Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Andrew John Simkin and Version 2 by Catherine Yang.
Carotenoids are important natural pigments found in all plants and some bacteria, algae and fungiand constitute one of the largest families of natural products, with more than 750 distinct compounds classified to date. Carotenoids have also been shown to have a significant impact on a number of human diseases, improving the survival rates of some cancers and slowing the progression of neurological illnesses.
- carotenoids
- flavour
- nutrition
1. Carotenoid Biosynthesis in Planta
The carotenoid biosynthetic pathway has been intensely studied since the early 1960s [1][2][3][9,49,50]. While the carotenoid biosynthetic genes are located in the nucleus, their precursor protein products are imported into the chloroplast where the mature proteins synthesis carotenoids [4][51]. In chloroplasts, carotenoids accumulate in the photosynthetic membranes in association with the photosynthetic reaction centres and light-harvesting complexes [5][6][7][8][26,52,53,54]. In fruits and flowers, petals chloroplasts differentiate into chromoplasts and carotenoids accumulate in the membranes or in oil bodies such as plastoglobules [9][10][20,22] and fibrils [11][21], or in other structures within the stroma.
Phytoene (Figure 1A), the first true carotenoid, is formed by the condensation of two molecules of geranylgeranyl diphosphate by the enzyme phytoene synthase (PSY; EC.2.5.1.32). Phytoene undergoes four consecutive desaturation steps catalysed by two enzymes, phytoene desaturase (PDS; EC.1.3.99.28), resulting in the formation of ζ-carotene (Figure 1B) via the intermediate phytofluene [12][13][55,56] and ζ-carotene desaturase (ZDS; EC.1.14.99.30) to form lycopene (Figure 1C), the red pigment responsible for the colour of tomatoes, via the intermediate neurosporene [14][15][57,58]. To maintain carotenoids in their trans form, ζ-carotene isomerase (Z-ISO; EC.5.2.1.12) [16][59] converts 9,15,9′-cis-z-carotene to 9,9′-cis-ζ--carotene via the isomerization of the 15-cis-double bond, and carotene isomerase (CRTISO; EC.5.2.1.13) [17][18][19][60,61,62] transforms 9,15,9′-tricis-ζ--carotene into 9,9′-dicis-ζ-carotene, 7,9,9′-tricis-neurosporene into 9-cis-neurosporene and 7,9-dicis-lycopene into all-trans-lycopene. These desaturation steps require the presence of the plastid terminal oxidase (PTOX; EC.1.10.3.11) as a co-factor [20][21][22][23][24][29,63,64,65,66].
Lycopene undergoes two cyclization reactions forming α- and β-carotene. Lycopene β-cyclase (LβCY; EC.5.1.1.19) introduces two β-rings to the ends of the Lycopene carbon chain forming β-carotene (β,β-carotene; Figure 1D) via the intermediate γ-carotene (β,ψ-carotene), which contains a single β-ring and one uncyclized end, known as psi (ψ) [25][67]. LβCY and lycopene ε-cyclase (LεCY; EC.5.1.1.18) form α-carotene (β,ε-carotene) (Figure 1E) by introducing one β-ring and one ε-ring respectively to lycopene via the intermediate δ-carotene (ε,ψ-carotene) with one ε-ring and one uncyclized ψ end [26][68].
In Lactuca sativa (lettuce), LεCY introduces two ε-rings, resulting in the formation of ε-carotene (ε,ε-carotene; Figure 1F) [27][69]. LεCY genes have been identified in plants, green algae and cyanobacteria (Prochlorococcus marinus), and likely arose following gene duplication of the β-cyclases and later functional divergence [28][29][30][31][70,71,72,73].
Oxygenated carotenoids are formed by the hydroxylation of the β- and ε-rings of the carotene carotenoids. β-carotene is converted to zeaxanthin (3,3′-dihydroxy-β,β-carotene) via cryptoxanthin (Figure 1G) by the action of β-carotene hydroxylase (βCHY; EC.1.14.15.24) [32][33][34][35][36][74,75,76,77,78], and α-carotene (β,ε-carotene) is hydroxylated by βCHY to form zeinoxanthin and then the ε-ring is hydroxylated by ε-carotene hydroxylase (εCHY; EC 1.14.99.45) to form lutein (dihydroxy-ε,ε-carotene) (Figure 1H) [37][38][39][79,80,81]. Lutein is essential for the assembly of the light-harvesting photosystems and plays a role in non-photochemical quenching [40][41][42][43][44][45][82,83,84,85,86,87].
Lutein has also been shown to enhance the stability of the antenna proteins [46][88], play a role in light harvesting by transferring energy to chlorophyll (Chl) [47][89] and to quench Chl triplet states in the light-harvesting complex, protecting it from photo-oxidative damage [48][90].
Zeaxanthin epoxidase (ZEP: EC.1.14.13.90) catalyses the epoxidation of the two hydroxylated β-rings of zeaxanthin in two steps to generate antheraxanthin (Figure 1J) and violaxanthin (Figure 1K; [49][50][91,92]. In high light, violaxanthin is converted back to zeaxanthin by the activity of violaxanthin de-epoxidase (VDE: EC.1.10.99.3). This inter-conversion of violaxanthin to zeaxanthin is called the xanthophyll cycle and is implicated in the adaptation of plastids to changing light conditions [51][52][53][93,94,95]. In a similar mechanism, ZEP and VDE catalyse the inter-conversion of Lutein to Lutein epoxide (Figure 1L) in a process first reported in green tomato fruit in 1975 [54][96].
The final carotenoid, neoxanthin (Figure 1M), is synthesized from violaxanthin by the enzyme neoxanthin synthase first cloned from tomato and potato (NYS: EC.5.3.99.9) [55][56][97,98]. In Capsicum annum, antheraxanthin and violaxanthin are modified by a unique enzyme, capsanthin/capsorubin synthase (CCS: EC.5.3.99.8), induced at the onset of ripening [57][99], resulting in the synthesis of capsanthin and capsorubin from antheraxanthin and violaxanthin, respectively (Figure 1N) [58][59][100,101]. CCS possesses 86.1% amino acid sequence similarity with the tomato βCHY, suggesting that the two genes evolved from a common ancestral form and that the CCS functional activity diverged at a later date [60][61][102,103].
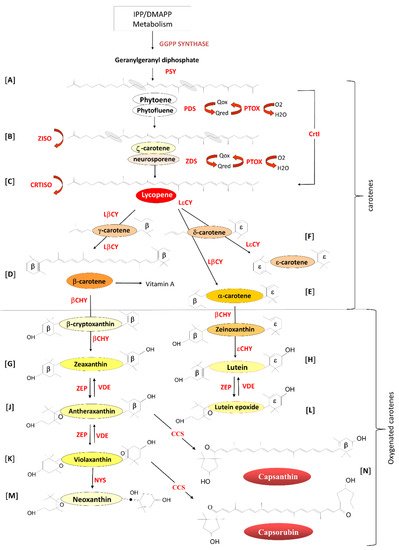
Figure 1. Overview of the biosynthesis of isoprenoids in plastids. PSY: Phytoene synthase. PDS: phytoene desaturase. ZDS: ζ-carotene desaturase. Z-ISO: ζ-carotene isomerase. PTOX: plastid terminal oxidase. CRTISO: carotene cis-trans isomerase. LβCY: lycopene β-cyclase. LεCY: lycopene ε-cyclase. βCHY: β-carotene hydroxylase. εCHY: ε-carotene hydroxylase. ZEP: zeaxanthin epoxidase. VDE: violaxanthin de-epoxidase. NYS: neoxanthin synthase. CCS: capsanthin/capsorubin synthase (adapted from Simkin et al. [62][48]. Letters A-N represent specific biosynthetic steps highlighted in the text.
2. Manipulating Carotenoid Content in Planta
Metabolic engineering has been used to generate a large number of crops with substantial increases in carotenoid content. Since carotenoid levels are determined by the rate of biosynthesis, the means of carotenoid sequestration and finally the rate of degradation, multiple avenues exist to increase carotenoid content in planta. The ‘push’ strategy uses methods to increase metabolic flux by over-expression of carotenoid biosynthesis enzymes. The ‘pull’ strategy increases carotenoid sink capacity and finally, the ‘block’ strategy seeks to reduce the rate of carotenoid turnover.
2.1. ‘Push’ Strategies for Increasing Carotenoid Content in Planta
Using genetic engineering to increase carotenoid content in fruit and staple crops has the potential to increase the availability of carotenoid substrates for the generation of a host of important volatile and non-volatile organic compounds and important nutritional components of foods. Genetic engineering of the carotenoid biosynthesis has been shown to create high carotenoid varieties of key staple crops such as flaxseed (Linum usitatissimum) [63][64][104,105], wheat (Triticum aestivum) [65][106], Sorghum [66][67][107,108], canola (Brassica napus) [68][109] and rice (Oryza sativa) [69][70][71][110,111,112], and root crops such as potato (Solanum tuberosum) [72][73][74][113,114,115] and cassava (Manihot esculenta) [73][114]. In addition, work to produce high carotenoid varieties of tomato (Solanum lycopersicum) has been well studied [10][75][76][22,116,117], (Table 1).
