Cardiogenic shock (CS) is associated with a high in-hospital mortality despite the achieved advances in diagnosis and management. Invasive mechanical ventilation and circulatory support constitute the highest step in cardiogenic shock therapy. Once established, taking the decision of weaning from such support is challenging. Intensive care unit (ICU) bedside echocardiography pro- vides noninvasive, immediate, and low-cost monitoring of hemodynamic parameters such as cardiac output, filling pressure, structural disease, congestion status, and device functioning. Supplemented by an ultrasound of the lung and diaphragm, it is able to provide valuable information about signs suggesting a weaning failure. The aim of this article was to review the state of the art taking into account current evidence and knowledge on ICU bedside ultrasound for the evaluation of weaning from mechanical ventilation and circulatory support in cardiogenic shock.
- cardiogenic shock weaning
- echocardiography
- ECMO
- Impella
1. Introduction
Cardiogenic shock (CS) is a complex syndrome defined as a low cardiac output leading to severe end-organ hypoperfusion and progressive failure. Despite the advances in diagnosing, monitoring, and management in the last decade, prognosis remains unacceptably poor with a 35–45% in-hospital mortality [1].
A total of 50–80% of patients with Society for Cardiovascular Angiography and Intervention (SCAI) classification stage C or D cardiogenic shock may require initiation of mechanical ventilation (MV) due to left-ventricular dysfunction and elevated filling pressure leading to pulmonary edema, oxygenation impairment, and the increased work of breathing with ventilatory muscle fatigue [2,3][2][3]. The early implantation of mechanical circulatory support devices has increased in recent years as initial vasopressor and inotropic therapies remain insufficient to stabilize the shock status [4]. A total of 19% of the patients with CS following an acute myocardial infarction in the CULPRIT-SHOCK trial received at least one mechanical circulatory support device [5]. Although the exact moment of initiation remains controversial and despite the lack of strong evidence in this field [1,4,6][1][4][6], there are a wide range of hemodynamic, echocardiographic, and clinical parameters that can be assessed to help the heart team make the decision. However, when myocardial contractility improves, the time of weaning from mechanical support, whether ventilatory or circulatory, remains less established, and the weaning criteria and protocols are highly variable across centers [7].
CS weaning includes the withdrawal of both ventilatory and circulatory mechanical support. Nevertheless, their removal does not have to be performed simultaneously. For instance, an awake ECMO strategy, with spontaneous patient breathing, is feasible and safe, being significantly associated with lower MV complication rates and short- and long-term mortality (61.9% vs. 26.7%) [8,9,10][8][9][10].
Echocardiography and right-heart catheterization are the cornerstone for hemodynamic assessment of myocardial improvement [8]. Moreover, lung and diaphragm ultrasound has emerged as a useful tool to predict outcomes of weaning in MV [11]. Although there is a lack of a gold standard [8], a broad range of helpful echocardiographic parameters enable an immediate, low-cost, and noninvasive bedside assessment for the best moment to wean the patient from circulatory and/or ventilatory support. Protocol-guided MV weaning has demonstrated a reduction in reintubation and post-extubation respiratory failure rates [12]. The aim of this study was to provide a review of the current evidence and knowledge on the role of ultrasound when assessing weaning from ventilatory and circulatory mechanical support.
2. Ultrasound Assessment in Mechanical Ventilation Weaning
Weaning from MV is challenging in all critically ill patients, even more so when recovering from CS, since concomitant left- and sometimes right-heart failure and diastolic dysfunction are associated with higher rates of extubation failure [13,14][13][14]. Heart failure is responsible for 60% of weaning failures [15].
Several echocardiographic parameters can be used to predict ventilatory support weaning failure, especially those that allow the estimation of filling pressure and diastolic dysfunction. The influence of the systolic ejection fraction remains unclear, with contradictory results [13,18,19,20][13][16][17][18].
