2. Current Insights
ATX encoded by
ENPP2 is a secreted glycoprotein that forms LPA
[24][42]. The ATX-LPA axis is related to many physiological processes, including embryonic development and wound healing. Dysregulation of ATX expression is connected with various pathological conditions such as cancer, inflammatory diseases and fibrosis
[3][4][5][3,4,5]. The exact mechanism by which
ENPP2 expression is regulated is still not fully understood, whereas recently, it has been proved that
ENPP2 is prone to epigenetic alterations
[13]. Still, very little information is available about its DNA methylation pattern and the consequent impact in gene expression in health and human pathology.
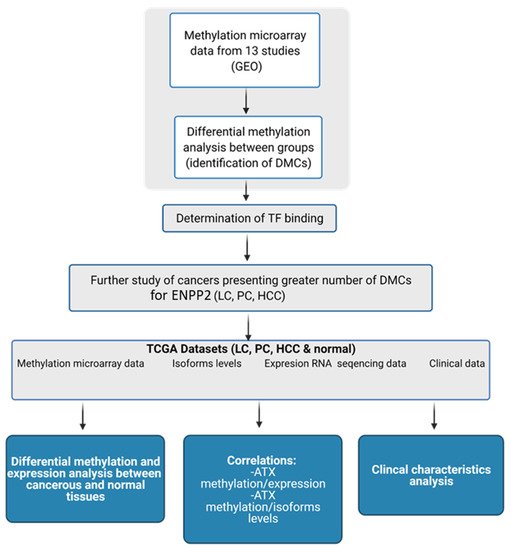
In the present study we adopted a bioinformatic in silico approach using publicly available datasets from healthy tissues and different cancer tissues and cell lines to analyze methylation patterns of
ENPP2. Our analysis showed a consistent methylation pattern throughout the gene’s regions across human tissues, i.e., increased methylation in the gene body and decreased methylation in TSS and the 1st exon. Given the fact that
ENPP2 is expressed in almost all tissues and biological fluids
[12][25][26][12,43,44], we can postulate that the decreased methylation in the TSS and 1st exon is associated with the active transcription of the gene in most human tissues.
Analysis of cancer datasets revealed aberrant
ENPP2 methylation, showing a malignant-specific profile throughout different cancer types. In general, methylation was increased in the TSS and 1st exon, regions known to hold an important role in gene expression, and decreased in the gene body region. A large number of DMCs were identified between malignant and respective benign tissues. Most importantly, all six DMCs of
ENPP2 located at TSS in the promoter or at the 1st exon showed increased methylation across different cancer types, including HCC, melanoma, CRC, LC and PC. These results corroborate and expand recent observations showing a hypermethylated
ENPP2 promoter in primary tumors of LC and squamous cell carcinoma patients
[27][45] and in breast cancer
[13][22][28][13,22,46].
Based on these interesting observations, we next performed in silico analysis of
ENPP2 methylation in datasets retrieved from the TCGA, focusing on those cancer types presenting the greatest number of DMCs, i.e., LC, PC and HCC. TCGA datasets are generally larger compared to those of other research efforts, allowing comparisons of stronger statistical relevance, and most significantly, they contain several clinical and demographic parameters of each patient. In addition, the datasets selected included also mRNA expression data and were therefore suitable for addressing an important objective of this study, i.e., if aberrant methylation is correlated to gene expression. Methylation, clinical and expression data were recovered for the three cancer types. Differential methylation analysis of
ENPP2 revealed that all emerged DMCs identified in transcription-related (TSS and 1st exon) regions were hypermethylated in all three cancers compared to healthy controls, confirming the analysis of the GEO datasets. In addition, the majority of DMCs located at the gene body were hypomethylated in the studied cancers in relation to controls. mRNA levels were decreased in PC and LC in relation to normal tissues. Collectively, our results indicate that the increased methylation of PA and 1st exon CGs is correlated with decreased expression in lung and prostate cancer. This is in line with previous studies in LC and BC showing that
ENPP2 is hypermethylated in tumor tissues in relation to normal, causing down regulation in gene expression
[13][27][13,45]. In PC, ATX protein was not or was weakly expressed in non-neoplastic epithelial cells and in high-grade intra-epithelial neoplasia, while in cancer cells ATX was only expressed in half of the tumors and was correlated with adverse tumor parameters
[29][47]. A relevant study in LC showed that ATX protein expression and activity was increased in LC tissues and sera
[30][48]. As far as HCC is concerned, our analysis showed upregulation of expression in HCC in relation to normal liver, showing a TSS and 1st exon methylation-independent and a cancer type-specific role of
ENPP2 expression regulation. In a previous study, ATX overexpression in HCC tissues was correlated with inflammation and liver cirrhosis. In addition, liver cancer cell lines presented stronger ATX expression in relation to normal hepatocytes
[31][49]. It should be noted that many authors have demonstrated that the relationship between mRNA expression and protein differs in many cancers. It has been reported in lung cancer and glioblastoma that, for many genes, mRNA expression is lower but protein levels are higher compared with the control
[32][33][34][35][50,51,52,53].
In agreement with the above findings, analysis using the UALCAN database showed that ENPP2 is hypermethylated and under-expressed in LC and PC, suggesting that DNA methylation regulates expression in LC and PC. However, no regulatory relation was observed between methylation and expression in HCC, as both were upregulated, pointing again to a cancer-specific methylation-independent ENPP2 regulation. Different mechanisms between cancer types are common. Here, our presented results from the cancer types studied indicate a cancer type-specific profile of ENPP2 methylation rather than a similar pan-cancer dysregulation. Without availability of suitable methylome datasets or targeted methylation studies of ENPP2 in each different cancer type, we cannot extrapolate conclusions between cancers.