Key staple crops such as rice (Oryza sativa), wheat, cassava and potato, which constitute a significant part of the diets of poorer communities, contain little or no carotenoids or carotenoid-derived compounds (CDCs). Early efforts to generate β-carotene enriched-rice (Oryza sativa), termed “golden rice” [69][70][71][110,111,112], by over-expressing multiple enzymatic steps in the pathway (Figure 1) successfully resulted in rice variety accumulating up to 18.4 µg/g of carotenoids (up to 86% β-carotene) [70][111]. In this instance, these authors over-expressed PSY with the expression of the Pantoea ananatis CrtI (EC 1.3.99.31). CrtI carries out the activities of four plant enzymes, namely PDS, Z-ISO, ZDS and CRTISO (Figure 1).
2.2. ‘Pull’ Strategies for Manipulating Carotenoid Storage in Planta
In transgenic tomato, expression of the Arabidopsis Or was shown to promote chloroplast to chromoplast differentiation inducing carotenoid accumulation at early fruit developmental [77][129]. Expression of AtOR under the control of an endosperm-specific promoter increased carotenoid content in corn by promoting the formation of carotenoid-sequestering plastoglobuli [78][133]. However, these authors showed that these increases were seen when the carotenoid pool was limited, but it had no effect when carotenoid levels where abundant [78][133]. In Arabidopsis, Zhou et al. [79][134] demonstrated that the Or protein interacts directly with PSY (see Figure 1), post-transcriptionally regulating carotenoid biosynthesis. Chayut et al. [80][135] demonstrated in melon (Cucumis melo) that CmOr is required to stabilize flux through the carotenoid biosynthetic pathway, but the increase in carotenoids is due to the inhibition of downstream metabolic turnover of β-carotene [80][135]. Or expression has also been shown to increase carotenoid content in the seeds of rice [81][127] and maise [78][133]. In rice, these increases in carotenoids were observed in conjunction with the over-expression of two photosynthetic genes ZmPSY and PaCrtI. When ZmPSY and PaCrtI were expressed together, rice grain accumulated up to 5.5 µg/g DW, increasing to 2.5µg/g DW when these genes were expressed along with the AtOr gene [81][127]. This is the first demonstration that a multi-gene approach, targeting both carotenoid synthesis and sequestration, has the potential to dramatically increase carotenoid levels in grain.
Furthermore, the over-expression of the pepper fibrillin in transgenic tomato showed that fibrillin proteins play a crucial role in development of plastoglobules and fibrils in differentiating chromoplast [10][22]. In transgenic tomato, over-expression of Fibrillin was shown to delay thylakoid loss during chloroplast to chromoplasts differentiation, increase plastoglobuli number and thereby increase the concentrations of carotenoids including β-carotene (+64%) and lycopene (+118%) [10][22]. These carotenoids were further shown to increase the pool of substrates for volatile formation, and fruit were shown to generate a 36% and 74% increase in β-carotene-derived volatiles β-ionone and β-cyclocitral, respectively. Furthermore, an increase in the lycopene-derived volatiles citral (+50%), 6-methyl-5-hepten-2-one (MHO; +122%) and the ζ-carotene-derived geranylacetone (+223%) were observed to be consistent with the increases in carotenoids in these fruit [10][22]. These results demonstrate that increasing carotenoid content in fruits, vegetables and other crops provides a substrate for the formation of important volatile and non-volatile organic compounds important to plant development, flavour and aroma.
2.3. ‘Block’ Strategies for Manipulating Carotenoid Storage in Planta
Carotenoid cleavage dioxygenases 4 knockout (ccd4-1) had an even higher impact on seed carotenoid levels. Total carotenoids in ccd4-1 increased by 270% and β-carotene alone increased by a remarkable 840% compared with the wild type [82][138]. The more significant carotenoid turnover in ccd4-1 mutants compared to ccd1-1 mutants may be linked to their subcellular location. CCD1 has been shown to be localized in the cytosol, where it may have access to carotenoids stored in the plastid envelope [83][84][85][40,42,139], whereas CCD4 has been shown to be localized to the chloroplast and plastoglobules [86][140] where carotenoids are stored, giving them easier access to these substrates. Combining ccd4-1 and ccd1-1 into a single background increased carotenoid levels in Arabidopsis seed by 360% compared with ~170% and 270% for ccd1-1 and ccd4-1 alone.
These data suggest that CCD1 and CCD4 are important actors in carotenoid turnover and that whilst CCD4 has a more important role, likely due to its chloroplastic localisation, the two work together, and combined ccd1 and ccd4 mutants have a synergistic effect on the accumulation of carotenoids in Arabidopsis seeds. Furthermore, a mutation in ccd4 in peach (Prunus persica) was shown to result in a yellow fleshed variety due to the accumulation of carotenoids compared to the white flesh of the wild type [87][141].
Furthermore, work to evaluate the impact of CCDs on carotenoid turnover, authors used transgenics to knockout (KO) CCD1 or CCD4 in planta. Ohmiya et al. [88][142] used RNAi to silence CCD4a in Chrysanthemum (Chrysanthemum morifolium) resulted in a change of petal colour from white to yellow and Campbell et al. [89][143] down-regulated CCD4 in potato tubers resulting in a yellow flesh variety .
Down-regulation of CCD1A and CCD1B in tomato (antisense construct) resulted in a significant reduction in the rates of emission of pseudoionone, geranylacetone and β-ionone in cut tomato fruits, volatiles generated by the 9–10(9′–10′) cleavage of lycopene, ζ-carotene and β-carotene, respectively. However, these authors did not observe significant changes in the carotenoid content of these fruits [83][40]. In tomato, CCD1A and CCD1B are not plastid-localized, and it is not unexpected that plants with greatly reduced CCD1 expression showed insignificant alterations in carotenoid content, given that tomato fruit accumulate a significant amount of carotenoids during ripening, and any small turnover may go unnoticed.
These areas of exploitation thus require additional research to explore the contribution of jointly manipulating ‘push’, ‘pull’ and ‘block’ mechanisms to increase carotenoid content to improve the nutritional quality of food stuffs. Carotenoids have furthermore been shown to have important health benefits when consumed as part of a balanced diet . Manipulating carotenoid biosynthesis and sequestration also offers the potential to modify the flavour and aroma of fruit, grain or leaves. However, it should be noted that blocking the carotenoids turnover could negatively impact CDCs nutritional importance. Carotenoids, via these activities of carotenoid cleavage enzymes, provide the building blocks for a number of volatile and non-volatile organic compounds of physiological importance for plant development.
3. ’Hidden Hunger’ and the Health Benefits of Carotenoids
It has been reported that although humans had access to more than 50 carotenoids in their diet, six major carotenoids persisted in blood plasma, including the colourless carotenoids phytoene and phytofluene, and the coloured carotenoids α-carotene, β-carotene, lycopene, β-cryptoxanthin, zeaxanthin and lutein [90][91][145,146].
Carotenoids such as β-carotene, α-carotene and β-cryptoxanthin with provitamin A activity are essential in the human diet [92][147]. Vitamin A, also known as retinol, is an essential micronutrient and is required for growth, development and vision and is important for immune system function [93][94][95][148,149,150]. Vitamin A, in the form of retinal, combines with the protein opsin to form rhodopsin, a pigment containing sensory protein that absorbs light, converting it to an electrical signal, and it is required for colour vision [96][151]. Most people suffering from a Vitamin A deficiency are often unaware of that deficiency and show no clinical symptoms in a phenomenon often called ‘hidden hunger’ [97][152]. Vitamin A deficiencies are more common in areas where cereals and tubers are relied upon for the vast majority of calories consumed, as they are a poor source of provitamin A carotenoids [97][152].
Genetically modified maize (Zea mays) [98][99][125,126] engineered to accumulate provitamin A carotenoids has shown to be effective at increasing the stores of vitamin A in the bodies of 5- to 7-year-old children [100][153]. This work has shown that β-carotene-fortified maize is as effective at controlling vitamin A deficiency as taking supplements [100][153]. Palmer et al. [101][154] showed that the consumption of β-carotene from fortified maize improved the visual function of children with a vitamin A deficiency. As such, crops such as ‘golden rice’ (see Section 2.2.1) biofortified with provitamin A, engineered by European scientists with the hope of combatting premature blindness, and in extreme cases, death by vitamin A deficiencies, have great potential to improve the health of populations that that subsist on nutrient-poor white rice [62][48]. However, 20 year later, golden rice is not readily available to those it was intended to help. Over the past 20 years, it has been reported that tens of millions of people across Asia (Bangladesh, China and South and Southeast Asia) have gone blind or died due to these delays [102][155]. Some critics described golden rice as a ‘hoax’ or ‘fool’s gold’ and eventually became a key piece of what supporters have described as propaganda against GM technologies, resulting in a 20-year delay in its introduction and what supporters have described as a crime against humanity [102][155].