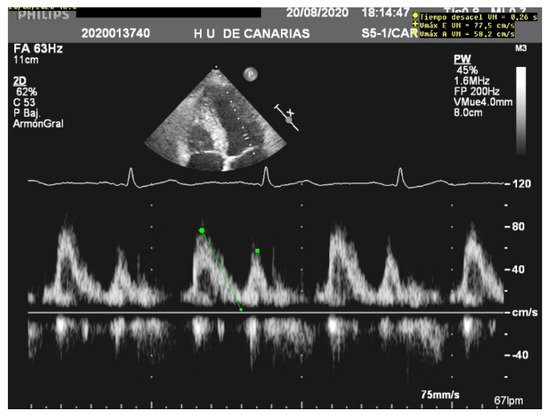
When assessing diastolic function, obtaining an E/e’ mitral ratio higher than 14.5 is associated with higher rates of weaning failure, even in atrial fibrillation [13,19,20][13][17][18], as are E waves higher than 0.87 m/s [13,21][13][19] ( Figure 1 ). However, this method is less reliable in acute decompensated heart failure and left ventricles with larger volumes, where significant mitral regurgitation can lead to underestimation, as well as in resynchronization therapy and wide QRS and the subsequent change in septal e’ due to its abnormal motion [22,23][20][21].
The lung ultrasound score (LUS) and modified lung ultrasound score (LUSm) are excellent predictors of weaning failure [11,21,25,26][11][19][22][23]. They allow a bedside quantification of lung aeration by examining 12 regions for the first or eight regions for the latter [8,27][8][24].
3. Ultrasound Assessment in Weaning from Temporary Mechanical Circulatory Support Devices
Furthermore, dynamic changes in tissular doppler parameters have been shown to predict successful weaning from ECMO, with an improvement in lateral e′ velocity. These parameters have been proposed as a more accurate predictor of myocardial reserve [32][25]. Diastolic parameters and the estimation of filling pressures, such as mitral E velocity or its time of deceleration, do not discriminate between successfully weaned patients and failed ones [33][26].
In addition, continuous hemodynamic assessment with transesophageal echocardiography, allowing a permanent evaluation of left- and right-ventricle function and volume status, was demonstrated to successfully predict ECMO weaning outcomes [36][27].
Although there has been an interest in studying the role of LUS role in veno-venous ECMO weaning, especially during the SARS-CoV-2 pandemic, no studies were found wherein lung ultrasound was proposed as a useful tool to predict the success of mechanical circulatory support weaning. The role of the diaphragm and its influence in veno-arterial ECMO weaning have also been studied, whereby a significant relationship was found between the diaphragm thickening fraction and left-ventricle ejection fraction, but without the predictability of successful weaning [37][28].
TEE is commonly used to assist placement, to guide management, and to reveal mechanical complications, as well as to assess the systolic function and concomitant valvulopathies and their severity [40,41][29][30].
4. Conclusions
Prediction of the extubation success can be assessed by bedside echocardiography to estimate diastolic function and filling pressures, suggesting a higher risk of poor outcomes in mechanical ventilatory support withdrawal in cases of an altered E/e’ ratio, mitral E wave, E/A pattern, left-atrial pressure, pulmonary capillary edge pressure, or TDI values. Supplemented with the estimation of the lung ultrasound score and an evaluation of diaphragm weakness, daily, immediate, low-cost, and noninvasive evaluation of ventilatory weaning opportunities can be assessed at the ICU.
Furthermore, when the cardiac index improvement is suspected and weaning from mechanical circulatory support is intended, echocardiography can be a useful tool, especially in ECMO weaning. Improvements of the ejection fraction, VTI, lateral e′ and tricuspid annular S′ velocities, and right-ventricle function are reliable parameters for assessing de-escalation on myocardial support. However, there are no feasible echocardiographic parameters to guide IABP weaning.
References
- Van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Council on Cardi-ovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline. Contemporary management of cardiogenic shock: A scientific statement from the American Heart Association. Circulation 2017, 136, e232–e268.
- The TRIUMPH Investigators. Effect of Tilarginine Acetate in Patients with Acute Myocardial Infarction and Cardiogenic Shock. JAMA 2007, 297, 1657.
- Alviar, C.L.; Rico-Mesa, J.S.; Morrow, D.A.; Thiele, H.; Miller, P.E.; Maselli, D.J.; van Diepen, S. Positive Pressure Ventilation in Cardiogenic Shock: Review of the Evidence and Prac-tical Advice for Patients with Mechanical Circulatory Support. Can. J. Cardiol. 2020, 36, 300–312.
