Glaucoma is an optic neuropathy characterized by high intra-ocular pressure (IOP) and progressive degeneration of retinal ganglionic cells (RGCs). Increased IOP and short-term IOP fluctuation are two of the most critical risk factors in glaucoma progression which can lead to visual field impairment and loss of visual function as a consequence of the damage of optic nerve. Histamine is produced within mast cell and neurons in the Central Nervous System (CNS) and it is a well-characterized neuromodulator. The secretion of histamine follows a circadian rhythm, regulates IOP and modulates retinal circuits and vision.
- histamine
- intraocular pressure (IOP)
- histamine H3R antagonists
- baro-protection
-
The Histaminergic System
1. The Histaminergic System
Histamine is a monovalent cationic biological amine synthesized from the essential amino acid (AA) L-histidine by L-histidine decarboxylase in professional and non-professional cells. The so-called "professional" histamine-producing cells, synthesize histamine rapidly and store it in granules from which the amine is rapidly secreted. "Non-professional" histamine producing cells generate histamine at 100- to 1000 fold lower concentrations and release it directly without a storage state. Histamine exerts its effects via four histamine receptors (H1-H4 Rs) which all belong to the large G-protein-coupled receptor (GPCR) family. Histamine H1 receptor (H1R) is ubiquitously expressed, specifically in the Central Nervous System (CNS), lungs and blood vessels; it couples to Gαq/11 proteins, causing phospholipase C (PLC) and protein kinase C (PKC) activation as well as inositol-1,4-5-trisphosphate (IP3) formation and intracellular Ca2+ release from intracellular stores [1]. Histamine H1R is a key regulator of inflammatory processes, Nf-kB expression and the feedback pathways are controlled by H1R antagonists. The typical signs of type I allergic reaction like pruritus, increased vascular permeability, and edema are caused by H1R activation, and the administration of H1R antagonists (so-called antihistamines) belongs to the most essential anti-allergic therapeutic interventions [2]. In the CNS, H1R are involved in locomotor activity, emotions, cognitive functions, arousal, sleep, circadian rhythm or pain perception [3]. Histamine H2[1][4] and in the CNS, where the receptor is found in cerebral cortex, caudate-putamen, hippocampus, and dentate nucleus of cerebellum, playing a role in neuronal plasticity [5]. Agonist binding to this receptor results in activation of Gαs-proteins that stimulate the adenylate cyclase-mediated production of the second messenger cAMP. The function of the H2R in the brain is less well documented than for H1R and includes modulation of cognitive processes, circadian rhythm, glucose metabolism and food intake [3]. Histamine H3R is mainly expressed in cortical and subcortical areas of the CNS, being involved in cognitive processes, wakefulness, and eating behaviors [6]. Histamine H3R acts as a presynaptic auto- and hetero-receptor and inhibits the release of histamine [7][8] and of other neurotransmitters and it is considered a potential target for treating several cerebral disorders [9]. Histamine H3R is coupled with Gi/o protein and inhibits the adenylate cyclase and the high voltage-activated Ca2+-channels that are responsible for regulating histamine synthesis and neurotransmitter release [1]. Despite the decade-long research on histamine H3R pharmacology, only the inverse H3R agonist pitolisant is currently used to treat narcoleptic patients [10]. Histamine H4R is the most recently discovered histamine receptor. The cloning of the histamine H4R provided a template for the search of other histamine receptors. This discovery culminated with six independent groups reporting the cloning of the histamine H4R in 2000 [1]. In humans, histamine H4R is mainly expressed peripherally in oral epithelium, bone marrow, and leukocytes [11]. This receptor modulates the migration and activation of a broad spectrum of immune cells (mast cells, basophils, eosinophils, monocytes, dendritic cells, NK, iNK T and γδ cells, CD8+ T cells, Treg, and Th2 cells) and it is thereby involved in allergic and immune-mediated disorders. It operates through Gai-dependent inhibition of adenylate cyclase and stimulation of diacylglycerol formation, calcium mobilization and activation of protein kinase C.
