Copper nanoclusters (Cu NCs) with their inherent optical and chemical advantages have gained increasing attention as a kind of novel material that possesses great potential, primarily in the use of contaminants sensing and bio-imaging. With a focus on environmental safety, this article comprehensively reviews the recent advances of Cu NCs in the application of various contaminants, including pesticide residues, heavy metal ions, sulfide ions and nitroaromatics. The common preparation methods and sensing mechanisms are summarized.
- sensor
- fluorescence
- pesticide
- heavy metal
1. Introduction
Metal nanoclusters (MNCs) with ultra-small and tunable sizes, excellent photoluminescent efficiency, long fluorescence lifespan, desirable physical and biochemical stability and relatively low toxicity, have prompted the great advancement of research in both theoretical and practical fields [1][2][1,2]. The last decade witnessed the successful synthesis of novel MNCs and their applications in fluorescent sensors mainly based on gold (Au) and silver (Ag) nanoclusters. Meanwhile, copper nanoclusters (Cu NCs) have gradually gained increasing attention due to their chemical similarity with Ag NCs and Au NCs, distinct fluorescent characteristics, and in particular the low-cost and easier accessibility of their precursors as well as facile preparation procedures [3][4][3,4]. With the help of various functional ligands, it is possible to tune their emission wavelengths and obtain highly photoluminescent nanoclusters, providing potential for large-scale applications. More importantly, Cu NCs possess additional merits over other noble metal clusters with their excellent biocompatibility [5][6][5,6]. The fluorescent probes based on Cu NCs have demonstrated their versatility in sensing, lighting and bioimaging in clinical diagnosis and treatment [6][7][8][9][6,7,8,9]. Meanwhile, the significance of monitoring and analyzing various contaminants for the purpose of environmental safety should also be emphasized due to the widespread application of Cu NCs in this field.
In this rentryview, we emphasize the recent progress of Cu NCs for application in environmental analysis. We first make a brief introduction of the common synthesis approaches of Cu NCs with a highlight on their intriguing optical properties. In the second section, we categorize the mainstream strategies based on Cu NCs in terms of sensing mechanisms. In the following part, we mainly present several typical novel Cu NCs targeting various contaminants in the environment, including pesticides, heavy metals, sulfide anions, as well as aromatic compounds. In the end, we conclude the article by discussing the challenges and prospects in the future development of Cu NCs as sensors for environmental pollutants.
2. Preparation Methods and Sensing Mechanism of Cu NCs
Many research groups developed GSH capped Cu NCs with orange or red fluorescence emission [10][11][12][36,37,38]. A one-pot sonochemical synthesis method was established for the preparation of GSH-Cu NCs by Wang et al. [13][39]. In this method, Cu (NO 3) 2 and GSH were mixed in water by 1: 4 ratio, and the pH of solution was adjusted to 6 with NaOH. The reaction was conducted in 15 min via ultrasonic irradiation, which is facile, fast and easy to operate. The as-synthesized GSH-Cu NCs exhibits bright red luminescence at λ max = 606 nm and quantum yield up to 5.3%. As mentioned above, there are also many fluorescent GSH-Cu NCs that have been reported with blue emission. The main difference in the synthesis process is that red emitting GSH-Cu NCs utilized GSH as both a stabilizing and a reducing agent in acidic condition, while blue emitting ones were synthesized in basic condition or reduced by AA. DNA template is also frequently exploited in the fabrication of Cu NCs with red emission [14][15][16][17][40,41,42,43]. Li et al. developed copper nanoclusters templated by poly(thymine)-DNA with a fluorescence emission of 627 nm [18][44]. The DNA-Cu NCs were produced by a facile reaction between copper sulfate and poly-T DNA in 3-(N-morpholino) propanesulfonic acid buffer with the help of sodium ascorbate. Intriguingly, a research group successfully synthesized Cu NCs with strong orange emission utilized egg white as template [19][45]. The formation of Cu NCs relies on the interaction between multiple functional groups in egg white and CuSO 4. Hydrazine hydrate was employed as reducing agent and NaOH was used to provide the basic environment. The reaction proceeded extremely fast, since it is carried out under microwave.