Carotenoids, such as phytoene, phytofluene, lycopene, lutein and astaxanthin, have been associated with a decreased in the risk of certain cancers, including colon [103][156], lung [104][157], and prostate cancer [105][106][107][158,159,160]. In elderly patients (64–75), a high intake of tomatoes, carrots and lycopene was associated with a decreased risk of prostate cancer compared to patients with a lower intake of these foods (~50% less tomatoes and 125% less carrots and a 23% lower carotenoid intake overall) [108][12]. For example, these authors found that patients with prostate cancer consumed 839 μg/day lycopene, 756 μg/day α-carotene and 4473 μg/day β-carotene compared to the general population with an intake of 1356, 919 and 5492 μg/day lycopene, α-carotene and β-carotene, respectively [108][12]. A low dietary intake of lycopene and a low plasma lycopene content have also been linked to increased mortality from oral cavity and pharynx cancer [109][161]. Furthermore, a study of 638 independently living 65–85 years old revealed that higher carotenoid (lycopene, lutein) serum levels and significantly higher levels of cholesterol adjusted α-tocopherol were correlated with higher cancer survival rates [110][162]. It has also been reported that lutein decreases the proliferation of breast cancer cells in a dose-dependent manner (6.25, 12.5, 25 and 50 μg/mL) and increases the expression of cellular antioxidant enzymes [111][163]. Further reports have shown that in human breast cell lines (e.g., MCF-7 or MDA-MB-235 cells) treatment with lycopene and β-carotene (0.5 to 10 μM), for 48 h and 96 h, inhibits cell proliferation [112][164]. Effectively, after 96h, treatment of MCF-7 cells with lycopene (2.5–10 μM) resulted in a 30% reduction in cell viability and a 20% reduction in MDA-MB-235 cell viability; however, the results obtained using MDA-MB-235 cells was only obtained with higher lycopene treatment [112][164]. Moreover, an additional cell line, MDA-MB-231, showed a 75% decrease in viability when treated with 10 μM lycopene after 96 h [112][164]. Similar results were found when these cell lines were treated with β-carotene. When treated with 10 μM β-carotene, a 40%, 30% and 70% reduction in MCF-7, MDA-MB-235 or MDA-MB-231 cell viability, respectively, was observed [112][164]. β-carotene at a concentration of 20 μM and has furthermore been shown to arrest the development of leukaemia cells (HL-60) by approximately 39% and significantly reduce their viability [113][165]. Phytofluene (10 μM) and ζ-carotene (10 μM) inhibited the cell growth of HL-60 cultures [114][166] (see Niranjana et al. [115][167] and Meléndez-Martínez et al. [90][145] for review).
Lycopene treatment (0–30 μM) over 0, 24, 48, and 96 h decreased the proliferation of SW480 cells 96 h after treatment with increasing effectiveness as lycopene levels increased from 10 to 30 μM [116][168]. Several other studies have also shown that lycopene (0–100 μM) inhibited cell growth in colorectal cancer cells (CRC) in a dose-dependent manner [117][169], and the proliferation of CRC was reduced by lycopene treatment to as low as 12 μM by Huang et al. [118][170]. A lycopene treatment of 20 mg/kg−1 in female Wistar rats has been shown to inhibit tumour growth [119][171] and protect against spontaneous ovarian cancer formation in laying hens (lycopene 26–52 mg/day/hen) [120][172].
It has been suggested that the preventive role of carotenoids against cancer is linked to their antioxidant activity and that regular consumption of carotenoids may alleviate oxidative stress. Lutein, zeaxanthin, and lycopene, for example, have been reported to decrease the inflammatory mediator’s production, as lycopene has been shown to have an anti-inflammatory effect on human colorectal cancer cells [116][168]. Lycopene and lutein have also been described as having the capacity to prevent oxidative stress-induced diseases such as cardiovascular disease in vivo (CVD) [121][122][123][124][125][173,174,175,176,177] and reduce LDL-cholesterol plasma levels [126][178]. Lutein has also been shown to reduce the risk of coronary artery disease [127][179] and may prevent atherosclerosis (condition where arteries become clogged with fatty deposits) development due to its anti-inflammatory and antioxidant properties and its ability to reduce the build-up of oxidized low-density lipoprotein (LDL) in the blood [128][180]. Lycopene has also been described as having preventive effects in atherosclerosis pathology [125][177]. High plasma lutein levels have also been found to reduce the risk of coronary heart disease and stroke [129][181] and decrease oxidative stress and apoptosis, protecting the myocardium from ischemia injury (inadequate blood supply to an organ i.e heart muscles) [124][176].
Carotenoids, lutein, zeaxanthin and β-carotene limit neuronal damage from free radicals, delaying the progression of neurological diseases, and dietary supplementation with lutein and zeaxanthin (2.02 mg/day) may prevent cognitive decline in those aged ≥ 60 years [130][182]. β-carotene has also been described as an Alzheimer’s disease antagonist [131][183], and high serum levels of lycopene, zeaxanthin and lutein have been linked to a reduction in mortality of Alzheimer’s sufferers [132][184].
It should also be noted that carotenoids have been linked to preventative roles in diabetes mellitus and osteoporosis, and numerous studies have suggested that carotenoids, including lutein and astaxanthin, could decrease age-associated decline in human skin cells and have a positive impact on the human life span (see Tan et al. [133][185], Rivera-Madrid et al. [134][186] and Milani et al. [135][187] for review), as well as having a beneficia effects on eye health and improving cognitive function (see Eggersdorfer et al. [136][188]).
The benefits noted above have suggested that increasing the levels of these beneficial carotenoids in the human diet could have a significant contribution to human health, and manipulating their metabolism would contribute greatly to this goal (see Section 2.2). Furthermore, manipulating terpenoid biosynthesis, either by increasing or decreasing specific carotenoid subsets, can lead to increases in nutritionally important compounds and flavour/aroma volatiles that could be used as a way to improve the quality in fresh produce such as tomatoes [10][22].
Carotenoid-derived apocarotenoids (CDCs) are formed by the oxidative cleavage of carbon–carbon double bonds in the carotenoid backbones either by carotenoid cleavage enzymes (CCDs) or via the exposure of carotenoids to ROS. Many of these apocarotenoids play key regulatory roles in plant development as growth simulators and inhibitors, signalling molecules, including as abscisic acid [137][138][139][37,38,189] and strigolactones [140][141][142][143][144][31,32,33,34,35], and have roles in plant defence against pathogens and herbivores [145][190]. Others act as flavour and aroma compounds in fruit pericarp, flowers and seeds [83][146][84][147][148][149][150][86][151][40,41,42,43,44,45,47,140,191]. The diverse variety of carotenoids (+700) means that the potential apocarotenoid products represent a significant number of natural compounds.
References
1. Goodwin TW: The Biochemistry of Carotenoids, vol 1, Plants. London: Chapman and Hall; 1980.
- Ruiz-Sola MÁ, Rodríguez-Concepción M: Carotenoid Biosynthesis in Arabidopsis: A Colorful Pathway. The Arabidopsis book 2012, 2012(10).
- Nisar N, Li L, Lu S, Khin Nay C, Pogson Barry J: Carotenoid Metabolism in Plants. Molecular plant 2015, 8(1):68-82.
- Britton G, Liaaen-Jensen S, Pfander H: Carotenoids: handbook. Birkhäuser: Springer, Basel AG; 2012.
- Paliwal C, Ghosh T, George B, Pancha I, Maurya R, Chokshi K, Ghosh A, Mishra S: Microalgal carotenoids: Potential nutraceutical compounds with chemotaxonomic importance. Algal Research 2016, 15:24-31.
- Meléndez-Martínez AJ, Mandić AI, Bantis F, Böhm V, Borge GIA, Brnčić M, Bysted A, Cano MP, Dias MG, Elgersma A et al: A comprehensive review on carotenoids in foods and feeds: status quo, applications, patents, and research needs. Crit Rev Food Sci Nutr 2021:1-51.
- Yabuzaki J: Carotenoids Database: structures, chemical fingerprints and distribution among organisms. Database 2017, 2017.
- Lerfall J: Carotenoids: Occurrence, Properties and Determination. In: Encyclopedia of Food and Health. Edited by Caballero B, Finglas PM, Toldrá F. Oxford: Academic Press; 2016: 663-669.