1a2. The Histaminergic System at ocular level
Retina, optic nerve and various brain structures of albino and pigmented rabbits contain histamine in the range of 40–400 ng/g of tissue; choroid tissue of both animal strains is characterized by amine contents several times higher. In the retina, no histamine-forming cells have been identified to date; however, retinopetal axons arising from the tuberomammillary nucleus extend across the inner plexiform layer, eliciting histamine responses in a range of inner retinal neurons [12]. The synthesis and release of histamine are controlled by presynaptic histamine H3 auto-receptors located in the CNS [7][8]. Histamine H1, H2 and H3R have been localized in the inner layer of ganglion cells in rodents and primate retinae [12][13]; the neuronal circuits involved in scotopic vision are altered by histamine release. In the retina, the stimulation of histamine H3R increases the delayed rectifier component of the voltage-dependent potassium conductance in ON bipolar cells [14], and in dark-adapted baboon retinas, histamine decreases the rate of maintained firing and the amplitude of the light responses of ON ganglion cells [15]. The retina of primates receives input from histaminergic neurons active during the day in the posterior hypothalamus; they receive input from the brain via axons emerging from the optic nerve. One set of retinopetal axons arises from perikarya in the posterior hypothalamus and uses histamine and projects via collaterals to many other targets in the CNS; they are components of the ascending arousal system, active when the animal is awake. Many of the effects of histamine on light responses suggest that retinopetal axons optimize retinal function at an ambient light intensity during the waking period. Histamine activates chloride channels, increasing chloride conductance, in the monopolar optic cells in insects, suggesting a role of histamine in photoreceptor response to light in flies [16]; moreover, severe mutation in the gene encoding for histamine receptors causes a defect in the transients of the electroretinogram derived from large monopolar cells of the first optic lamina [17]. Histamine reduces the amplitude of light responses in monkey retinal ganglionic cells (RGCs), this finding is consistent with a role for retinopetal axons in light adaptation in these diurnal animals [18]. This evidence suggests s that histamine acts primarily via volume transmission in the primate retina, increasing the operating range of cones and conserving ATP in bright, ambient light. The histamine system is deeply implicated in circadian rhythm and fulfills a significant role in maintaining waking [19]. During the day, histamine tone plays a role in maintaining the IOP balance, and histamine is responsible for ciliary muscle contraction in human eyes and, therefore, in IOP reduction [20].Taken together, this evidence supports the potential role of histamine in the regulation of IOP. Moreover, aqueous humor (AH) production or outflow can be influenced by histamine, which is the neurotransmitter of the ascending arousal system, and the contribution of retinopetal axons to vision can be predicted from the well-known effects of histamine on the neurons of the retina. Ocular administration of histamine triggers local inflammation in a non-specific manner; the severity of conjunctivitis is dose-dependent; however, histamine is well tolerated, although transient blepharitis, aqueous flare, and ocular hypertension occur in some experimental situations [21]. Total protein content and serum albumin levels increase after histamine administration, as lacrimal albumin levels during naturally acquired conjunctivitis and lacrimal albumin concentration decrease in parallel with the reduction in the conjunctivitis score [21]. In the conjunctival immediate hypersensitivity reaction (type I allergy), histamine is released from degranulated mast cells in the early and late phases [22]. Histamine levels in the tears of kerato-conjunctivitis (KCV) patients are higher than in control subjects, and histamine H1 receptors are over-expressed in the active phase of the disease [23].