On account of the prominent fluorescence property and cost-effectiveness of Cu NCs, numerous Cu NCs-based sensing probes have been developed for multitude of analytes. The majority of Cu NCs-based fluorescent sensors follow turn-off mechanism that the analytes are detected due to the decrease in fluorescence intensity.
Inner filter effect (IFE) is a most common strategy in the development of turn-off sensors [20][21][22][23][48,49,50,51]. The inner filter effect refers to the fluorescence quenching process that the quencher absorbs the emission or excitation light of fluorophore. A study in this context established a label-free assay for nitrofurantoin using adenosine-stabilized Cu NCs [24][16]. Nitrofurantoin’s UV absorption band located at 250–430 nm happens to overlap the Cu NCs’ fluorescence excitation and emission spectra. In this way, the excitation and emission light could be shielded by nitrofurantoin, leading to decrease in fluorescence intensity.
Ratiometric fluorescence sensors have also attracted increasing research interest since it exhibits dominant advantages in accuracy, sensitivity and stability. In a ratiometric sensing approach, the detection is achieved by the intensity ratio of dual-emission peaks, which could eliminate the interference of environment and probe concentrations by self-calibration. A CuNC@AF660 sensor was fabricated for ratiometric sensing of calcium ions. The fluorescence intensity of Cu NCs emission peak was enhanced gradually with the increase in Ca 2+ concentration through ion-induced AIE mechanism. The Alex Fluor 660 NHS ester fabricated on Cu NCs provides the inner-reference signal.
3. Sensing Applications Based on Cu NCs
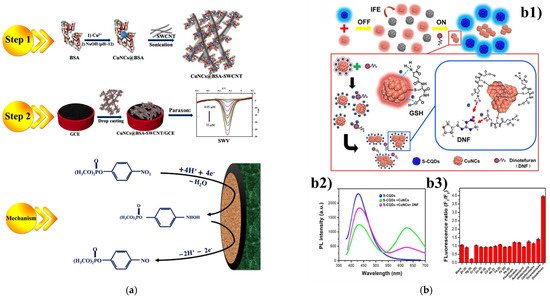
In this section, we mainly highlight the Cu NCs-based detection systems targeting common pesticides ( Table 1 ), e.g., paraoxon, dinotefuran (DNF), o-phenylenediamine (OPD), dithiocarbamates (DTCs), thiram, paraquat, fluazina, nitrofurantoin (NFT). Copper nanocluster was employed in the construction of an enzyme-free electrochemical biosensor toward paraoxon as the model of organophosphates (OP) [25][12]. The biocompatible nanocomposite Cu NCs@BSA-SWCNT was synthesized by combining bovine serum albumin (BSA) template-capped Cu nanoclusters (Cu NCs@BSA) and single-walled carbon nanotubes (SWCNT), which demonstrated remarkable sensitivity and high electrocatalytic property toward the reduction of paraoxon ( Figure 1 a). The entrapped Cu NCs rendered high electrical conductivity and concentrated the redox active centers on the surface of the probe, while the SWCNT further enhanced the electrocatalytic activity along with conductivity of the glassy carbon electrode (GCE) surface. The linear range was 0.5–35 μM, with a limit of detection of 12.8 nM. Moreover, this electrochemical nanocomposite was found to be able to effectively determine paraoxon with satisfied recoveries ranged from 93% to 104% in a real water sample. In order to detect and monitor the residues of dinotefuran (DNF), which has been widely used in agriculture, novel sensing probes and platforms based on fluorescent copper nanoclusters have been constructed. Yang et al. [26][67] established a dual-emission ratiometric fluorescent probe by integrating sulfur-doped carbon quantum dots (S-CQDs) and Cu NCs with mixed fluorescent signals ( Figure 1 b). The as-developed hybrid (S-CQDs/Cu NCs) was observed to demonstrate desirable sensitivity and selectivity towards DNF with linear