Cancer-associated fibroblasts (CAFs), as a critical component of the tumor stroma, are strong promoters of various tumor behaviors, including tumorigenesis, growth, invasion, and/or metastasis, because they produce abundant extracellular matrices (ECMs) and mediate the proliferation, apoptosis, migration, and stemness of tumor cells.
Clinically, numerous studies have shown that CAFs can reduce the efficacy of a variety of anti-tumor treatments, including chemotherapy, radiotherapy, biotherapy, and/or targeted therapy, subsequently leading to therapeutic resistance or even failure. For instance, CAFs can prevent the penetration of chemotherapeutic drugs by synthesizing ECMs, promote the growth and metabolism of tumor cells by paracrine signaling/exosome secretion, and prevent the eradication of tumors by therapeutic agents .
- cancer-associated fibroblasts
- cancer
- nanomaterials
1. Introduction
Cancer-associated fibroblasts (CAFs), as a critical component of the tumor stroma, are strong promoters of various tumor behaviors, including tumorigenesis, growth, invasion, and/or metastasis, because they produce abundant extracellular matrices (ECMs) and mediate the proliferation, apoptosis, migration, and stemness of tumor cells [1][2][1,2]. Clinically, numerous studies have shown that CAFs can reduce the efficacy of a variety of anti-tumor treatments, including chemotherapy, radiotherapy, biotherapy, and/or targeted therapy, subsequently leading to therapeutic resistance or even failure [3]. For instance, CAFs can prevent the penetration of chemotherapeutic drugs by synthesizing ECMs, promote the growth and metabolism of tumor cells by paracrine signaling/exosome secretion, and prevent the eradication of tumors by therapeutic agents [4][5][4,5]. Further, CAFs can reduce the sensitivity of tumors to radiotherapy by promoting the epithelial–mesenchymal transition (EMT) of tumor cells and the survival of cancer stem cells (CSCs) [6][7][6,7]. Thus, in the past decades, the design, improvement, and application of biological materials targeting CAFs have attracted much attention for their potential to optimize the therapeutic efficacy of anti-cancer treatments.
At present, studies on biological materials targeting CAFs can generally be divided into three aspects depending on their purpose: high-throughput screening and sequencing, three-dimensional (3D) culture technologies, and nanomaterials for therapeutic applications. Recently, by using a variety of microarrays, differential expression/infiltration patterns of CAFs were detected and analyzed at gene, protein, and tissue levels [8][9][10][8,9,10]. Differentially expressed genes and/or proteins may be targeted as therapeutic markers of crosstalk between CAFs and tumor cells, or applied as indicators for evaluating different functional therapeutic parameters, such as efficacy, complications, and survival rate. Moreover, by improving and optimizing cultivation technology or scaffold materials, 3D culture models of in vivo tumors have been used to explore different effects of CAFs on tumor cells [11]. Importantly, several metal nanoparticles and drug-carrying systems have been developed to target and eliminate CAFs or inhibit their functions to improve anti-cancer therapy [12].
Although the application trend of these biological materials targeting CAFs is promising for anti-tumor treatment, huge challenges must be overcome before they can be used in clinical practice. Due to the fact that the spatial structures differ between existing scaffold materials in co-culture models and the natural ECM, the cell composition of these models, typically from cell lines, is relatively uniform [13], which greatly differs from the organization of tumor tissues. Additionally, nanotherapy, when targeting CAFs, has encountered therapeutic resistance and has even caused adverse reactions in bone marrow [14][15][14,15], thus largely limiting its clinical applications. In this review, we systematically summarize recent findings related to biological materials targeting CAFs, from basic research to the clinical setting, to better understand their applications. We then offer new perspectives in this field and propose potential treatment strategies.
2. Gene Chips and Protein Chips Targeting CAFs
To date, one of the most difficult aspects of targeting CAFs in anti-tumor treatment design has been the difficultly in locating the “Achilles’ heel” among thousands of mutant genes and proteins. Thus, biochips have been widely used to rapidly and accurately identify potential candidates in CAFs for anti-tumor treatments ( Figure 1 ). Typically, a biochip consists of an array of molecular sensors placed on a small surface with a strong substrate to analyze genes, proteins, and/or tissues, enabling the simultaneous execution of many biological or chemical reactions in a relatively short time in a high-throughput process [16]. Different types of biochips, including gene and protein chips, are basically composed of immobilized biomolecules and solid support materials to analyze CAFs [10][16][17][18][10,16,17,18 ]. In general, the sample preparation and testing procedures for the biochip analysis of CAFs are similar to those for other cells[19][20][19,20]; however, the analysis of CAFs with biochips still exhibits some idiosyncrasies when compared to other types of cells.
