Micronutrients such as selenium, fluoride, zinc, iron, and manganese are minerals that are crucial for many body homeostatic processes supplied at low levels. The importance of these micronutrients starts early in the human life cycle and continues across its different stages.
1. Introduction
Minerals are inorganic elements essentially required by humans to carry out important functions throughout the human life, with approximately 20 mineral elements discovered to be vital for their electrolyte balance, structural and functional roles
[1]. The most abundant elements are carbon, hydrogen, oxygen and nitrogen, accounting for approximately 96% of the human body weight although they are not listed as nutrient minerals, whereas macro minerals (i.e., magnesium, sodium, calcium, sulfur, chlorine, phosphorus and potassium) and micro minerals (i.e., manganese, zinc, cobalt, copper, molybdenum, fluoride, iron, iodine and selenium) make up the remaining percentage
[2].
Several health benefits have been associated with micronutrients’ (minerals and vitamins) intake attributed to their functions in the enzyme system as cofactors and coenzymes, healthy bones and teeth formation, maintenance of body tissues as well as other physiological and biochemical functions
[3]. These elements are derived from different dietary sources typically required by humans at different levels (generally less than 100 milligrams per day) to prevent against these deficiencies by nutrient supplementations and or fortifications
[4,5,6][4][5][6]. Examples include salt iodization, iron and folate supplementation programs for child-bearing women, micronutrient powders and bio-fortification of crops
[3].
Deficiency of trace element nutrients is more likely to be encountered in pregnant and lactating women than normal adults which can only be met through eating a balanced diet
[7]. For example, hypocalcemia negatively influence the permeability of membranes and the power of smooth muscle contractions, which can impact blood pressure and could initiate preterm uterine contractions
[8]. Furthermore, aging process involves cellular senescence in body tissues and organs ultimately leading to the production of reactive oxygen species (ROS) which impairs proper function of cells
[9,10][9][10]. Therefore, adequate daily intake is crucial. For example, copper is essential for maintaining cognitive function in elderly people and selenium plays an important role in boosting the immune system
[11,12][11][12]. Additionally, these microminerals can prevent deterioration of age-related diseases (i.e., the intravenous iron helps in heart failure patients)
[13]. Consequently, monitoring nutrient intake, especially crucial minerals and vitamins, is required in geriatric preparations.
2. Selenium
Selenium (Se) takes part in selenoproteins (selenium-dependent enzymes) which functions as redox regulator, antioxidant, in immunoglobin production, lymphocyte proliferation and as an antiviral and anticancer element inside the body
[14,15,16][14][15][16]. In nature, selenium exists in organic (selenomethionine and selenocysteine) and inorganic (selenate and selenite) forms, with both to represent good dietary sources of selenium. Plants accumulate inorganic selenites from the soil and convert them to organic forms which suggests that the latter will most likely be the major dietary source of selenium
[17,18][17][18].
Although selenium is an essential trace mineral required in small quantities, it has potential toxicity at high levels of consumption. The optimal recommended daily consumption of selenium is 55 µg, although generally, recommended dietary allowance (RDA) may differ depending on several factors such as geographical area and selenium level in the diet
[19]. Recommended dietary allowances of selenium
[17] is summarized in
Table 1. Excess accumulation of selenium has been linked to hepatic necrosis, and cardiac muscle dystrophy
[20]. In pigs, acute selenosis develops after an high dietary consumption of more than 20 mg per kg of body weight of the element selenium
[21]. Chronic selenosis develops after consuming diets containing 5–20 ppm selenium for an extended period of time in pigs
[21]. Selenium poisoning is characterized by symptoms such as blindness, stiffness of the bone, severe anemia and hair loss
[22], more likely to exist in regions with extremely high selenium levels above average and in individuals that consume up to 5 mg per day or from atmospheric inhalation of as much as 0.2 mg/m
3 [23]. Serum concentration of 60–120 ng/mL is the best indicator for selenium status inside the body
[24].
Table 1.