Histamine H1Rs are localized on horizontal cells and in a small number of amacrine cells, whereas histamine H2Rs appear closely associated with synaptic ribbons inside cone pedicles [13]. Histamine H1 and H2 receptors in the iris arterioles and H2 receptors in the iridal venules modulate vascular tone in rats [24]. Several ocular hypertensive effects have been reported in chronic glaucoma patients following the use of cimetidine and ranitidine, two histamine H2R antagonists used for peptic ulcer treatment [25]; on the contrary, recent studies have failed to demonstrate the significant action of topical administered H2 blockers on IOP in humans [26]. Histamine H1 and H2R antagonists possess anticholinergic activity that may induce glaucoma. Promethazine, an antipsychotic drug with antihistamine activity, has been shown to produce an idiopathic swelling of the lens that could increase the risk of angle-closure glaucoma. Topical administration of ranitidine produces vasoconstriction in both the arterioles and the venules of the iris, suggesting a predominant role of histamine H2R in the vasculature of the iris [24].
Histamine H4 receptors are expressed mostly in T and B-cells, monocytes, eosinophils, dendritic and natural killer cells, therefore playing an important role in the modulation of immune system. Not surprisingly, this selective localization suggested therapeutic use in inflammatory disorders and autoimmune diseases [27]. However, evidence also demonstrated the topological and functional localization in this receptor in human CNS [28]; infiltrating inflammatory cells in sub-conjunctival tissues of KCV patients strongly express histamine H4R [29]. The down-regulation of this receptor, mainly expressed in immune cells, leads to a decreased eosinophil infiltration into the conjunctival tissue. Therefore, the expression level of H4R on the ocular surface may be a useful biomarker for atopic KCV in clinical examinations [23]. Moreover, in an animal model of autoimmune encephalomyelitis (EAE), histamine H4 signaling exerts control over the abundance of regulatory T cells in secondary lymphoid tissues, regulates their chemotaxis and suppressive ability. The deficiency of histamine H4R leads to lower infiltration of regulatory T cells into the CNS during the acute phase of the disease [30]. In another paper, the H4R antagonist JNJ7777120 administrated to EAE mice caused a general worsening of disease symptoms, thus suggesting a protective role of these receptors in the contest of EAE [31].
23. The Role of Histamine H3 Receptors in the Control of Intraocular Pressure
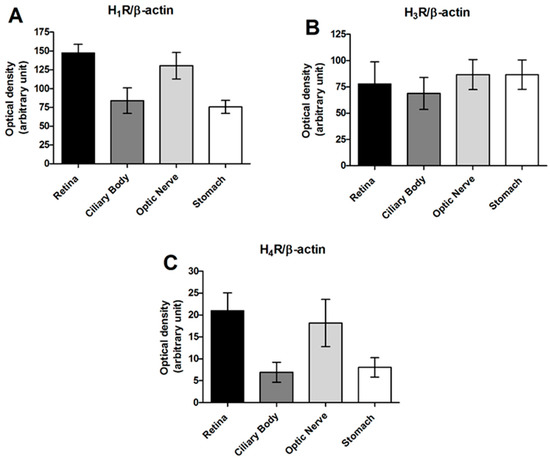
Topical treatment with H3R antagonists was effective in reducing IOP both in transient and stable ocular hypertensive (OHT) rabbit models. Both imidazole (ciproxifan) and non-imidazole compounds, such as DL-76 (1-[3-(4-tert-butylphenoxy)propyl]piperidine hydrogen oxalate, [35]) and GSK189254, at an equimolar concentration (1%) reduced IOP following a single acute challenge or after repeated doses in transient or stable IOP raise models in rabbits [35]. After 50 µL of 5% hypertonic saline injection in the eye’s anterior chamber, IOP increased from 16.8 ± 5.6 mmHg to 39.63 ± 4.85 mmHg. This value remained stable until 120 min and decaying after this time to reach baseline values at 240 min. All the compounds reduced IOP in a statistically significant manner with a different profile; ciproxifan and DL-76 were more effective than GSK189254 (Table 1A). As observed in the transient ocular hypertensive model, all the compounds, at a 1% dose, caused a significant reduction in IOP in the carbomer-induced chronic model in male New Zealand White (NZW) rabbits after seven days of treatment. Ciproxifan and DL-76 were the most effective compounds. The effect of timolol at a 1% dose, the gold standard treatment, is also reported (Table 1B).