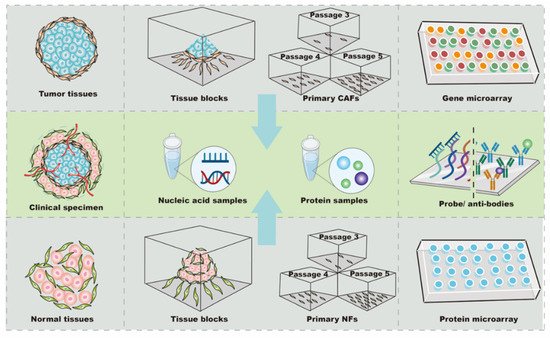
CAFs and normal fibroblasts (NFs) derived from primary culture are the main cellular sources for biochip detection ( Figure 1 ). The majority of CAFs are isolated from fresh tumor specimens. To analyze differences in profiles, it is necessary to use NFs as controls. Since the tumor and its surrounding tissues are usually surgically removed together, tumor-adjacent tissue located more than 2–5 cm away from the primary site is the most common source of NFs [21][22][21,22,23]. Additionally, NFs can be obtained from the skin of the head and neck, gingiva, buccal mucosa, or foreskin tissue after circumcision [23][24][25][24,25,26]. Further, commercial cell lines of NFs can be used as controls. For instance, NFs purchased from Jingkang (Shanghai, China) were used to study the miRNA differences in CAF-derived exosomes in breast cancer [7] [7]. The obtained expression profiles were very sensitive to the controls; even in the same study, using NFs from different sources led to different biochip results. In support of this finding, Enkelmann et al. found that miR-16 was up-regulated in bladder CAFs when compared with foreskin fibroblasts, but not when compared with fibroblasts from normal urothelial tissue [24][25]. Intriguingly, sequence analysis further revealed that miR-16 was decreased in foreskin tissue compared with urothelial tissue, which may explain this discrepancy [24][25]. These results suggest that miR-16 expression in bladder CAFs may not be significantly changed, and the agents targeting miR-16 in bladder CAFs might be off-target or less effective. Similarly, Nakagawa et al. used NFs from the liver and skin as controls in separate analyses, and the results showed that reticulon 1 (RTN1), dickkopf homolog 1 (DKK1), hypothetical protein PP1044 (PP1044), and cyclin-dependent kinase inhibitor 2A (CDKN2A) were up-regulated in CAFs when compared with liver NFs, whereas proteoglycan 1 (PRG1), ankyrin 3 (ANK3), and monocyte chemotactic protein 1 (MCP1) were increased when compared with skin NFs [19]. Notably, vascular cell adhesion molecule 1 (VCAM1) was up-regulated in CAFs compared with NFs from both the liver and skin [19], indicating that VCAM1 was consistently increased in CAFs and might be a candidate target for anti-tumor therapy in metastatic colon cancer. In summary, these findings show that CAFs and NFs have a high cellular heterogeneity, and the expression profiles of NFs are variable depending on their tissue source. Accurately obtaining potential candidate targets in CAFs requires control NFs from at least two sources during high-throughput screening, or the preliminary results from biochips need to be re-validated by other methods before processing.
In most studies, CAFs were passaged three to five times before analysis, while 10 passages were considered acceptable for primary cells [22][23]. The total RNA of the primary cultured fibroblasts was extracted by Trizol Reagent (Invitrogen) or commercial kits, such as the RNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA) and miRVana Isolation Kit (Ambion, Life Technologies, Milan, Italy) [7][21][26][7,21,28]. For the protein arrays, the conditioned medium was collected after 2000× g centrifugation and stored at –80 °C if hybridization with biochips could not be performed immediately [17]. Recently, many studites have been tended to use arrays from Affymetrix (Thermo Fisher Scientific Inc., Santa Clara, CA, USA) and Agilent (Agilent Technologies, Santa Clara, CA, USA) to explore mRNA and/or miRNA expression [27][28][22,30], and, to date, the cytokine antibody chip from RayBio (RayBio, AAH-BLG-493) has mainly been used to detect the level of protein secreted by CAFs [20][29][20,34]. In summary, CAF analysis using biochips depends on cell isolation, culture, and identification. As a result of cellular heterogeneity, all or a combination of α-SMA, FAP, and/or PDGFRα/β should be used for identification. Biochips from different manufacturers, such as Affymetrix, Agilent, and RayBio, need to be chosen according to the type of expression profile being analyzed.
The differential expression obtained from biochips reflects relative changes and needs to be validated by quantitative methods. For gene chips, quantitative polymerase chain reaction (qPCR) is commonly used, and western blotting (WB) and/or enzyme-linked immunosorbent assay (ELISA) are used in the detection of protein levels. In most cases, the qPCR or WB results have corresponded to the gene expression obtained in the biochip analysis. Zhao et al. randomly chose miRNAs for further analysis by RT-qPCR, and the data were highly similar to the biochip results [26][23]. Similarly, in another study, ELISA and WB indicated that the concentration of plasminogen activator inhibitor-1(PAI-1) was higher in the conditioned medium of CAFs, which was also observed in the cytokine array results [30][17]. However, Enkelmann et al. provided evidence that, whereas biochip results indicated that miR143 and miR145 were down-regulated in CAFs in bladder cancer, qPCR showed no notable changes in the same miRNAs [31][25], indicating that foreskin fibroblasts might not be suitable as a control in this study, or that the difference might be caused by a sample size bias. Supporting this notion, Utaijaratrasmi et al. found that the expression of miR-486 was different in two normal skin fibroblasts [32][24], suggesting that the sample size needs to be expanded when discrepancies appear. Taken together, the expression profiles of CAFs from biochips can strongly vary as a result of different factors, from the sample size to controls; thus, further validation by other methods needs to be performed to confirm the results obtained using biochips.