- Tehrani, B.N.; Truesdell, A.G.; Psotka, M.A.; Rosner, C.; Singh, R.; Sinha, S.S.; Damluji, A.A.; Batchelor, W.B. A Standardized and Comprehensive Approach to the Management of Cardiogenic Shock. JACC Heart Fail. 2020, 8, 879–891.
- Feistritzer, H.J.; Desch, S.; Freund, A.; Poess, J.; Zeymer, U.; Ouarrak, T.; Schneider, S.; de Waha-Thiele, S.; Fuernau, G.; Eitel, I.; et al. Prognostic Impact of Active Mechanical Circulatory Support in Cardiogenic Shock Complicating Acute Myocardial Infarction, Results from the Culprit-Shock Trial. J. Clin. Med. 2020, 9, 1976.
- Thiele, H.; Ohman, E.; de Waha-Thiele, S.; Zeymer, U.; Desch, S. Management of cardiogenic shock complicating myocardial in-farction: An update 2019. Eur Heart J. 2019, 40, 2671–2683.
- Dandel, M.; Hetzer, R. Myocardial recovery during mechanical circulatory support: Weaning and explantation criteria. Heart Lung Vessel. 2015, 7, 280–288.
- Dandel, M.; Javier, M.F.D.M.; Delmo, E.M.J.; Loebe, M.; Hetzer, R. Weaning from ventricular assist device support after recovery from left ventricular failure with or without secondary right ventricular failure. Cardiovasc. Diagn. Ther. 2021, 11, 226–242.
- Ellouze, O.; Lamirel, J.; Perrot, J.; Missaoui, A.; Daily, T.; Aho, S.; Petrosyan, A.; Guinot, P.G.; Bouchot, O.; Bouhemad, B. Extubation of patients undergoing extracorporeal life support. A retrospective study. Perfusion 2018, 34, 50–57.
- Montero, S.; Huang, F.; Rivas-Lasarte, M.; Chommeloux, J.; Demondion, P.; Bréchot, N.; Hékimian, G.; Franchineau, G.; Persichini, R.; Luyt, C.É.; et al. Awake venoarterial extracorporeal membrane oxygenation for refractory cardi-ogenic shock. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 585–594.
- Tenza-Lozano, E.; Llamas-Alvarez, A.; Jaimez-Navarro, E.; Fernández-Sánchez, J. Lung and diaphragm ultrasound as predictors of success in weaning from mechanical ventilation. Crit. Ultrasound J. 2018, 10, 1–9.
- Nitta, K.; Okamoto, K.; Imamura, H.; Mochizuki, K.; Takayama, H.; Kamijo, H.; Okada, M.; Takeshige, K.; Kashima, Y.; Satou, T.; et al. A comprehensive protocol for ventilator weaning and extubation: A prospective ob-servational study. J. Intensive Care 2019, 7, 1–9.
- Sanfilippo, F.; Di Falco, D.; Noto, A.; Santonocito, C.; Morelli, A.; Bignami, E.; Scolletta, S.; Vieillard-Baron, A.; Astuto, M. Association of weaning failure from mechanical ventilation with transthoracic echo-cardiography parameters: A systematic review and meta-analysis. Br. J. Anaesth. 2021, 126, 1319–1330.
- Roche-Campo, F.; Bedet, A.; Vivier, E.; Brochard, L.; Dessap, A.M. Cardiac function during weaning failure: The role of diastolic dysfunction. Ann. Intensive Care 2018, 8, 1–11.
- Liu, J.; Shen, F.; Teboul, J.-L.; Anguel, N.; Beurton, A.; Bezaz, N.; Richard, C.; Monnet, X. Cardiac dysfunction induced by weaning from mechanical ventilation: Incidence, risk factors, and effects of fluid removal. Crit. Care 2016, 20, 1–14.
- Caille, V.; Amiel, J.-B.; Charron, C.; Belliard, G.; Vieillard-Baron, A.; Vignon, P. Echocardiography: A help in the weaning process. Crit. Care 2010, 14, R120.
- Moschietto, S.; Doyen, D.; Grech, L.; Dellamonica, J.; Hyvernat, H.; Bernardin, G. Transthoracic Echocardiography with Doppler Tissue Imaging predicts weaning failure from mechanical ventilation: Evolution of the left ventricle relaxation rate during a spontaneous breathing trial is the key factor in weaning outcome. Crit. Care 2012, 16, 1–10.