Table 1. (A) Transient ocular hypertensive model and (B) carbomer-induced glaucoma model in male NZW rabbits.
| A | IOP-Lowering Effect | |||||
| Compound | Basal | After Saline |
Post Treatment (60 min) | ΔΔIOP (60 min) |
Post Treatment (120 min) | ΔΔIOP (120 min) |
| IOP, mmHg | IOP, mmHg | IOP, mmHg | mmHg | IOP, mmHg | mmHg | |
| Vehicle | 15 ± 0.3 | 36 ± 7.5 | 38 ± 3.9 | 0 ± 3.9 | 28 ± 5.7 | 0 ± 5.7 |
| Ciproxifan | 13 ± 4.3 | 38 ± 6.2 | 22 ± 5.3 | −18.9 ± 5.3 ** | 17 ± 5.5 | −16.4 ± 5.5 ** |
| DL-76 | 15 ± 4.3 | 34 ± 6.2 | 23 ± 7.4 | −15.4 ± 7.5 ** | 20 ± 3.0 | −16.1 ± 3.1 ** |
| GSK189254 | 14 ± 5.6 | 37 ± 5.5 | 32 ± 4.3 | −8.5 ± 4.3 * | 25 ± 6.7 | −9.9 ± 6.7 * |
| Timolol | 14 ± 2.5 | 38 ± 5.7 | 21 ± 3.8 | −16.5 ± 3.8 ** | 19 ± 4.6 | −14.8 ± 4.7 ** |
| B | IOP-Lowering Effect | |||||
| Compound | Basal | After Carbomer |
Post-Treatment (7 days) |
ΔΔIOP (7 days) |
||
| IOP, mmHg | IOP, mmHg | IOP, mmHg | mmHg | |||
| Vehicle | 15 ± 0.3 | 38 ± 2.8 | 41 ± 7.3 | 0 ± 2.12 | ||
| Ciproxifan | 13 ± 5.1 | 36 ± 4.1 | 20 ± 2.9 | −19 ± 2.9 ** | ||
| DL-76 | 12 ± 3.5 | 34 ± 2.8 | 23 ± 3.8 | −15.7 ± 2.7 * | ||
| GSK189254 | 14 ± 0.0 | 40 ± 1.4 | 26 ± 2.8 | −14.5 ± 3.8 * | ||
| Timolol | 15 ± 0.7 | 41 ± 5.6 | 27 ± 2.1 | −13.5 ± 2.1 * | ||
Ocular hypotensive efficacy is expressed in mmHg. The average difference in IOP between drug-treated eyes or vehicle-treated eyes and their respective pre-treatment value are shown in the following formula: Efficacy = (IOPdrug − IOPpredose drug) − (IOPveh − IOP predose veh). (A) ** p < 0.01 ciproxifan, DL-76 1% and Timolol 1% at 60′ and 120′; * p < 0.05 GSK189254 1% at 60′ and 120′ versus vehicle; (B) ** p < 0.01 ciproxifan; * p < 0.05 DL-76, Timolol, GSK189254 at day 7 versus vehicle. All the results are expressed as mean ± SEM (n = 6). The significance of differences was assessed by two-way ANOVA for multiple comparisons followed by the Bonferroni post hoc test [35] modified.
Interestingly, the IOP-lowering activity of ciproxifan and DL-76 were suppressed by the pre-treatment with 1% imetit, a histamine H3R agonist, providing the specificity of histamine H3R antagonism action (Figure 2).
