cholera [8]. Conventional culturing methods failed to identify Vibrio spp. in seaweeds collected in Ireland [49][18] or Norway [50][21].
2.3. Aeromonas sp.
The genus Aeromonas belongs to the family Aeromonadaceae, and is a group of Gram-negative, rod-shaped, oxidase- and catalase-positive and facultatively anaerobic bacteria [51][52][76,77]. Members of this genus are ubiquitous aquatic bacteria and thus common in environments such as fresh-, brackish and marine water, and also found as inhabitants of aquatic animals [52][77]. Aeromonas spp. are potential foodborne pathogens and known to cause gastrointestinal as well as extra-intestinal infections in humans [53][78]. Most studies have dealt with A. hydrofila, which have been implicated in many seafood-borne outbreaks [54][79]. The occurrence of Aeromonas spp. has been frequently reported in water and food, including RTE seafood [55][56][57][80,81,82]. Currently not much is known on the role of seaweeds as responsible food for infections. However, based on their indigenous aquatic prevalence, Aeromonas spp. could be expected to colonize seaweeds and possibly follow the raw materials to processing. Furthermore, the ability of some Aeromonas sp. to survive and even grow at chilled temperatures gives reason for concern for seaweed and other seafood products. A. hydrophila was isolated from e.g., Ulva reticulata harvested in Malaysia [58][83], and Aeromonas spp., in concentrations up to log 5.9 cfu/g, from mauro prepared from Chondrus crispus and Chondracanthus teedii sold by fishmongers or from street stalls in Sicily, Italy [59][25].3. Processing and Factors That Control Microbial Growth in Seaweed
3.1. Drying
Drying may inhibit all microbial growth including yeast and mold by reducing the water activity (aW) to 0.6 or below, while bacteria of relevance are inhibited at much higher aW according to Table 12. The optimal aW for a food product is usually a compromise between several priorities. At aW below 0.30, lipid oxidation will occur and Maillard reaction has an optimum at aW = 0.65 [60][114] and high-temperature drying should therefore not be used down to this level. Seaweed processors will, in general, avoid drying to lower moisture content than needed for the preservation of the products as the weight loss and drying costs represent a direct economic loss. Determination of the optimal aW and moisture content is therefore essential. To achieve this, the relationship between the moisture content of the seaweeds and aW has to be determined but literature on this has not been found. Some correlations have been documented for other foods, e.g., algae and fish by the method of da Silva et al. [61][115]. A more fundamental understanding of the relation of water content, aW, and water structure in foods has been presented by Mathlouthi, 2001 [60][114] who proposed a method for determining the correlations and validated it for sugars. The surface-to-volume ratio is very high for most seaweeds and the drying time is relatively short which makes it feasible to dry at low temperatures (<< 60 °C) without risking microbial growth during drying. Typical low-temperature drying methods are sun drying and drying with dehumidified air but may also be achieved by electromagnetic drying by microwaves or radio frequency. The latter may also be used for high-temperature drying alone or in combination with hot air drying, infrared drying, or alternatively by superheated steam drying. These high-temperature drying methods may be designed to inactivate both bacteria and spores of bacteria. This may be of interest when the dried seaweeds are intended for use as ingredients in moist foods intended to have a shelf life after the addition of the seaweeds.3.2. Thermal Processing
Blanching and boiling of seaweeds are done for several purposes including the inactivation of microorganisms and inactivating inherent enzymes causing the breakdown of the product. Brown seaweeds commonly have an unacceptable high concentration of iodine which may be reduced by up to 94% by boiling for a few minutes. However, boiling causes loss of flavonoids and water-soluble nutrients which limits the prevalence [62][116]. There are currently few thermally processed seaweed products in the market compared to dried seaweed, but they are found as ingredients in canned (e.g., mackerel in tomato sauce), pasteurized (e.g., fish burgers), fried and boiled (e.g., soup) products. The edible seaweed laver (Porphyra umbilicalis), commonly named nori, is cultivated and consumed in East Asia [63][117] and is one of the most commonly used seaweeds for human consumption. It is manufactured as dried and/or processed products and is in great demand as side dishes and snacks. Dried laver may be a contamination source to kimbab and in rolled sushi [64][118], but Choi et al., 2014 [65][89] showed that heat-processed laver (260 to 400 °C, 2 to 10 s) had reduced aerobic bacterial counts, and no non-spore-forming pathogens (coliforms, L. monocytogenes, S. aureus, Salmonella spp. and V. parahaemolyticus).3.3. Fermentation
Successful fermentation stabilizes the raw seaweed biomass by producing lactic acid and quickly reducing the pH of the seaweeds to below 4.3, where most potentially pathogenic bacteria are inactivated at refrigeration temperatures (pH 3.7 for ambient temperatures, cf. Table 12). Lactic acid fermentation of seaweed is a recent strategy and quite limited information is available on culture conditions [66][67][122,123]. The absence of natural lactic acid bacteria (LAB) microflora and simple sugars in most seaweeds, as opposed to terrestrial plants, may have limited development of this technique in the former [67][123]. Fermentation may be a preferred processing technique for seaweeds because several seaweed species are sensitive to both thermal treatment and freezing that often diminishes the sensorial properties, appearance, and nutritional value of the products. However, as shown by Uchida et al., 2007 [66][122], LAB fermentation of Undaria pinnatifida is not straightforward due to the selective survival of potential pathogenic spore-forming Bacillus spp. through the drying process that could not be effectively outcompeted by the LAB starter culture during fermentation. When cultivated seaweed was mixed with sauerkraut at a ratio of up to 1:1, LAB fermentation proved successful by resulting in sufficiently low pH and thus maintained acceptable microbial and sensorial quality up to 60 days post-inoculation [67][123]. Heat treatment (95 °C for 15 min) followed by fermentation using a commercial Lactobacillus plantarum starter culture led to a drop in pH and stabilization at pH 4.5 after 40 h in Saccharina latissima [68][124], and although this is above the limit set at 4.3 in regards to the growth of B. cereus (Table 12), no colonies with the morphology of B. cereus were observed [68][124].
3.4. Freezing
During the freezing of seaweeds, most of the water content is immobilized around the freezing point of seawater which depends on the salt content of the actual seaweed, usually between 0 °C and −2 °C. Water bound to other molecules has shown a freezing depression in the range −12 °C to −25 °C before rinsing, but after proper rinsing and loss of salts, the freezing point is increased to 0 °C [69][125]. This change in the freezing point is important for the availability of water to microorganisms.
There is surprisingly little literature available on the freezing of seaweeds, possibly due to the limited changes during long-time frozen storage. Del Olmo, Pico, and Nunez, 2019 [70][126] documented 72% retention of polyphenols and 79% retention of antioxidant capacity after 180 days of storage at −24 °C. While freezing to a temperature below −25 °C is an effective measure to protect against microbial growth during storage, the damage to the cell structure during freezing and thawing may make the plant more accessible to microorganisms after thawing. During thawing, the drip loss released from the seaweeds may provide a pathway for the microorganisms.
Rapid freezing and thawing are recommended to minimize the risk of microbial growth as well as to limit the drip loss as much as possible. This may be achieved by thin layer band freezers or in vertical plate freezers if the width of the blocks is limited to keep the freezing time below a few hours. Block freezing on racks without air circulation and other methods needing several days to freeze the product will be less effective than rapid freezing with respect to food safety.