- Ruiz-Bailén, M.; Cobo-Molinos, J.; Castillo-Rivera, A.; Pola-Gallego-De-Guzmán, M.D.; Cárdenas-Cruz, A.; Martínez-Amat, A.; Sevilla-Martínez, M.; Hernández-Caballero, C. Stress echocardiography in patients who experienced mechanical ventilation weaning failure. J. Crit. Care 2017, 39, 66–71.
- Haji, K.; Haji, D.; Canty, D.J.; Royse, A.G.; Green, C.; Royse, C.F. The impact of heart, lung and diaphragmatic ultrasound on prediction of failed extubation from mechanical ventilation in critically ill patients: A prospective observational pilot study. Crit. Ultrasound J. 2018, 10, 1–12.
- Mullens, W.; Borowski, A.; Curtin, R.; Thomas, J.; Tang, W. Tissue Doppler Imaging in the Estimation of Intracardiac Filling Pres-sure in Decompensated Patients with Advanced Systolic Heart Failure. Circulation 2009, 119, 62–70.
- Ritzema, J.L.; Richards, A.M.; Crozier, I.G.; Frampton, C.F.; Melton, I.C.; Doughty, R.N.; Stewart, J.T.; Eigler, N.; Whiting, J.; Abraham, W.T.; et al. Serial Doppler Echocardiography and Tissue Doppler Imaging in the Detection of Elevated Directly Measured Left Atrial Pressure in Ambulant Subjects with Chronic Heart Failure. JACC Cardiovasc. Imaging 2011, 4, 927–934.
- Xu, X.; Wu, R.; Zhang, Y.-J.; Li, H.-W.; He, X.-H.; Wang, S.-M. Value of Combination of Heart, Lung, and Diaphragm Ultrasound in Predicting Weaning Outcome of Mechanical Ventilation. Med. Sci. Monit. 2020, 26, e924885-1.
- Llamas-Álvarez, A.M.; Tenza-Lozano, E.M.; Latour-Pérez, J. Diaphragm and Lung Ultrasound to Predict Weaning Outcome. Chest 2017, 152, 1140–1150.
- Soummer, A.; Perbet, S.; Brisson, H.; Arbelot, C.; Constantin, J.-M.; Lu, Q.; Rouby, J.-J. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress. Crit. Care Med. 2012, 40, 2064–2072.
- Kim, D.; Jang, W.J.; Park, T.K.; Cho, Y.H.; Choi, J.-O.; Jeon, E.-S.; Yang, J.H. Echocardiographic Predictors of Successful Extracorporeal Membrane Oxygenation Weaning After Refractory Cardiogenic Shock. J. Am. Soc. Echocardiogr. 2021, 34, 414–422.e4.
- Aissaoui, N.; El-Banayosy, A.; Combes, A. How to wean a patient from veno-arterial extracorporeal membrane oxygenation. Intensive Care Med. 2015, 41, 902–905.
- Cavarocchi, N.C.; Pitcher, H.T.; Yang, Q.; Karbowski, P.; Miessau, J.; Hastings, H.M.; Hirose, H. Weaning of extracorporeal membrane oxygenation using continuous hemodynamic transesophageal echocardiography. J. Thorac. Cardiovasc. Surg. 2013, 146, 1474–1479.
- Moury, P.H.; Zunarelli, R.; Bailly, S.; Durand, Z.; Béhouche, A.; Garein, M.; Durand, M.; Vergès, S.; Albaladejo, P. Diaphragm Thickening During Venoarterial Extracorporeal Membrane Oxygenation Weaning: An Observational Prospective Study. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1981–1988.
- Crowley, J.; Cronin, B.; Essandoh, M.; D’Alessandro, D.; Shelton, K.; Dalia, A.A. Transesophageal Echocardiography for Impella Placement and Management. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2663–2668.
- Montisci, A.; Bertoldi, L.; Price, S.; Hassager, C.; Møller, J.; Pappalardo, F. Intensive care unit management of percutaneous mechanical circulatory supported patients: The role of imaging. Eur. Heart J. Suppl. 2021, 23 (Suppl. A), A15–A22.