- Sun T, Tadmor Y, Li L: Pathways for Carotenoid Biosynthesis, Degradation, and Storage. Methods Mol Biol 2020, 2083:3-23.
- Cheng HM, Koutsidis G, Lodge JK, Ashor AW, Siervo M, Lara J: Lycopene and tomato and risk of cardiovascular diseases: A systematic review and meta-analysis of epidemiological evidence. Critical Reviews in Food Science and Nutrition 2019, 59(1):141-158.
- Langi P, Kiokias S, Varzakas T, Proestos C: Carotenoids: From Plants to Food and Feed Industries. Methods Mol Biol 2018, 1852:57-71.
- Van Hoang D, Pham NM, Lee AH, Tran DN, Binns CW: Dietary Carotenoid Intakes and Prostate Cancer Risk: A Case-Control Study from Vietnam. Nutrients 2018, 10(1):70.
- Hou L-l, Gao C, Chen L, Hu G-q, Xie S-q: Essential role of autophagy in fucoxanthin-induced cytotoxicity to human epithelial cervical cancer HeLa cells. Acta Pharmacologica Sinica 2013, 34(11):1403-1410.
- Satomi Y: Antitumor and Cancer-preventative Function of Fucoxanthin: A Marine Carotenoid. Anticancer Research 2017, 37(4):1557-1562.
- Koklesova L, Liskova A, Samec M, Zhai K, Abotaleb M, Ashrafizadeh M, Brockmueller A, Shakibaei M, Biringer K, Bugos O et al: Carotenoids in Cancer Metastasis-Status Quo and Outlook. Biomolecules 2020, 10(12).
- Havaux M: Carotenoids as membrane stabilisers in chloroplasts. Trends in Plant Science 1998, 3:147-151.
- Gruszecki WI, Strzałka K: Carotenoids as modulators of lipid membrane physical properties. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2005, 1740(2):108-115.
- Johnson QR, Mostofian B, Fuente Gomez G, Smith JC, Cheng X: Effects of carotenoids on lipid bilayers. Phys Chem Chem Phys 2018, 20(5):3795-3804.
- Mostofian B, Johnson QR, Smith JC, Cheng X: Carotenoids promote lateral packing and condensation of lipid membranes. Phys Chem Chem Phys 2020, 22(21):12281-12293.
- Simkin AJ, Laizet Y, Kuntz M: Plastid lipid associated proteins of the fibrillin family: structure, localisation, functions and gene expression. In: Recent Research Developmetns in Biochemistry. Edited by Pandalai SG, vol. 5. India: Research Signpost; 2004: 307-316.
- Deruere J, Romer S, d'Harlingue A, Backhaus RA, Kuntz M, Camara B: Fibril assembly and carotenoid overaccumulation in chromoplasts: a model for supramolecular lipoprotein structures. The Plant cell 1994, 6(1):119-133.
- Simkin AJ, Gaffe J, Alcaraz JP, Carde JP, Bramley PM, Fraser PD, Kuntz M: Fibrillin influence on plastid ultrastructure and pigment content in tomato fruit. Phytochemistry 2007, 68(11):1545-1556.
- Frank HA, Cogdell RJ: Carotenoids in photosynthesis. Photochem Photobiol 1996, 63(3):257-264.
- Hashimoto H, Uragami C, Cogdell RJ: Carotenoids and Photosynthesis. Subcell Biochem 2016, 79:111-139.
- Ledford HK, Niyogi KK: Singlet oxygen and photo-oxidative stress management in plants and algae. Plant Cell Environ 2005, 28:1037-1045.
- Telfer A: What is beta-carotene doing in the photosystem II reaction centre? Philosophical transactions of the Royal Society of London Series B, Biological sciences 2002, 357(1426):1431-1439; discussion 1439-1440, 1469-1470.
- Sandmann G, Böger P: Inhibition of carotenoid biosynthesis by herbicides. In: Target Sites of Herbicides Action Edited by Böger P, Sandmann G. Boca Raton, FL: CRC Press; 1989: 25-44.
- Simkin AJ, Breitenbach J, Kuntz M, Sandmann G: In vitro and in situ inhibition of carotenoid biosynthesis in Capsicum annuum by bleaching herbicides. Journal of agricultural and food chemistry 2000, 48(10):4676-4680.
- Josse EM, Simkin AJ, Gaffe J, Laboure AM, Kuntz M, Carol P: A plastid terminal oxidase associated with carotenoid desaturation during chromoplast differentiation. Plant physiology 2000, 123(4):1427-1436.
- Wilson F, Harrison K, Armitage AD, Simkin AJ, Harrison RJ: CRISPR/Cas9-mediated mutagenesis of phytoene desaturase in diploid and octoploid Strawberry. BMC Plant Methods 2019, 15:45.
- Booker J, Auldridge M, Wills S, McCarty D, Klee H, Leyser O: MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr Biol 2004, 14(14):1232-1238.
- Snowden KC, Simkin AJ, Janssen BJ, Templeton KR, Loucas HM, Simons JL, Karunairetnam S, Gleave AP, Clark DG, Klee HJ: The Decreased apical dominance1/Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, root growth, and flower development. The Plant cell 2005, 17(3):746-759.
- Schwartz SH, Qin X, Loewen MC: The biochemical characterization of two carotenoid cleavage enzymes from Arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching. The Journal of biological chemistry 2004, 279(45):46940-46945.
- Lopez-Obando M, Ligerot Y, Bonhomme S, Boyer F-D, Rameau C: Strigolactone biosynthesis and signaling in plant development. Development 2015, 142(21):3615-3619.
- Vogel JT, Walter MH, Giavalisco P, Lytovchenko A, Kohlen W, Charnikhova T, Simkin AJ, Goulet C, Strack D, Bouwmeester HJ et al: SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. The Plant journal : for cell and molecular biology 2010, 61(2):300-311.
- Jia KP, Baz L, Al-Babili S: From carotenoids to strigolactones. J Exp Bot 2018, 69(9):2189-2204.
- Qin X, Zeevaart JAD: The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proceedings of the National Academy of Sciences 1999, 96(26):15354-15361.
- Parry AD, Babiano MJ, Horgan R: The role of cis-carotenoids in abscisic acid biosynthesis. Planta 1990, 182:118-128.
- Dong T, Park Y, Hwang I: Abscisic acid: biosynthesis, inactivation, homoeostasis and signalling. Essays Biochem 2015, 58:29-48.
- Simkin AJ, Schwartz SH, Auldridge M, Taylor MG, Klee HJ: The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles beta-ionone, pseudoionone, and geranylacetone. Plant J 2004, 40(6):882-892.
- Simkin AJ, Underwood BA, Auldridge M, Loucas HM, Shibuya K, Schmelz E, Clark DG, Klee HJ: Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of beta-ionone, a fragrance volatile of petunia flowers. Plant physiology 2004, 136(3):3504-3514.
- Auldridge ME, Block A, Vogel JT, Dabney-Smith C, Mila I, Bouzayen M, Magallanes-Lundback M, DellaPenna D, McCarty DR, Klee HJ: Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. The Plant journal : for cell and molecular biology 2006, 45(6):982-993.
- Auldridge ME, McCarty DR, Klee HJ: Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr Opin Plant Biol 2006, 9(3):315-321.
- Schwartz SH, Qin X, Zeevaart JA: Characterization of a novel carotenoid cleavage dioxygenase from plants. J Biol Chem 2001, 276(27):25208-25211.
- Giberti S, Giovannini D, Forlani G: Carotenoid cleavage in chromoplasts of white and yellow-fleshed peach varieties. J Sci Food Agric 2019, 99(4):1795-1803.
- Simkin AJ, Moreau H, Kuntz M, Pagny G, Lin C, Tanksley S, McCarthy J: An investigation of carotenoid biosynthesis in Coffea canephora and Coffea arabica. J Plant Physiol 2008, 165(10):1087-1106.
- Simkin A, McCarthy J, Petiard V, Lin C, Tanksley S: Polynucleotides encoding carotenoid and apocartenoid biosynthetic pathway enzymes in coffee. Patent WO2007028115A2. In.; 2007.
- Simkin AJ: Genetic Engineering for Global Food Security: Photosynthesis and Biofortification. Plants 2019, 8(12):586.
- Mezzomo N, #xe1, lia, Ferreira SRS: Carotenoids Functionality, Sources, and Processing by Supercritical Technology: A Review. Journal of Chemistry 2016, 2016:16.
- Cunningham FX, Gantt E: Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Phys 1998, 49(1):557-583.
- Hirschberg J: Carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol 2001, 4(3):210-218.