Recommended dietary allowance for selenium and iodine.
| |
Selenium |
Iodine |
| Life Stage |
Age |
Males (µg/day) |
Females (µg/day) |
Age |
Males (µg/day) |
Females (µg/day) |
| Infants |
0–6 months |
15 |
15 |
0–6 months |
110 |
110 |
| 7–12 months |
20 |
20 |
7–12 months |
130 |
130 |
| Children |
1–3 years |
20 |
20 |
1–8 years |
90 |
90 |
| 4–8 years |
30 |
30 |
9–13 years |
120 |
120 |
| 9–13 years |
40 |
40 |
14–18 years |
150 |
150 |
| Adolescents |
14–18 years |
55 |
55 |
≥19 years |
150 |
150 |
| Adults |
19–50 years |
55 |
55 |
- |
- |
- |
| Pregnant |
- |
- |
60 |
- |
|
220 |
| Breastfeeding |
- |
- |
70 |
- |
- |
290 |
Selenium deficiency does not always manifest with an obvious illness, although individuals with selenium deficiency often experience more physiological stress. Insufficient selenium can adversely affect enzyme activities that depend on selenium i.e., iodothyronine deiodinases, selenoprotein W and glutathione peroxidases (GPx1 and GPx3)
[25].
Patients on long-term total parenteral nutrition (TPN) without selenium as part of the nutrients show symptoms such as inflammation of the heart muscle, muscle weakness and wasting
[25]. Likewise, Keshan disease, a type of cardiomyopathy, is fatal and predominant in selenium-deficient individuals. Supplementation of selenium in diet has been reported to prevent development of Keshan disease although unable to reverse cardiac muscle damage. Selenium deficiency is also common in patients with Kashin–Beck disease (KBD), a condition characterized by osteoarthritis and dwarfism
[26,27][26][27]. Since selenium is important in many body functions such as the immune modulatory functions, it is thus important to understand how it changes at different life stages as illustrated in the next subsections.
3. Iodine
Iodine is a trace mineral essential in the biosynthesis of thyroid hormone and to regulate basal metabolic rate; thyroid hormones are required for normal neurodevelopment and growth especially in children
[35,36][28][29]. Iodine in food exists in different chemical forms as iodide, inorganic iodine (I
2), iodate, as well as salts of potassium and sodium. In the gastrointestinal tract, iodate is reduced and absorbed as iodide while in the duodenum and stomach, iodide is rapidly absorbed and circulates into the thyroid gland with the remainder excreted in the urine
[37][30].
Iodine deficiency disorder (IDD) has become a public health problem affecting people of all age groups especially children and breastfeeding mothers
[38][31]. Under normal circumstance, the thyroid gland of a healthy individual is expected to encompass 70–80% of the total iodine in the body, which is about 15–20 mg while the body uses about 80 µg to produce thyroid hormones In the case of deficiency, the hypothalamus–pituitary–thyroid pathway becomes activated such that the volume of the thyroid gland increases until goiter becomes obvious, to be managed with surgery and replacement of thyroid hormone
[35][28]. However in a patient with chronic iodine deficiency, iodine level in the thyroid can be as low as 1 mg leading to a spectrum of IDD which includes hypothyroidism, goiter, mental retardation and other growth abnormalities
[39,40][32][33]. Recommended dietary intake of iodine
[41][34] across the human life is summarized in
Table 1.
As with most micronutrients, the daily requirements for iodine changes at different life stages, generally ranging between 90 µg to 290 µg. Daily iodine consumption below 50 µg may cause deficiency, while more than 350 µg may lead to excess iodine and above 600 µg can cause damage to the thyroid function
[35][28] suggestive for the value of RDA levels in order to avoid deficiencies and potential toxicity.
4. Iron
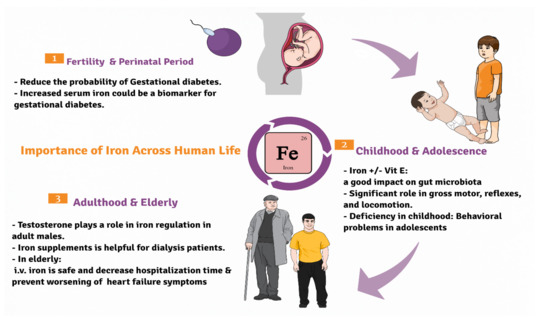
Iron is an essential micromineral involved in many crucial body functions,
Figure 1. It is found in diets mainly in meat, legumes and leafy vegetables. Primarily, most of the body iron is present in the form of hemoglobin in red blood corpuscles, where it plays a major role in oxygen transfer process
[50][35]. Overall, adult females typically require more iron than men attributed to the fact that adult females loose blood during menstrual cycle
[51][36]. Recommended dietary allowances
[52][37] of iron throughout life is illustrated in
Table 2.