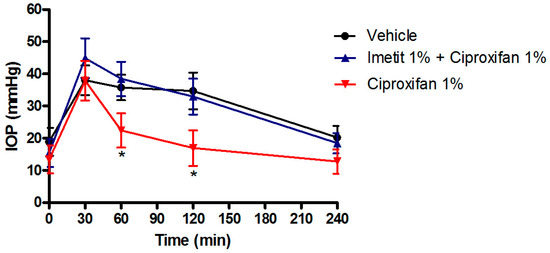
Figure 2. IOP lowering effect of ciproxifan in the transient ocular hypertensive model in NZW rabbits. The effect of ciproxifan is suppressed by pre-treatment with 1% imetit. * p < 0.05 ciproxifan 1% at 60′ and 120′ vs. vehicle and imetit 1% + ciproxifan 1%. All the results are expressed as mean ± SEM (n = 6). Two-way ANOVA followed by Bonferroni post hoc test.
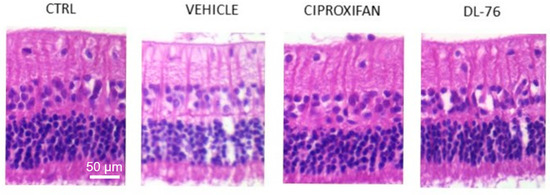
Figure 3. Representative images of hematoxylin/eosin-stained histological sections of retinae from different treated groups. RGCs are visible in the upper layer. The histological sections of Vehicle, Ciproxifan and DL-76 panels were prepared from carbomer-induced glaucoma models in male NZW rabbits with stable elevated IOP [35] modified.
In conclusion, these observations clearly confirm that histamine plays an important role in IOP regulation. Topical treatments with histamine H3R antagonists were effective in reducing IOP both in transient and stable ocular hypertensive animal models, preventing RGC loss by an improvement of vascular performance of the central ophthalmic artery and these molecules could represent a future therapy for glaucoma.
References
- Panula, P., Chazot, P. L., Cowart, M., Gutzmer, R., Leurs, R., Liu, W. L. S.; International union of basic and clinical pharmacology. XCVIII. histamine receptors. Pharmacol. Rev. 2015, 67, 601–655, doi:10.1124/pr.114.010249..
- Simons, F. E. R., and Simons, K. J.; Histamine and H1-antihistamines: Celebrating a century of progress. J. Allergy Clin. Immunol. 2011, 128, 1139-1150, doi:10.1016/j.jaci.2011.09.005.
- Schneider EH, Neumann D, Seifert R; Modulation of behavior by the histaminergic system: lessons from HDC-, H3R- and H4R-deficient mice. Neurosci Biobehav Rev 2014, 47, 101-21, doi: 10.1016/j.neubiorev.2014.07.020.
- Neumann D, Schneider EH, Seifert R; Analysis of histamine receptor knockout mice in models of inflammation. J. Pharmacol. Exp. Ther. 2014, 348, 2-11, doi: 10.1124/jpet.113.204214.
- Haas HL, Sergeeva OA, Selbach O; Histamine in the nervous system. Physiol Rev 2008, 88, 1183-241, doi: 10.1152/physrev.00043.2007.
- Nieto-Alamilla G, Márquez-Gómez R, García-Gálvez AM, Morales-Figueroa GE, Arias-Montaño JA.; The Histamine H3 Receptor: Structure, Pharmacology, and Function. Mol Pharmacol. 2016, 90(5), 649-673, doi: 10.1124/mol.116.104752.
- Arrang, JM., Garbarg, M. & Schwartz, JC; Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor.. Nature 1983, 302, 832–837, doi.org/10.1038/302832a0.
- Arrang JM, Garbarg M, Schwartz JC; Autoregulation of histamine release in brain by presynaptic H3-receptors. Neuroscience 1985, 15(2), 553-62, doi: 10.1016/0306-4522(85)90233-7.
- Nuutinen S, Panula P; Histamine in neurotransmission and brain diseases. Adv Exp Med Biol. 2010, 709, 95-107, doi: 10.1007/978-1-4419-8056-4_10..