- Ruban AV, Lee PJ, Wentworth M, Young AJ, Horton P: Determination of the stoichiometry and strength of binding of xanthophylls to the photosystem II light harvesting complexes. Journal of Biological Chemistry 1999, 274(15):10458-10465.
- Ruban AV, Young AJ, Pascal AA, Horton P: The Effects of Illumination on the Xanthophyll Composition of the Photosystem II Light-Harvesting Complexes of Spinach Thylakoid Membranes. Plant physiology 1994, 104(1):227-234.
- Thayer SS, Björkman O: Carotenoid distribution and deepoxidation in thylakoid pigment-protein complexes from cotton leaves and bundle-sheath cells of maize. Photosynthesis research 1992, 33(3):213-225.
- Bartley GE, Viitanen PV, Bacot KO, Scolnik PA: A tomato gene expressed during fruit ripening encodes an enzyme of the carotenoid biosynthesis pathway. Journal of Biological Chemistry 1992, 267(8):5036-5039.
- Hugueney P, Römer S, Kuntz M, Camara B: Characterization and molecular cloning of a flavoprotein catalyzing the synthesis of phytofluene and ζ-carotene in Capsicum chromoplasts. European journal of biochemistry 1992, 209(1):399-407.
- Bartley GE, Ishida BK: Zeta-carotene desaturase from tomato. Plant Physiology 1999, 121:1383.
- Albrecht M, Klein A, Hugueney P, Sandmann G, Kuntz M: Molecular cloning and functional expression in E. coli of a novel plant enzyme mediating ξ-carotene desaturation. FEBS letters 1995, 372(2-3):199-202.
- Chen Y, Li F, Wurtzel ET: Isolation and Characterization of the Z-ISO Gene Encoding a Missing Component of Carotenoid Biosynthesis in Plants Plant Physiology 2010, 153(1):66-79.
- Yu Q, Ghisla S, Hirschberg J, Mann V, Beyer P: Plant Carotene Cis-Trans Isomerase CRTISO: A new member of the FAD RED dependent flavoproteins catalysing non-redox reactions. Journal of Biological Chemistry 2011, 286(10):8666-8676.
- Isaacson T, Ronen G, Zamir D, Hirschberg J: Cloning of tangerine from Tomato Reveals a Carotenoid Isomerase Essential for the Production of β-Carotene and Xanthophylls in Plants. The Plant Cell 2002, 14(2):333-342.
- Park H, Kreunen SS, Cuttriss AJ, DellaPenna D, Pogson BJ: Identification of the Carotenoid Isomerase Provides Insight into Carotenoid Biosynthesis, Prolamellar Body Formation, and Photomorphogenesis. The Plant Cell 2002, 14(2):321-332.
- Carol P, Stevenson D, Bisanz C, Breitenbach J, Sandmann G, Mache R, Coupland G, Kuntz M: Mutations in the Arabidopsis Gene IMMUTANS Cause a Variegated Phenotype by Inactivating a Chloroplast Terminal Oxidase Associated with Phytoene Desaturation. The Plant cell 1999, 11(1):57-68.
- Josse E-M, Alcaraz J-P, Labouré A-M, Kuntz M: In vitro characterization of a plastid terminal oxidase (PTOX). European journal of biochemistry 2003, 270(18):3787-3794.
- Kuntz M: Plastid terminal oxidase and its biological significance. Planta 2004, 218(6):896-899.
- Kambakam S, Bhattacharjee U, Petrich J, Rodermel S: PTOX Mediates Novel Pathways of Electron Transport in Etioplasts of Arabidopsis. Mol Plant 2016, 9(9):1240-1259.
- Cunningham FX, Pogson B, Sun Z, McDonald KA, DellaPenna D, Gantt E: Functional analysis of the beta and epsilon lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. The Plant cell 1996, 8(9):1613-1626.
- Ronen G, Cohen M, Zamir D, Hirschberg J: Regulation of carotenoid biosynthesis during tomato fruit development: expression of the gene for lycopene epsilon-cyclase is down-regulated during ripening and is elevated in the mutant Delta. The Plant Journal 1999, 17(4):341-351.
- Cunningham FX, Gantt E: One ring or two? Determination of ring number in carotenoids by lycopene ɛ-cyclases. Proceedings of the National Academy of Sciences 2001, 98(5):2905-2910.
- Partensky F, Hoepffner N, Li W, Ulloa O, Vaulot D: Photoacclimation of Prochlorococcus sp. (Prochlorophyta) Strains Isolated from the North Atlantic and the Mediterranean Sea. Plant physiology 1993, 101(1):285-296.
- Krubasik P, Sandmann G: Molecular evolution of lycopene cyclases involved in the formation of carotenoids with ionone end groups. Biochemical Society transactions 2000, 28(6):806-810.
- Hess WR, Rocap G, Ting CS, Larimer F, Stilwagen S, Lamerdin J, Chisholm SW: The photosynthetic apparatus of Prochlorococcus: Insights through comparative genomics. Photosynthesis research 2001, 70(1):53-71.
- Stickforth P, Steiger S, Hess WR, Sandmann G: A novel type of lycopene ε-cyclase in the marine cyanobacterium Prochlorococcus marinus MED4. Arch Microbiol 2003, 179(6):409-415.
- Sandmann G: Carotenoid biosynthesis in microorganisms and plants. European journal of biochemistry 1994, 223(1):7-24.
- Sun Z, Gantt E, Cunningham FX: Cloning and Functional Analysis of the β-Carotene Hydroxylase of Arabidopsis thaliana. Journal of Biological Chemistry 1996, 271(40):24349-24352.
- Kim J, DellaPenna D: Defining the primary route for lutein synthesis in plants: the role of Arabidopsis carotenoid beta-ring hydroxylase CYP97A3. Proceedings of the National Academy of Sciences 2006, 103(9):3474-3479.
- Tian L, DellaPenna D: Characterization of a second carotenoid β-hydroxylase gene from Arabidopsis and its relationship to the LUT1 locus. Plant molecular biology 2001, 47(3):379-388.
- Tian L, Magallanes-Lundback M, Musetti V, DellaPenna D: Functional Analysis of β- and ε-Ring Carotenoid Hydroxylases in Arabidopsis. The Plant cell 2003, 15(6):1320-1332.
- Tian L, DellaPenna D: Progress in understanding the origin and functions of carotenoid hydroxylases in plants. Arch Biochem Biophys 2004, 430(1):22-29.
- Tian L, Musetti V, Kim J, Magallanes-Lundback M, DellaPenna D: The Arabidopsis LUT1 locus encodes a member of the cytochrome P450 family that is required for carotenoid ε-ring hydroxylation activity. Proceedings of the National Academy of Sciences 2004, 101(1):402-407.
- Inoue K: Carotenoid hydroxylation--P450 finally! . Trends Plant Science 2004, 9:515-517.
- Pogson BJ, Niyogi KK, Björkman O, DellaPenna D: Altered xanthophyll compositions adversely affect chlorophyll accumulation and nonphotochemical quenching in <em>Arabidopsis</em> mutants. Proceedings of the National Academy of Sciences 1998, 95(22):13324-13329.
- Lokstein H, Tian L, Polle JEW, DellaPenna D: Xanthophyll biosynthetic mutants of Arabidopsis thaliana: altered nonphotochemical quenching of chlorophyll fluorescence is due to changes in Photosystem II antenna size and stability. Biochimica et Biophysica Acta (BBA) - Bioenergetics 2002, 1553(3):309-319.
- Dall'Osto L, Fiore A, Cazzaniga S, Giuliano G, Bassi R: Different roles of alpha- and beta-branch xanthophylls in photosystem assembly and photoprotection. The Journal of biological chemistry 2007, 282(48):35056-35068.
- Dall'Osto L, Lico C, Alric J, Giuliano G, Havaux M, Bassi R: Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC plant biology 2006, 6:32.
- García-Plazaola JI, Matsubara S, Osmond CB: The lutein epoxide cycle in higher plants: its relationships to other xanthophyll cycles and possible functions. Funct Plant Biol 2007, 34:759-773.
- Li Z, Ahn TK, Avenson TJ, Ballottari M, Cruz JA, Kramer DM, Bassi R, Fleming GR, Keasling JD, Niyogi KK: Lutein accumulation in the absence of zeaxanthin restores nonphotochemical quenching in the Arabidopsis thaliana npq1 mutant. The Plant cell 2009, 21(6):1798-1812.
- Jahns P, Wehner A, Paulsen H, Hobe S: De-epoxidation of violaxanthin after reconstitution into different carotenoid binding sites of light-harvesting complex II. The Journal of biological chemistry 2001, 276(25):22154-22159.
- Matsubara S, Krause GH, Aranda J, Virgo A, Beisel KG, Jahns P, Winter K: Sun-shade patterns of leaf carotenoid composition in 86 species of neotropical forest plants. Functional Plant Biology 2009, 36:20-36.