range from 10 to 500 μM. In this nanocomposite, IFE caused the decrease in fluorescent signals of S-CQDs with the addition of Cu NCs. In the presence of dinitefuran, the IFE between S-CQDs and Cu NCs would be weakened due to the aggregation of Cu NCs, leading to the restoration of S-CODs fluorescence. In the case of honey as the real sample, this ratiometric fluorescent S-CQDs/Cu NCs showed good analysis performance for the detection of DNF. Besides, another ratiometric detection system was proposed for o-phenylenediamine (OPD) based on the use of copper nanoclusters [27]. This method achieved signal amplification and ideal sensitivity through the combined influence of the oxidation reaction and FRET effect. With addition of OPD into Cu NCs, the fluorescence intensity of the Cu NCs at 432 nm decreased, while the oxidized OPD (oxOPD) showed strong fluorescence at 557 nm. This detection strategy was able to determine OPD in real water samples with an ultralow limit of detection of 0.096 g L −1 . Furthermore, a rapid and sensitive detection method of dithiocarbamates (DTCs) with dual functionality in fluorescence and colorimetry was established utilizing CTAB -entrapped Cu NCs [28][68]. Owing to the fluorescence quenching of the Cu NCs with addition of DTCs, the detection system demonstrated remarkable sensitivity and selectivity toward DTCs with a linear range from 1 to 100 mg kg −1 and a low limit of detection of 0.63 mg kg −1 .
| No | Analytes | Sensors | Ex./Em. Maxima (nm) | Sensing Mechanism | Linear Range | Limit of Detection (LOD) | Real Sample | Ref. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Paraoxon | Cu NCs @ BSA-SWCNT/GCE | 325/420 | electrochemical method | 0.05–0.5 μM 0.5–35 μM | 12.8 nM | water | [25] | [12] | ||||||||
| 2 | Thiram Paraquat |
Egg white- Cu NCs | 344/600 | turn off | 0.5–1000 μM 0.2–1000 μM |
70 nM 49 nM | water | [19] | [45] | ||||||||
| 3 | Metham sodium | CTAB-Cu NCs | 254/620 | fluorescent based colorimetric method | 1–100 mg kg | −1 | 0.63 mg kg | −1 | apple, pear and cherry tomato | [28] | [68] | ||||||
| 4 | Fluazina | L-Cys-Cu NCs | 365/497 | turn off | 0.05–25 µM | 1.4 nM | pears and cabbage | [29] | [33] | ||||||||
| 5 | o-Phenylenediamine | GSH-Cu NCs | 334/432 | ratiometric | 0.15–110 μg L | −1 | 93 ng L | −1 | industry water | [27] | |||||||
| 6 | Nitrofurantoin | Adenosine-Cu NCs | 285/417 | turn off | 0.05–4.0 μM | 30 nM | lake water | [24] | [16] | ||||||||
| 7 | Dinotefuran | S-CQDs/Cu NCs | 330/430 | ratiometric | 10–500 μM | 7.04 μM | honey | [26] | [67] | ||||||||
| 8 | AChE Methamidophos |
DNA-Cu/Ag NCs | 480/565 | turn off turn on |
0.05–2.0 U L | −1 | — |
0.05 UL | −1 | 0.075 mg L | −1 | (IC | 50 | ) | water and vegetable | [30] | [69] |
| 10 | AChE | L-His-Cu/Ag NCs | 390/485 | turn off | 0.1–1.0 UL | −1 | and 1.0–7.0 UL | −1 | 0.03 UL | −1 | — | [31] | [34] | ||||
| 9 | AChE | PVP-Cu NCs | 370/438 | ratiometric | 2.0–70 UL | −1 | 0.56 UL | −1 | human serum sample | [32] | [14] | ||||||
| 11 | AChE | PEI-Cu NCs | 365/495 | turn on | 3–200 UL | −1 | 1.38 UL | −1 | human serum sample | [33] | [32] |
Heavy metals are notorious and hazardous contaminants in environment due to their low degradability, acute toxicity, high bioaccumulation, and other factors. Herein, we summarized nanoprobes based on Cu NCs for the determination of several representative heavy metal ions including mercury ions (Hg 2+ ), lead ions (Pb 2+ ), chromate anions (Cr 6+ ) and copper ions (Cu 2+ ) ( Table 2 ).