The same correlation pattern was noticed for
ENPP2 isoforms in all cancer types studied. Interestingly, there was a significant negative correlation between mRNA expression (gene and isoform alpha and beta) and promoter methylation in four CGs (cg02156680, cg02709432, cg04452959 and cg06998282) in PRAD. In LC samples, the methylation of cg06998282 and cg02709432 was negatively correlated with the expression of
ENPP2 and also with isoform beta and gamma. Finally, in the case of HCC, only the methylation of cg06998282 was negatively correlated with the expression of
ENPP2 and isoform beta. The above findings indicate that the promoter methylation of specific CGs is negatively correlated with
ENPP2 and isoform expression differs between cancers, with cg02709432 being a common site in PC and LC but not in the case of HCC. This CG is located at a site that can bind E2F-1 TF, which has been shown to be inhibited by CG methylation
[36][54], and Sp1 TF, which has been found to regulate ENPP2 transcription
[37][55]. Thus, we hypothesized that as the level of methylation increases, methylation of cg02709432 hinders the binding of the TFs to the promoter, thus leading to reduction in ENPP2 gene and isoforms expression.
The expression pattern of isoforms differs between tissues as high expression levels of isoform beta were found in peripheral tissues and plasma, while isoform gamma was mostly found in the brain, and isoform alpha is considered to be the most under-expressed in brain and peripheral tissue in comparison to the other two
[38][56]. According to a relevant study, isoform alpha has a deletion of exon 12, isoform beta a deletion of exons 12 and 21 and isoform gamma a deletion of exon 21
[12], leading to different splice variants. None of the identified DMCs were located at these regions, explaining similar patterns of
ENPP2 mRNA and isoform expression.
DNA methylation within promoters is known to modulate the binding of TFs to regulatory elements, thus resulting in transcriptional repression
[39][57]. In our study, we predicted 39 TFs which can regulate transcription through binding to
ENPP2 promoter’s DMCs. Therefore, any aberrant methylation events in these DMCs during pathological transformation may block TF binding and related transcription. This is further supported by reports involving the identified TFs in
ENPP2 and ATX expression. Indeed, among the predicted TFs, NF kappaB, AP-2 and E2F have been previously shown to be sensitive to CG methylation with consequent inhibition of their DNA binding activities
[36][54]. Another TF predicted to bind DMCs of
ENPP2, NFAT1, has been shown to mediate ATX overexpression in MDA-MB-435 cells
[40][58]. It has also been shown that blocking the expression of NFAT1 results in downregulation of ATX expression, leading to inhibition of melanoma and metastasis
[41][35]. High C-Jun levels seem to enhance
ENPP2 expression
[42][59]. Interestingly, SP was found to regulate
ENPP2 transcription in neuroblastoma cells by activating a CRE/AP-1-like element at position −142 to −149 and a GAbox at position −227 to −235 near the TSS of
ENPP2 [37][55]. This is in accordance with our finding that Sp1 can bind near the cg02709432 located at TSS200.
In order to assess any correlation of
ENPP2 methylation to tumor prognosis, clinical characteristics analysis was performed and showed that increased methylation of some CGs was correlated with poor tumor parameters. Indeed, in PC it was associated with larger tumors and non-response to pharmacotherapy, in LC it was connected to the advanced cancer stage and in HCC it was associated with macro-invasion. Hence,
ENPP2 methylation in the identified CGs could be pursued further and be evaluated in clinical cancer samples as biomarkers of cancer progression and poor outcome. In addition, these results corroborate previous data showing that low mRNA expression was associated with worse prognosis in LC
[27][45].
The involvement of ENPP2 methylation in tumor progression and prognosis was also addressed by analyzing methylomes from cell lines presenting a more or less aggressively invasive phenotype, revealing several DMCs. Higher methylation was observed in the more aggressive in relation to less aggressive HCC and PC cell lines, indicating a connection of ENPP2 methylation with worse prognostic behavior, in accordance with our findings in the clinical samples.
Finally, analysis of colon cell lines treated with DNA methyltransferase inhibitors showed that 5-AZA caused a decrease of methylation in all CGs in relation to untreated controls in the three studied cell lines, showing a clear demethylation effect in the
ENPP2 gene. Given the contribution of
ENPP2 in a variety of pathologies, further studies could assess a methylation-based reprogramming of
ENPP2 via a variety of methylation inhibitors. Similarly, previous studies have demonstrated that targeting the ATX-LPA-LPP axis is an attractive strategy for introducing new therapeutic choices
[43][44][60,61].
In conclusion, healthy tissues presented increased methylation of ENPP2 in the gene body and decreased in the promoter and 1st exon connected to the active transcription of the gene in most human tissues. A different pattern was described in HCC, melanoma, CRC, LC and PC, showing a malignant-specific profile of ENPP2 methylation. Further analysis of independent TCGA datasets confirmed these results as increased methylation of promoter and 1st exon CGs and decreased ENPP2 mRNA expression in PC and LC in relation to healthy tissues were found. Furthermore, increased methylation of ENPP2 was connected to poor prognostic parameters in the same cancers, which was also supported by analysis of cell line datasets. We also found a negative correlation between mRNA expression at gene and isoform levels and methylation of PA CGs that present TF binding sites. In specific, we postulate that the methylation of promoter CGs may hinder the binding of TFs, and thus, the expression of ENPP2 and isoforms may be reduced.
Our findings contribute to the understanding of methylation events and regulatory mechanism of ENPP2 in cancer and provide a full description of DMCs to be further validated in functional and clinical studies.