Figure 1. Diagrammatic sketch showing the importance of iron across life cycle of humans beginning from the fertile stage, childhood to the elderly.
Table 2.
Recommended dietary allowance for iron and vitamin B12.
| |
Iron |
Vit. B12 (Cobalt) |
| Life Stage |
Age in Years |
Males (mg/day) |
Females (mg/day) |
Age in Years |
Males (µg/day) |
Females (µg/day) |
| Children |
1–3 |
9 |
9 |
1–3 |
0.9 |
0.9 |
| 4–8 |
10 |
10 |
4–8 |
1.2 |
1.2 |
| 9–13 |
8 |
8 |
9–13 years |
1.8 |
1.8 |
| Adolescents |
14–18 |
11 |
15 |
14–18 years |
2.4 |
2.4 |
| Adults |
19–50 |
8 |
18 |
19 years |
2.4 |
2.4 |
| 51–>70 |
8 |
8 |
- |
- |
- |
][38]. Cobalt, as one of the essential minerals for human health, possesses a key function in several biochemical activities such as biosynthesis of nucleic acids and amino acids, as well as erythrocytes production
[74][39]. Nowadays, lots of cobalt (Co)-containing dietary supplements are sold on the market, and many producers have suggested a daily dose up to 1 mg to aid in metabolizing fat and carbohydrates, protein and red blood cell formation
[75][40]. Cobalt enters the body through several routes, including food, the respiratory system, the skin, and biomaterials. Recommended dietary allowances
[76][41] of cobalt across life cycle are listed in
Table 2.
6. Fluoride
Water, pharmaceuticals, herbicides, insecticides, fertilizer residues, dental restorative materials, dental goods (toothpastes and mouth rinses), pediatric supplements, beverages prepared with fluoridated water, and food are all sources of sodium fluoride (NaF)
[80][42]. The environmental protection agency (EPA) recommends acceptable fluoride levels in drinking water to be around 4.0 mg/L, with majority of bottled water fluoridation at 1.5 mg/L according to the world health organization (WHO)
[81][43]. Fluoride deficiency is known to lead to dental caries and can be overcome by water fluorination. However, concerns regarding the unsafety and toxicity due to the over use of fluoride rendered many countries to prohibit fluorination
[82][44]. Recommended dietary allowances
[83][45] of fluoride are displayed in
Table 3.
Table 3. Recommended dietary allowance for fluoride and zinc.
| |
Fluoride |
Zinc |
| Life Stage |
Age in Years |
Males (mg/day) |
Females (mg/day) |
Age in Years |
Males (mg/day) |
Females (mg/day) |
Manganese is a mineral that is required for intracellular activity. It is an enzyme activator and a component of various enzymes, including arginase, glutamine synthases (GS), pyruvate carboxylase, and Mn superoxide dismutase. Mn is required for development, digestion, reproduction, antioxidant defense, energy synthesis, immunological response, and neural activity modulation
[119,120][48][49].
Dietary manganese does not cause any adverse health effects and has no risk at the usual intake levels with an estimated adequate intake (AI) level of 2.3 mg/day. However, excess Mn tends to accumulate in the liver, pancreas, bone, kidney and brain causing hepatic cirrhosis, polycythemia, and Parkinsonism-like symptoms. Mn deficiency is rare, but if it occurs it is associated with symptoms such as impaired growth, skeletal abnormalities, depressed reproductive function, ataxia of the newborn and faults in lipid and carbohydrate metabolism
[121][50]. Regarding the recommended dietary allowances of manganese throughout life
[122][51], refer to
Table 4.
Table 4. Recommended dietary allowance for manganese and copper.