| No | Analytes | Sensors | Ex./Em. Maxima (nm) | Sensing Mechanism | Linear Range | Limit of Detection (LOD) | Real Sample | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Hg | 2+ | Cu NCs@P-8B | 400/535 | turn off | 10–100 μM | 10 μM | aqueous solution | [34] | [74] | ||
| 2 | Hg | 2+ | Curcuminoids-Cu NCs | 350/440 | turn off | 0.5 nM–25 µM | 0.12 nM | water | [35] | [54] | ||
| 3 | Hg | 2+ | Cu NCs | 340/560 | turn off | 2–40 μM | 23 nM | water | [36] | [72] | ||
| 4 | Hg | 2+ | + | Trypsin-Cu NCs | 360/567 | turn off | 0.1−100 μM | 30 nM | human urine and serum samples | [37] | [28] | |
| 5 | Hg | 2+ | TdT-INAA-DNA-Cu NCs | 343/600 | turn off | 0.2–500 nM | 76 pM | environmental water | [15] | [41] | ||
| 6 | Hg | 2+ | GSH–Cu NCs | 360/445 | turn off | 10 nM–10 μM | 3.3 nM | water and rice | [38] | [13] | ||
| 7 | Hg | 2+ | poly(30T) DNA-Cu NCs | 340/650 | turn on | 50 pM–2.5 μM and 2.5–500 μM | 16 pM | lake water | [14] | [40] | ||
| 8 | Hg | 2+ | DTT-Cu NCs/CNNS nanocomposite | 395/615 | electrochemiluminescence | 0.05–10 nM | 0.01 nM | lake and tap water | [39] | [71] | ||
| 9 | Hg | 2+ | Metallothionein–Cu NCs | — | UV-VIS | 97 nm–2.3 μM and 3.1–15.6 μM | 43.8 nM | environmental water | [40] | [82] | ||
| 10 | Hg | 2+ | GSH-Cu NCs | 375/440 | turn off | 0.04−60 μM | 22 nM | water | [41] | [26] | ||
| 11 | Hg | 2+ | Cytosine rich- ssDNA-Cu/Ag NCs | 470/550 | turn off | 40–550 nM | 2.4 nM | lake and tap water | [42] | [31] | ||
| 12 | Hg | 2+ | apt-Cu@Au NCs | 470/656 | ratiometric | 0.1–9.0 μM | 4.92 nM | porphyra | [43] | [63] | ||
| 13 | Hg | 2+ | 4-chlorothiophenol-Cu NCs | 330/605 | turn off | 1–500 nM | 0.3 nM | environmental water | [44] | [83] | ||
| 14 | Hg | 2+ | BSA-Cu NCs | 320/420 | turn off | 0.01 nM–10 μM | 4.7 pM | water | [45] | [19] | ||
| 15 | Hg | 2+ | BSA-Cu NCs | 395/645 | turn off | 20–1000 nM | 0.2 nM | — | [46] | [84] | ||
| 16 | Hg | 2+ | L-Cys-Cu NCs | 375/480 | turn off | 0.1–1000 μM | 24 nM | human urine sample | [47] | [17] | ||
| 17 | Hg | 2+ | dsDNA-Cu NCs | 570/595 | turn off | 0.04−8 nM | 4 pM | water | [48] | [70] | ||
| 18 | Hg | 2+ | Ag/Cu NCs | colorimetric | turn on | 0.1–700 nM | 0.05 nM | aqueous sample | [49] | [85] | ||
| 19 | Hg | 2+ | CDs-CuNCs | 345/430,647 | ratiometric | 0–4000 nM | 0.31 nM | Tap, lake water | [50] | [86] | ||
| 20 | Hg | 2+ | BSA-Cu NCs/ BSA-Au NCs | 365/398,616 | ratiometric | 0.06–1 µM and 1–4 µM | 19.4 nM | Tap, mineral, lake water | [51] | [87] | ||
| 21 | Pb | 2+ | BSA-Cu NCs | 324/401 (fluorescent); 324/396 (light scattering) |
turn off; turn on |
30–180 nM; 3–21 nM |
10 nM; 1 nM |
environmental water | [52] | [88] | ||
| 22 | Pb | 2+ | BSA-Cu NCs | 325/410 | turn off | 0–200 ppm | — | — | [53] | [18] | ||
| 23 | Pb | 2+ | GSH-Cu NCs | 360/607 | turn on | 200–700 μM | 106 μM | water | [54] | [57] | ||
| 24 | Pb | 2+ | Cu NCs-CNQDs | 365/468, 632 | ratiometric | 0.01–2.5 mg L | −1 | 0.0031 mg L | −1 | porphyra | [55] | [77] |
| 25 | Pb | 2+ | Metallothionein–Cu NCs | — | UV-VIS | 0.7–96 μM | 142 nM | environmental water | [40] | [82] | ||
| 26 | Pb | 2+ | dsDNA-Cu NCs | 340/605 | turn off | 0–150 pM | 5.2 pM | tap water | [17] | [43] | ||