- Dauvilliers Y, Bassetti C, Lammers GJ, Arnulf I, Mayer G, Rodenbeck A, Lehert P, Ding CL, Lecomte JM, Schwartz JC; HARMONY I study group.; Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013, 12, 1068-75, doi: 10.1016/S1474-4422(13)70225-4.
- Thurmond RL; The histamine H4 receptor: from orphan to the clinic.. Front Pharmacol 2015, 6, 65, doi: 10.3389/fphar.2015.00065.
- Greferath U, Kambourakis M, Barth C, Fletcher EL, Murphy M; Characterization of histamine projections and their potential cellular targets in the mouse retina. Neuroscience 2009, 158(2), 932-44, doi: 10.1016/j.neuroscience.2008.10.034..
- Vila A, Satoh H, Rangel C, Mills SL, Hoshi H, O'Brien J, Marshak DR, Macleish PR, Marshak DW; Histamine receptors of cones and horizontal cells in Old World monkey retinas. J Comp Neurol 2012, 520(3), 528-43, doi: 10.1002/cne.22731.
- Yu YC, Satoh H, Wu SM, Marshak DW; Histamine enhances voltage-gated potassium currents of ON bipolar cells in macaque retina. Invest Ophthalmol Vis Sci 2009, 50(2), 959-65, doi: 10.1167/iovs.08-2746.
- Akimov NP, Marshak DW, Frishman LJ, Glickman RD, Yusupov RG; Histamine reduces flash sensitivity of on ganglion cells in the primate retina. Invest Ophthalmol Vis Sci 2010, 51(7), 3825-34, doi: 10.1167/iovs.09-4806.
- Skingsley D.R., Laughlin S.B., Hardie R.C.; Properties of histamine-activated chloride channels in the large monopolar cells of the dipteran compound eye: A comparative study. . J. Comp. Physiol. A. 1995, 176, 611–623, doi:10.1007/BF01021581..
- Coombe P.E.; The large monopolar cells L1 and L2 are responsible for ERG transients in Drosophila. J. Comp. Physiol. A 1986, 159, 655–665, doi:10.1007/BF00612038..
- Gastinger M., Tian N., Horvath T., Marshak D.; Retinopetal axons in mammals: Emphasis on histamine and serotonin. Curr. Eye Res. 2006, 31, 655–667, doi:10.1080/02713680600776119..
- Lin J.S., Sergeeva O.A., Haas H.L.; Histamine H3 receptors and sleep-wake regulation. J. Pharmacol. Exp. Ther 2011, 336, 17–23, doi:10.1124/jpet.110.170134.
- Markwardt K.L., Magnino P.E., Pang I.H.; Histamine induced contraction of human ciliary muscle cells. Exp. Eye Res 1997, 64, 713–717, doi:10.1006/exer.1996.0255.
- Sebbag L., Allbaugh R.A., Weaver A., Seo Y.J., Mochel J.P.; Histamine-Induced Conjunctivitis and Breakdown of Blood–Tear Barrier in Dogs: A Model for Ocular Pharmacology and Therapeutics. Front. Pharmacol. 2019, 10, 1-2, doi:10.3389/fphar.2019.00752.
- Montan P.G., Van Hage-Hamsten M.; Eosinophil cationic protein in tears in allergic conjunctivitis. Br. J. Ophthalmol. 1996, 80, 556–560, doi:10.1136/bjo.80.6.556. .
- Inada N., Shoji J., Shiraki Y., Aso H., Yamagami S.; Histamine H1 and H4 receptor expression on the ocular surface of patients with chronic allergic conjunctival diseases. Allergol. Int. 2017, 66, 586–593, doi:10.1016/j.alit.2017.03.004. .