- Standfuss J, Terwisscha van Scheltinga AC, Lamborghini M, Kuhlbrandt W: Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 A resolution. The EMBO journal 2005, 24(5):919-928.
- Marin E, Nussaume L, Quesada A, Gonneau M, Sotta B, Hugueney P, Frey A, Marion-Poll A: Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in abscisic acid biosynthesis and corresponding to the ABA locus of Arabidopsis thaliana. The EMBO journal 1996, 15(10):2331-2342.
- Bouvier F, d'Harlingue A, Hugueney P, Marin E, Marion-Poll A, Camara B: Xanthophyll Biosynthesis: Cloning, expression, functional reconstitution, and regulation of β-cyclohexenyl carotenoid epoxidase from pepper (Capsicum annuum). Journal of Biological Chemistry 1996, 271(46):28861-28867.
- Esteban R, Moran JF, Becerril JM, García-Plazaola JI: Versatility of carotenoids: An integrated view on diversity, evolution, functional roles and environmental interactions. Environ Exp Bot 2015, 119:63-75.
- Brüggemann W, Bergmann M, Nierbauer K-U, Pflug E, Schmidt C, Weber D: Photosynthesis studies on European evergreen and deciduous oaks grown under Central European climate conditions: II. Photoinhibitory and light-independent violaxanthin deepoxidation and downregulation of photosystem II in evergreen, winter-acclimated European Quercus taxa. Trees (Berl West) 2009, 23(5):1091-1100.
- Hieber AD, Bugos RC, Yamamoto HY: Plant lipocalins: violaxanthin de-epoxidase and zeaxanthin epoxidase. Biochim Biophys Acta 2000, 1482(1-2):84-91.
- Rabinowitch HD, Budowski P, Kedar N: Carotenoids and epoxide cycles in mature-green tomatoes. Planta 1975, 122(1):91-97.
- Bouvier F, D’Harlingue A, Backhaus RA, Kumagai MH, Camara B: Identification of neoxanthin synthase as a carotenoid cyclase paralog. European journal of biochemistry 2000, 267(21):6346-6352.
- Al-Babili S, Hugueney P, Schledz M, Welsch R, Frohnmeyer H, Laule O, Beyer P: Identification of a novel gene coding for neoxanthin synthase from Solanum tuberosum. FEBS letters 2000, 485(2-3):168-172.
- Kuntz M, Chen HC, Simkin AJ, Römer S, Shipton CA, Drake R, Schuch W, Bramley PM: Upregulation of two ripening-related genes from a non-climacteric plant (pepper) in a transgenic climacteric plant (tomato). The Plant Journal 1998, 13(3):351-361.
- Bouvier F, Hugueney P, D'Harlingue A, Kuntz M, Camara B: Xanthophyll biosynthesis in chromoplasts: isolation and molecular cloning of an enzyme catalyzing the conversion of 5,6-epoxycarotenoid into ketocarotenoid. The Plant Journal 1994, 6(1):45-54.
- Lefebvre V, Kuntz M, Camara B, Palloix A: The capsanthin-capsorubin synthase gene: a candidate gene for the y locus controlling the red fruit colour in pepper. Plant molecular biology 1998, 36(5):785-789.
- Ronen G, Carmel-Goren L, Zamir D, Hirschberg J: An alternative pathway to β-carotene formation in plant chromoplasts discovered by map-based cloning of <em>Beta</em> and <em>old-gold</em> color mutations in tomato. Proceedings of the National Academy of Sciences 2000, 97(20):11102-11107.
- Hugueney P, Badillo A, Chen H-C, Klein A, Hirschberg J, Camara B, Kuntz M: Metabolism of cyclic carotenoids: a model for the alteration of this biosynthetic pathway in Capsicum annuum chromoplasts. The Plant Journal 1995, 8(3):417-424.
- Fujisawa M, Misawa N: Enrichment of carotenoids in flaxseed by introducing a bacterial phytoene synthase gene. Methods Mol Biol 2010, 643:201-211.
- Fujisawa M, Watanabe M, Choi SK, Teramoto M, Ohyama K, Misawa N: Enrichment of carotenoids in flaxseed (Linum usitatissimum) by metabolic engineering with introduction of bacterial phytoene synthase gene crtB. Journal of bioscience and bioengineering 2008, 105(6):636-641.
- Cong L, Wang C, Chen L, Liu H, Yang G, He G: Expression of phytoene synthase1 and Carotene Desaturase crtI Genes Result in an Increase in the Total Carotenoids Content in Transgenic Elite Wheat (Triticum aestivum L.). Journal of agricultural and food chemistry 2009, 57(18):8652-8660.
- Che P, Zhao Z-Y, Glassman K, Dolde D, Hu TX, Jones TJ, Gruis DF, Obukosia S, Wambugu F, Albertsen MC: Elevated vitamin E content improves all-trans β-carotene accumulation and stability in biofortified sorghum. Proceedings of the National Academy of Sciences 2016, 113(39):11040-11045.
- Lipkie TE, De Moura FF, Zhao Z-Y, Albertsen MC, Che P, Glassman K, Ferruzzi MG: Bioaccessibility of Carotenoids from Transgenic Provitamin A Biofortified Sorghum. Journal of agricultural and food chemistry 2013, 61(24):5764-5771.
- Shewmaker CK, Sheehy JA, Daley M, Colburn S, Ke DY: Seed-specific overexpression of phytoene synthase: increase in carotenoids and other metabolic effects. The Plant journal : for cell and molecular biology 1999, 20(4):401-412X.
- Ye X, Al-Babili S, Kloti A, Zhang J, Lucca P, Beyer P, Potrykus I: Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 2000, 287(5451):303-305.
- Paine JA, Shipton CA, Chaggar S, Howells RM, Kennedy MJ, Vernon G, Wright SY, Hinchliffe E, Adams JL, Silverstone AL et al: Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nature biotechnology 2005, 23(4):482-487.
- Al-Babili S, Hoa TT, Schaub P: Exploring the potential of the bacterial carotene desaturase CrtI to increase the beta-carotene content in Golden Rice. J Exp Bot 2006, 57(4):1007-1014.
- Diretto G, Al-Babili S, Tavazza R, Papacchioli V, Beyer P, Giuliano G: Metabolic engineering of potato carotenoid content through tuber-specific overexpression of a bacterial mini-pathway. Plos One 2007, 2(4):e350.
- Beyene G, Solomon FR, Chauhan RD, Gaitan-Solis E, Narayanan N, Gehan J, Siritunga D, Stevens RL, Jifon J, Van Eck J et al: Provitamin A biofortification of cassava enhances shelf life but reduces dry matter content of storage roots due to altered carbon partitioning into starch. Plant biotechnology journal 2017.
- Ducreux LJM, Morris WL, Hedley PE, Shepherd T, Davies HV, Millam S, Taylor MA: Metabolic engineering of high carotenoid potato tubers containing enhanced levels of β-carotene and lutein. J Exp Bot 2004, 56(409):81-89.
- Römer S, Fraser PD, Kiano JW, Shipton CA, Misawa N, Schuch W, Bramley PM: Elevation of the provitamin A content of transgenic tomato plants. Nature biotechnology 2000, 18(6):666-669.
- Fraser PD, Romer S, Shipton CA, Mills PB, Kiano JW, Misawa N, Drake RG, Schuch W, Bramley PM: Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner. Proceedings of the National Academy of Sciences 2002, 99(2):1092-1097.
- Fraser PD, Enfissi EM, Halket JM, Truesdale MR, Yu D, Gerrish C, Bramley PM: Manipulation of phytoene levels in tomato fruit: effects on isoprenoids, plastids, and intermediary metabolism. Plant Cell 2007, 19(10):3194-3211.
- McQuinn RP, Wong B, Giovannoni JJ: AtPDS overexpression in tomato: exposing unique patterns of carotenoid self-regulation and an alternative strategy for the enhancement of fruit carotenoid content. Plant Biotechnol J 2018, 16(2):482-494.
- McQuinn RP, Gapper NE, Gray AG, Zhong S, Tohge T, Fei Z, Fernie AR, Giovannoni JJ: Manipulation of ZDS in tomato exposes carotenoid- and ABA-specific effects on fruit development and ripening. Plant Biotechnology Journal 2020, 18(11):2210-2224.
- D’Ambrosio C, Giorio G, Marino I, Merendino A, Petrozza A, Salfi L, Stigliani AL, Cellini F: Virtually complete conversion of lycopene into β-carotene in fruits of tomato plants transformed with the tomato lycopene β-cyclase (tlcy-b) cDNA. Plant Sci 2004, 166(1):207-214.