| 27 | Pb | 2+ | GSH-Cu NCs | 420/606 | turn off | 1–160 nM | 1 nM | — | [13] | [39] | ||
| 28 | Pb | 2+ | Cu NASs | 340/590 | turn off | 2–100 nM | 0.75 nM | aqueous sample | [56] | [89] | ||
| 29 | Cr | 2 | O | 72− | GSH@CDs-Cu NCs | 360/450,750 | ratiometric | 0–20 μM | 0.9 μM | tap water, spring water samples and human urine | [57] | [78] |
| 30 | Cr(VI) | DAMP-Cu NCs | 357/428 | turn off | 0–150 μM | 8.5 μM | water | [58] | [24] | |||
| 31 | Cr(VI) | Thiosalicylic acid/Cysteamine-Cu NCs | 355/411 | turn off | 0.1–1000 μM | 30 nM | water | [59] | [21] | |||
| 32 | Cr(VI) | Cysteamine-Au/Cu NCs | 350/436 | turn off | 0.2–100 μM | 80 nM | water and human urine sample | [60] | [23] | |||
| 33 | Cr(Ⅵ) | Cu NCs@TA | 360/430 | turn off | 0.03–60 µM | 5 nM | water sample | [61] | [90] | |||
| 34 | Cu | 2+ | D-Penicillamine -Cu NCs | 391/673 | turn on | 0.95–6.35 ppm | 0.3 ppm | tap water | [62] | [91] | ||
| 35 | Cu | 2+ | CdSe QDs @ hPEI-Cu NCs | 380/495,625 | ratiometric | 0.022–8.8 μM | 8.9 nM | river water | [63] | [81] | ||
| 36 | Cu | 2+ | GSH- Cu NCs | 330/615 | turn on | 0.25–10 μM | 170 nM | chalcocite | [12] | [38] | ||
| 37 | Cu | 2+ | DNA-Cu/Ag NCs | 480/576 | turn on | 5–200 nM | 2.7 nM | soil and pond water | [16] | [42] | ||
| 38 | Cu | 2+ | Cytidine-Cu NCs | 300/380 | turn on | 0.05–2.0 µM | 32 nM | lake water | [64] | [58] | ||
| 39 | Cu | 2+ | BSA-Cu NCs | 340/420 | turn off | 0.02–34 μM | 1 nM | tap water | [65] | [20] | ||
| 40 | Cu | 2+ | BSA-Cu NCs/ BSA-Au NCs | 365/398,616 | ratiometric | 0.1–1 µM and 1–5 µM | 23.4 nM | Tap, mineral, lake water | [51] | [87] |
Chromium (Cr (VI)) has been extensively used in the modern industrial production and has also been a common contaminant in the environment. Considering its hugely harmful effect on human health, various analytical methods for the detection of chromium have been designed and, similar to other heavy metal contaminants, many novel sensors based on metal nanoclusters for Cr (VI) have been constructed. Bai and coworkers [57][78] proposed a ratiometric fluorescent probe for the convenient and effective detection of Cr 2O 72− or Cd 2+ by integrating GSH-based carbon dots with copper nanoclusters. As shown in Figure 5 a, the carbon dots-stabilized copper nanoclusters (GSH@CDs-Cu NCs) exhibited two obvious emission peaks at 450 nm and 750 nm, respectively. Owing to fluorescence quenching or enhancement of the nanohybrid, the GSH@CDs-Cu NCs showed good sensitivity and selectivity toward Cr 2O 72− with a linear range from 2 to 40 μmol L −1 and a detection limit of 0.9 μmol L −1 , and Cd 2+ with a linear range from 0 to 20 μmol L −1 and a detection limit of 0.6 μmol L −1 . In addition, successful application of the fluorescence test strips was achieved in the rapid detection of Cr 2O 72− ions with distinct fluorescent color changes from pink to purple under UV light. In the test of real samples, the recovery rates of the target analytes in various water samples were in the range from 102% to 109% with the relative standard deviations (RSD) smaller than 4.5%. And the recovery rates of the target analytes ranged from 97%to 108% with the RSD smaller than 3.5%. Through the one-pot