- Luncă D.-C., Păunescu H., Mușat O., Fulga I.; The histaminergic control of the iridal vascular tone in rats and its influencing by topical administration of olopatadine and ranitidine. Rom. J. Ophthalmol. 2019, 63, 23–28, doi:10.22336/rjo.2019.5..
- Lachkar Y., Bouassida W.; Drug-induced acute angle closure glaucoma. Curr. Opin. Ophthalmol. 2007, 18, 129–133, doi:10.1097/ICU.0b013e32808738d5..
- Razeghinejad M.R., Myers J.S., Katz L.J.; Iatrogenic glaucoma secondary to medications. Am. J. Med. 2011, 124, 20–25, doi:10.1016/j.amjmed.2010.08.011..
- Zampeli E., Tiligada E.; The role of histamine H4 receptor in immune and inflammatory disorders. Br. J. Pharmacol. 2009, 157, 24–33, doi:10.1111/j.1476-5381.2009.00151.x..
- Strakhova MI, Nikkel AL, Manelli AM, Hsieh GC, Esbenshade TA, Brioni JD, Bitner RS.; Localization of histamine H4 receptors in the central nervous system of human and rat. Brain Res. 2009, 1250, 41-8, doi:10.1016/j.brainres.2008.11.018. .
- Leonardi A., Di Stefano A., Vicari C., Motterle L., Brun P.; Histamine H4 receptors in normal conjunctiva and in vernal keratoconjunctivitis. Allergy Eur. J. Allergy Clin. Immunol. 2011, 66, 1360–1366, doi:10.1111/j.1398-9995.2011.02612.x..
- del Rio R, Noubade R, Saligrama N, Wall EH, Krementsov DN, Poynter ME, Zachary JF, Thurmond RL, Teuscher C.; Histamine H4 receptor optimizes T regulatory cell frequency and facilitates anti-inflammatory responses within the central nervous system. J Immunol. 2012, 188, 541-7., doi:10.4049/jimmunol.1101498. .
- Ballerini C, Aldinucci A, Luccarini I, Galante A, Manuelli C, Blandina P, Katebe M, Chazot PL, Masini E, Passani MB.; Antagonism of histamine H4 receptors exacerbates clinical and pathological signs of experimental autoimmune encephalomyelitis. Br. J. Pharmacol. 2013, 170, 67-77, doi:10.1111/bph.12263.
- Smith SD, Gregory DS.; A circadian rhythm of aqueous flow underlies the circadian rhythm of IOP in NZW rabbits. Invest Ophthalmol Vis Sci. 1989, 30, 775-8, PMID: 2703321.
- Tripathi R.C., Tripathi B.J.; Human trabecular endothelium, corneal endothelium, keratocytes, and scleral fibroblasts in primary cell culture. A comparative study of growth characteristics, morphology, and phagocytic activity by light and scanning electron microscopy. Exp. Eye Res. 1982, 35, 611–624, doi:10.1016/S0014-4835(82)80074-2..
- Caprioli J, Coleman AL.; Intraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology 2008, 115, 1123-1129.e3., doi:10.1016/j.ophtha.2007.10.031..
- Lanzi C., Lucarini L., Durante M., Sgambellone S., Pini A., Catarinicchia S., Łażewska D., Kieć-Kononowicz K., Stark H., Masini E.; Role of Histamine H3 Receptor Antagonists on Intraocular Pressure Reduction in Rabbit Models of Transient Ocular Hypertension and Glaucoma. Int. J. Mol. Sci. 2019, 20, 981, doi:10.3390/ijms20040981..
- Impagnatiello F., Giambene B., Lanzi C., Pini A., Somma T., Bastia E., Ongini E., Galassi F., Masini E.; The nitric oxide donating triamcinolone acetonide NCX 434 does not increase intraocular pressure and reduces endothelin-1 induced biochemical and functional changes in the rabbit eye. Br. J. Ophthalmol. 2012, 96, 757–761, doi:10.1136/bjophthalmol-2011-300404..