- Morris WL, Ducreux LJM, Hedden P, Millam S, Taylor MA: Overexpression of a bacterial 1-deoxy-D-xylulose 5-phosphate synthase gene in potato tubers perturbs the isoprenoid metabolic network: implications for the control of the tuber life cycle. J Exp Bot 2006, 57(12):3007-3018.
- Schmidt MA, Parrott WA, Hildebrand DF, Berg RH, Cooksey A, Pendarvis K, He Y, McCarthy F, Herman EM: Transgenic soya bean seeds accumulating β-carotene exhibit the collateral enhancements of oleate and protein content traits. Plant biotechnology journal 2015, 13(4):590-600.
- Paul JY, Khanna H, Kleidon J, Hoang P, Geijskes J, Daniells J, Zaplin E, Rosenberg Y, James A, Mlalazi B et al: Golden bananas in the field: elevated fruit pro-vitamin A from the expression of a single banana transgene. Plant biotechnology journal 2017, 15(4):520-532.
- Naqvi S, Zhu C, Farre G, Ramessar K, Bassie L, Breitenbach J, Perez Conesa D, Ros G, Sandmann G, Capell T et al: Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proceedings of the National Academy of Sciences 2009, 106(19):7762-7767.
- Aluru M, Xu Y, Guo R, Wang Z, Li S, White W, Wang K, Rodermel S: Generation of transgenic maize with enhanced provitamin A content. J Exp Bot 2008, 59(13):3551-3562.
- Bai C, Capell T, Berman J, Medina V, Sandmann G, Christou P, Zhu C: Bottlenecks in carotenoid biosynthesis and accumulation in rice endosperm are influenced by the precursor-product balance. Plant Biotechnol J 2016, 14(1):195-205.
- Lu S, Van Eck J, Zhou X, Lopez AB, O'Halloran DM, Cosman KM, Conlin BJ, Paolillo DJ, Garvin DF, Vrebalov J et al: The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high levels of beta-carotene accumulation. The Plant cell 2006, 18(12):3594-3605.
- Yazdani M, Sun Z, Yuan H, Zeng S, Thannhauser TW, Vrebalov J, Ma Q, Xu Y, Fei Z, Van Eck J et al: Ectopic expression of ORANGE promotes carotenoid accumulation and fruit development in tomato. Plant biotechnology journal 2019, 17(1):33-49.
- Li L, Yang Y, Xu Q, Owsiany K, Welsch R, Chitchumroonchokchai C, Lu S, Van Eck J, Deng X-X, Failla M et al: The Or Gene Enhances Carotenoid Accumulation and Stability During Post-Harvest Storage of Potato Tubers. Molecular plant 2012, 5(2):339-352.
- Lopez AB, Van Eck J, Conlin BJ, Paolillo DJ, O'Neill J, Li L: Effect of the cauliflower Or transgene on carotenoid accumulation and chromoplast formation in transgenic potato tubers. J Exp Bot 2008, 59(2):213-223.
- Park S, Kim HS, Jung YJ, Kim SH, Ji CY, Wang Z, Jeong JC, Lee HS, Lee SY, Kwak SS: Orange protein has a role in phytoene synthase stabilization in sweetpotato. Sci Rep 2016, 6:33563.
- Berman J, Zorrilla-López U, Medina V, Farré G, Sandmann G, Capell T, Christou P, Zhu C: The Arabidopsis ORANGE (AtOR) gene promotes carotenoid accumulation in transgenic corn hybrids derived from parental lines with limited carotenoid pools. Plant Cell Rep 2017, 36(6):933-945.
- Zhou X, Welsch R, Yang Y, Álvarez D, Riediger M, Yuan H, Fish T, Liu J, Thannhauser TW, Li L: Arabidopsis Or proteins are the major posttranscriptional regulators of phytoene synthase in controlling carotenoid biosynthesis. Proceedings of the National Academy of Sciences 2015, 112(11):3558-3563.
- Chayut N, Yuan H, Ohali S, Meir A, Sa'ar U, Tzuri G, Zheng Y, Mazourek M, Gepstein S, Zhou X et al: Distinct Mechanisms of the ORANGE Protein in Controlling Carotenoid Flux. Plant Physiol 2017, 173(1):376-389.
- Osorio CE: The Role of Orange Gene in Carotenoid Accumulation: Manipulating Chromoplasts Toward a Colored Future. Front Plant Sci 2019, 10:1235.
- Park SC, Kim SH, Park S, Lee HU, Lee JS, Park WS, Ahn MJ, Kim YH, Jeong JC, Lee HS et al: Enhanced accumulation of carotenoids in sweetpotato plants overexpressing IbOr-Ins gene in purple-fleshed sweetpotato cultivar. Plant Physiol Biochem 2015, 86:82-90.
- Gonzalez-Jorge S, Ha S-H, Magallanes-Lundback M, Gilliland LU, Zhou A, Lipka AE, Nguyen Y-N, Angelovici R, Lin H, Cepela J et al: Carotenoid cleavage dioxygenase4 Is a Negative Regulator of β-Carotene Content in Arabidopsis Seeds. The Plant Cell 2013, 25(12):4812-4826.
- Frusciante S, Diretto G, Bruno M, Ferrante P, Pietrella M, Prado-Cabrero A, Rubio-Moraga A, Beyer P, Gomez-Gomez L, Al-Babili S et al: Novel carotenoid cleavage dioxygenase catalyzes the first dedicated step in saffron crocin biosynthesis. Proc Natl Acad Sci U S A 2014, 111(33):12246-12251.
- Rubio A, Rambla JL, Santaella M, Gomez MD, Orzaez D, Granell A, Gomez-Gomez L: Cytosolic and plastoglobule-targeted carotenoid dioxygenases from Crocus sativus are both involved in beta-ionone release. J Biol Chem 2008, 283(36):24816-24825.
- Falchi R, Vendramin E, Zanon L, Scalabrin S, Cipriani G, Verde I, Vizzotto G, Morgante M: Three distinct mutational mechanisms acting on a single gene underpin the origin of yellow flesh in peach. The Plant Journal 2013, 76(2):175-187.
- Ohmiya A, Kishimoto S, Aida R, Yoshioka S, Sumitomo K: Carotenoid Cleavage Dioxygenase (CmCCD4a) Contributes to White Color Formation in Chrysanthemum Petals. Plant Physiology 2006, 142(3):1193-1201.
- Campbell R, Ducreux LJM, Morris WL, Morris JA, Suttle JC, Ramsay G, Bryan GJ, Hedley PE, Taylor MA: The metabolic and developmental roles of carotenoid cleavage dioxygenase4 from potato. Plant physiology 2010, 154(2):656-664.
- Ohmiya A, Sumitomo K, Aida R: Yellow jimba: suppression of carotenoid cleavage dioxygenase (CmCCD4a) expression turns white Chrysanthemum petals yellow. Journal of the Japanese Society for Horticultural Science 2009, 78(4):450-455.
- Meléndez-Martínez AJ, Mapelli-Brahm P, Benítez-González A, Stinco CM: A comprehensive review on the colorless carotenoids phytoene and phytofluene. Archives of Biochemistry and Biophysics 2015, 572:188-200.
- Meléndez-Martínez AJ, Mapelli-Brahm P, Stinco CM: The colourless carotenoids phytoene and phytofluene: From dietary sources to their usefulness for the functional foods and nutricosmetics industries. Journal of Food Composition and Analysis 2018, 67:91-103.
- Olson JA: Provitamin A Function of Carotenoids: The Conversion of β-Carotene into Vitamin A. The Journal of Nutrition 1989, 119(1):105-108.
- West CE, Rombout JH, van der Zijpp AJ, Sijtsma SR: Vitamin A and immune function. Proc Nutr Soc 1991, 50(2):251-262.
- Tanumihardjo SA: Vitamin A: biomarkers of nutrition for development. The American Journal of Clinical Nutrition 2011, 94(2):658S-665S.
- Rando RR: The chemistry of vitamin A and vision. Angew Chem Int 1990, 29:461-480.
- von Lintig J, Vogt K: Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem 2000, 275(16):11915-11920.
- Hodge J: Hidden hunger: Approaches to tackling micronutrient deficiencies. In: Nourishing millions: Stories of change in nutrition. Edited by Gillespie S, Hodge J, Yosef S, Pandya-Lorch R. Washington, D.C: International Food Policy Research Institute (IFPRI); 2016: 35-46.
- Gannon B, Kaliwile C, Arscott SA, Schmaelzle S, Chileshe J, Kalungwana N, Mosonda M, Pixley KV, Masi C, Tanumihardjo SA: Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: a community-based, randomized placebo-controlled trial. Am J Clin Nutr 2014, 100(6):1541–1550.
- Palmer AC, Healy K, Barffour MA, Siamusantu W, Chileshe J, Schulze KJ, West KP, Jr., Labrique AB: Provitamin A Carotenoid-Biofortified Maize Consumption Increases Pupillary Responsiveness among Zambian Children in a Randomized Controlled Trial. J Nutr 2016, 146(12):2551-2558.