galvanic reduction approach, gold-copper nanoclusters capped by cysteamine (CA-AuCu NCs) were prepared for the detection of chromium(VI) and dopamine, the levels of which are critical to the development of severe diseases [60][23]. The distinct optical properties of this probe made it an ideal candidate in the determination of the target analytes in real samples, such as tap water, lake water, sea water and human urine. In particular, this switch-off probe was responsive in the linear range of 0.2–100 μm for Cr (VI) and with a detection limit of 80 nM.
The prevalence of copper ion residues in the food and in the environment is another looming danger due to damage to the human liver and the nervous system caused by excessive Cu 2 + . Typically, Alzheimer’s disease (AD), as a common neurodegenerative disease, might be caused by excessive accumulation of copper ion and corresponding oxidative stress [66][67][79,80]. Hyperbranched polyethyleneimine-protected copper nanoclusters (hPEI-Cu NCs) were integrated with the silica-coated CdSe quantum dots (QDs) to obtain a novel ratiometric and visual analytical probe toward copper ions through the simple and green one-pot chemical reduction method at room temperature [63][81]. This QDs@Cu NCs probe exhibited high sensitivity and visual detection capability toward Cu 2 + , which could be attributed to the self-calibration function of the dual-emission fluorescence signals inherent in the ratio-based fluorescence probe. The fluorescence of hPEI-Cu NCs was quenched due to the interaction of its amine groups with Cu 2+ , while the fluorescence intensity of QDs remained unchanged, and the distinct color changes could be easily observed by the naked eye under the UV-light. The linear range of the probe in Cu 2+ concentrations was, approximately, from 22 nM to 8.8 μM, with an estimated detection limit of 8.9 nM ( Figure 5 b). When applied in the real water samples with different concentrations of Cu 2 + , the as-synthesized probe exhibited satisfactory recovery.
4. Conclusions and Perspectives
This rentryview summarized and reported the latest development of fluorescent copper nanoclusters utilized in monitoring various types of environmental contaminants. Despite the fascinating advances, there is still a long and arduous way ahead in further improving Cu NCs to make them ideal materials for pollutant detection, quantitative determination, and, even, decomposition and removal. Firstly, the sizes of the current Cu NCs designed fall into a rather wide range, and the desirable optical properties of most Cu NCs heavily rely on excitation. Both of these two aspects may largely constrain the possibility of efficient application of Cu NCs in environmental analysis on a large scale. Secondly, efforts are imperative to further enhance the chemical and optical stability, as well as quantum yield of Cu NCs, so as to improve their feasibility as sensors in more complex, real environments, even with complicated, interfering factors. Lastly, the functions of the current probes are expected to be extended from pure detection of environmental pollutants to simultaneous detection, degradation and, even, removal.