| |
Manganese |
Copper |
| Life Stage |
Age in Years |
Males (mg/day) |
Females (mg/day) |
Age in Years |
Males (µg/day) |
Females (µg/day) |
| Children |
1–3 |
0.7 |
0.7 |
1–3 |
3 |
3 |
| Children |
1–3 |
1.2 |
1.2 |
1–3 |
340 |
340 |
| 4–8 |
1 |
1 |
4–8 |
5 |
5 |
| 4–8 |
1.5 |
1.5 |
4–8 |
440 |
440 |
9–13 |
2 |
2 |
9–13 years |
8 |
8 |
| 9–13 |
1.9 |
1.6 |
9–13 |
700 |
700 |
Adolescents |
14–18 |
3 |
3 |
| Adolescents | 14–18 years |
11 |
9 |
| 14–18 |
2.2 |
1.6 |
14–18 |
890 |
890 |
Adults |
≥19 |
4 |
3 |
≥19 years |
11 |
9 |
| Adults |
≥19 |
2.3 |
1.8 |
≥19 |
900 |
900 |
Pregnant |
≥19 years |
- |
3 |
≥19 years |
- |
11 |
Pregnant |
| Pregnant |
≥19 years |
- |
19–50 |
- |
Breastfeeding |
≥19 years27 |
|
|
2.6 |
| - |
3 |
≥19 years |
- |
12 |
Breastfeeding |
19–50 |
- |
9 |
|
|
2.8 |
5. Cobalt
Cobalt (Co) is a relatively rare metal in the Earth’s crust that mammals require in the form of cobalamin (vitamin B12). Cobalt is found in the human body at small levels, with 85% in the form of vitamin B12 primarily derived from drinking or eating Co containing foods
[73
7. Zinc
Zinc is the most abundant micronutrient in the human body after iron and one of the chief trace elements in the human body detected at approximately 1.4–2.3 g Zn in the body of an adult. High levels of zinc are present in all body tissues at highest level (85%) in muscles and bones, followed by prostate and the eye. It catalyses the activity of several enzymes involved in both protein structure folding and gene expression regulation. Zinc is also needed structurally for of zinc-containing proteins, namely zinc finger proteins (ZFP), biggest superfamily of nucleic acid-binding proteins. It is also fundamental for cell growth, differentiation and homeostasis, asides from its unique role in connective tissue growth and maintenance and immune system integrity
[98][46]. For recommended dietary allowance of zinc
[99][47], refer to
Table 3.
8. Manganese (Mn)
| Breastfeeding |
| ≥19 years |
| - |
| 2.6 |
≥19 years |
- |
1300 |
9. Copper (Cu)
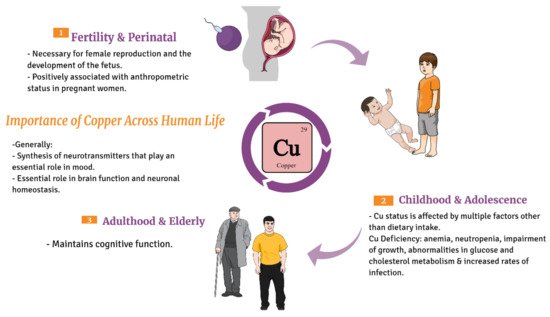
Copper is essential for the survival of humans;
Figure 2. It is involved in many biochemical processes supporting life, such as antioxidant defense, mitochondrial respiration, development of connective tissue, melanin biosynthesis, iron homeostasis, and peptide hormone processing
[131][52]. It acts as cofactor in tyrosine hydroxylase and dopamine hydroxylase enzymes, which participate in the synthesis of neurotransmitters that play an essential role in mood. Ions such as copper plays an essential role in brain function and neuronal homeostasis, and long-term imbalance of these metals has been linked to neurodegeneration and neurological disorder
[132][53]. Regarding the recommended dietary allowance
[133][54] of copper throughout life, see
Table 4.
Figure 2. Diagrammatic sketch showing the importance of copper across life cycle of humans beginning from the fertile stage, childhood to the elderly.
10. Molybdenum
Molybdenum is a trace element that is abundant in many animal and plant dietary sources with legumes having the highest amount reaching up to 87 mg per 100 g wet weight
[144,145][55][56]. Regarding its functions for humans, it is involved mainly as a cofactor in enzymes that metabolizes few chemicals such as purine, sulfur-containing amino acids, N-heterocyclic and N-hydroxylated compounds
[145,146,147][56][57][58]. Like other nutrients, the WHO sets a daily requirement of molybdenum for adults at 100 to 300 µg/day
[148][59]. Due to its distribution in a wide range of food materials, its deficiency is extremely rare and related mainly to a genetic disorder, termed as molybdenum cofactor deficiency, in which molybdenum fails to form its cofactor and consequently the linked enzymes fail to do their functions leading to the accumulation of undesirable substances
[145,149][56][60]. In addition, its toxicity is also rare since the urinary system is sensitive towards increasing levels of molybdenum where an increase in its excretion takes place if its levels are elevated
[145,150][56][61].