- Regis E: Golden Rice: The Imperiled Birth of a GMO Superfood: Johns Hopkins University Press; 2019.
- Liu X, Song M, Gao Z, Cai X, Dixon W, Chen X, Cao Y, Xiao H: Stereoisomers of Astaxanthin Inhibit Human Colon Cancer Cell Growth by Inducing G2/M Cell Cycle Arrest and Apoptosis. Journal of Agricultural and Food Chemistry 2016, 64(41):7750-7759.
- Shareck M, Rousseau M-C, Koushik A, Siemiatycki J, Parent M-E: Inverse Association between Dietary Intake of Selected Carotenoids and Vitamin C and Risk of Lung Cancer. Frontiers in Oncology 2017, 7(23).
- Ansari M, Ansari S: Lycopene and prostate cancer. Future Oncology 2005, 1(3):425-430.
- Rafi MM, Kanakasabai S, Gokarn SV, Krueger EG, Bright JJ: Dietary Lutein Modulates Growth and Survival Genes in Prostate Cancer Cells. Journal of Medicinal Food 2015, 18(2):173-181.
- Kotake-Nara E, Kushiro M, Zhang H, Sugawara T, Miyashita K, Nagao A: Carotenoids affect proliferation of human prostate cancer cells. J Nutr 2001, 131(12):3303-3306.
- Mayne ST, Cartmel B, Lin H, Zheng T, Goodwin WJ, Jr.: Low plasma lycopene concentration is associated with increased mortality in a cohort of patients with prior oral, pharynx or larynx cancers. J Am Coll Nutr 2004, 23(1):34-42.
- De Waart F, Schouten E, Stalenhoef A, Kok F: Serum carotenoids, α-tocopherol and mortality risk in a prospective study among Dutch elderly. International Journal of Epidemiology 2001, 30(1):136-143.
- Chang J, Zhang Y, Li Y, Lu K, Shen Y, Guo Y, Qi Q, Wang M, Zhang S: NrF2/ARE and NF-κB pathway regulation may be the mechanism for lutein inhibition of human breast cancer cell. Future Oncology 2018, 14(8):719-726.
- Gloria NF, Soares N, Brand C, Oliveira FL, Borojevic R, Teodoro AJ: Lycopene and beta-carotene induce cell-cycle arrest and apoptosis in human breast cancer cell lines. Anticancer Res 2014, 34(3):1377-1386.
- Upadhyaya KR, Radha KS, Madhyastha HK: Cell cycle regulation and induction of apoptosis by beta-carotene in U937 and HL-60 leukemia cells. J Biochem Mol Biol 2007, 40(6):1009-1015.
- Nara E, Hayashi H, Kotake M, Miyashita K, Nagao A: Acyclic carotenoids and their oxidation mixtures inhibit the growth of HL-60 human promyelocytic leukemia cells. Nutr Cancer 2001, 39(2):273-283.
- Niranjana R, Gayathri R, Nimish Mol S, Sugawara T, Hirata T, Miyashita K, Ganesan P: Carotenoids modulate the hallmarks of cancer cells. Journal of Functional Foods 2015, 18:968-985.
- Cha JH, Kim WK, Ha AW, Kim MH, Chang MJ: Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr Res Pract 2017, 11(2):90-96.
- Lee H.S, Jung J.I, Kang Y.H, Khachik F, J.H. Y: Effect of Lycopene on the Insulin-like Growth Factor-I Receptor Signaling Pathway in Human Colon Cancer HT-29 Cells. Journal of the Korean Society of Food Science and Nutrition 2003, 32(3):437-443.
- Huang RF, Wei YJ, Inbaraj BS, Chen BH: Inhibition of colon cancer cell growth by nanoemulsion carrying gold nanoparticles and lycopene. Int J Nanomedicine 2015, 10:2823-2846.
- Sahin K, Tuzcu M, Sahin N, Akdemir F, Ozercan I, Bayraktar S, Kucuk O: Inhibitory effects of combination of lycopene and genistein on 7,12- dimethyl benz(a)anthracene-induced breast cancer in rats. Nutr Cancer 2011, 63(8):1279-1286.
- Sahin K, Yenice E, Tuzcu M, Orhan C, Mizrak C, Ozercan IH, Sahin N, Yilmaz B, Bilir B, Ozpolat B et al: Lycopene Protects Against Spontaneous Ovarian Cancer Formation in Laying Hens. J Cancer Prev 2018, 23(1):25-36.
- Thies F, Mills LM, Moir S, Masson LF: Cardiovascular benefits of lycopene: fantasy or reality? Proceedings of the Nutrition Society 2017, 76(2):122-129.
- Alvi SS, Iqbal D, Ahmad S, Khan MS: Molecular rationale delineating the role of lycopene as a potent HMG-CoA reductase inhibitor: in vitro and in silico study. Natural Product Research 2016, 30(18):2111-2114.
- Sandoval V, Rodríguez-Rodríguez R, Martínez-Garza Ú, Rosell-Cardona C, Lamuela-Raventós RM, Marrero PF, Haro D, Relat J: Mediterranean Tomato-Based Sofrito Sauce Improves Fibroblast Growth Factor 21 (FGF21) Signaling in White Adipose Tissue of Obese ZUCKER Rats. Molecular Nutrition & Food Research 2018, 62(4):1700606.
- Gammone MA, Riccioni G, D’Orazio N: Carotenoids: potential allies of cardiovascular health? Food & Nutrition Research 2015, 59.
- Costa-Rodrigues J, Pinho O, Monteiro PRR: Can lycopene be considered an effective protection against cardiovascular disease? Food Chemistry 2018, 245:1148-1153.
- Cheng HM, Koutsidis G, Lodge JK, Ashor A, Siervo M, Lara J: Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis 2017, 257:100–108.
- Chung RWS, Leanderson P, Lundberg AK, Jonasson L: Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis 2017, 262:87–93.
- Kishimoto Y, Taguchi C, Saita E, Suzuki-Sugihara N, Nishiyama H, Wang W, Masuda Y, Kondo K: Additional consumption of one egg per day increases serum lutein plus zeaxanthin concentration and lowers oxidized low-density lipoprotein in moderately hypercholesterolemic males. Food Research International 2017, 99:944-949.
- Leermakers ET, Darweesh SK, Baena CP, Moreira EM, Melo van Lent D, Tielemans MJ, Muka T, Vitezova A, Chowdhury R, Bramer WM et al: The effects of lutein on cardiometabolic health across the life course: a systematic review and meta-analysis1,2. The American Journal of Clinical Nutrition 2016, 103(2):481-494.
- Christensen K, Gleason CE, Mares JA: Dietary carotenoids and cognitive function among US adults, NHANES 2011–2014. Nutritional Neuroscience 2020, 23(7):554-562.
- Krishnaraj RN, Kumari SSS, Mukhopadhyay SS: Antagonistic molecular interactions of photosynthetic pigments with molecular disease targets: a new approach to treat AD and ALS. Journal of Receptors and Signal Transduction 2016, 36(1):67-71.
- Min J, Min K: Serum Lycopene, Lutein and Zeaxanthin, and the Risk of Alzheimer's Disease Mortality in Older Adults. Dementia and Geriatric Cognitive Disorders 2014, 37(3-4):246-256.
- Tan BL, Norhaizan ME: Carotenoids: How Effective Are They to Prevent Age-Related Diseases? Molecules (Basel, Switzerland) 2019, 24(9):1801.
- Rivera-Madrid R, Carballo-Uicab VM, Cárdenas-Conejo Y, Aguilar-Espinosa M, Siva R: 1 - Overview of carotenoids and beneficial effects on human health. In: Carotenoids: Properties, Processing and Applications. Edited by Galanakis CM: Academic Press; 2020: 1-40.
- Milani A, Basirnejad M, Shahbazi S, Bolhassani A: Carotenoids: biochemistry, pharmacology and treatment. Br J Pharmacol 2017, 174(11):1290-1324.
- Eggersdorfer M, Wyss A: Carotenoids in human nutrition and health. Arch Biochem Biophys 2018, 652:18-26.
- Tan B-C, Joseph LM, Deng W-T, Liu L, Li Q-B, Cline K, McCarty DR: Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. The Plant Journal 2003, 35(1):44-56.
- Hou X, Rivers J, León P, McQuinn RP, Pogson BJ: Synthesis and Function of Apocarotenoid Signals in Plants. Trends in Plant Science 2016, 21(9):792-803.
- Simkin AJ, Kuntz M, Moreau H, McCarthy J: Carotenoid profiling and the expression of carotenoid biosynthetic genes in developing coffee grain. Plant Physiol Biochem 2010, 48(6):434-442.