3. Three-Dimensional Co-Cultivation Materials
The tumor microenvironment (TME) is complex in composition and structure, which can affect tumor growth, metastasis, and the cellular phenotypes of tumor and stromal cells [33][35]. Traditional co-cultivation techniques have been primarily carried out on a two-dimensional (2D) plane, which is quite different from the growth mode of cells in vivo [34][36], leading to deviations from the actual situation in terms of cell growth, differentiation, and interaction. In recent years, with the development of materials research, the emergence of multiple scaffold materials has made it possible to construct 3D co-culture models for in vitro studies in cell, tissue, and/or organ cultures, and 3D co-cultivation appears to have a promising future in facilitating the translation of basic research to the clinical setting. At present, the types of 3D co-culture models related to CAFs can generally be divided into scaffold-free co-culture systems, scaffold-based co-culture models, and microfluidic platform co-cultivation technology ( Figure 2 ).
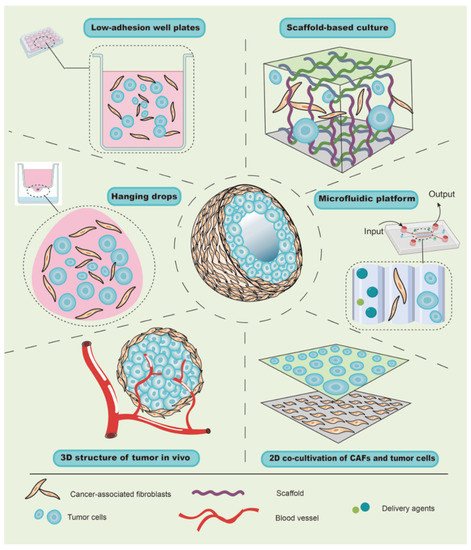
Due to buoyancy and gravity, suspended cells can aggregate into small spherical cell clumps without the aid of foreign scaffold materials [35][37]; therefore, this method has been widely used to explore the role of CAFs in tumor growth using hanging-drop and low-adherence plates. For instance, due to the effect of surface tension, liquid can hang onto the lid to form hanging drops, and the cells therein can gather into a spherical shape under the action of gravity [34][36]. Similarly, by mixing and adding the same number of cancer cells and CAFs from the primary culture into hanging-drop plates, Ma et al. transferred and harvested a cell suspension containing spheroids after 72 h. After imaging by microscopy, it wahe authors found that CAFs could promote the growth of gastric cancer cells and increase the diameter of the spheres [36][38]. Further, when a well plate is covered with an inert substrate (usually polystyrene), cells are unable to adhere to the plate wall, and the suspended cells aggregate into visible spheroids. Zhou et al. harvested similar spheroids consisting of melanoma cells and CAFs of melanoma for 48 h by inoculating a mixture of cell suspension into 96-well plates with low-cell-adhesion surfaces [37][39]. Although centrifugation will accelerate the formation of spheroids, it is limited in the hanging drop, which increases the experimental period. Taken together, in contrast to traditional direct or indirect co-culture, the scaffold-free culture allows cell spheres to grow and form a spatial structure, which is more similar to tumors in vivo and can be used to evaluate the promoting effect of CAFs on tumor growth. Notably, when the cell spheroids are suspended in the medium, their motility cannot be evaluated or controlled, which limits the further analysis of the role of CAFs in tumor behaviors, such as invasion, metastasis, and so onetc.
Since a scaffold material with a porous structure provides space and support for the growth of cells, the growth patterns of cells in a scaffold-based culture differ from those in a 2D co-culture. For example, Phan-Lai et al. co-cultured mouse mammary carcinoma (MMC) cells and mouse CAFs in both 2D plates and chitosan–alginate scaffold, and they observed that MMC cells formed tumor spheroids and had slower growth in the scaffold-based culture than in the 2D co-culture [38][46]. Intriguingly, scaffold-based culture technology can be combined with a scaffold-free culture to explore the role of CAFs in tumor cell invasion and migration. For instance, Pankova et al. used the hanging-drop method to acquire tumor spheroids, and then embedded them in rat-tail collagen gel pre-inoculated with CAFs, which increased the protrusions of spheroids and enhanced the invasive activity of cancer cells [39][47]. These findings suggest that the scaffold-based co-culture has multiple advantages, including the rapid formation of spheroids, well-controlled cellular motility, and the 3D structure and spatial organization, in order to study tumorigenesis, invasion, and metastasis.
Furthermore, the scaffold material can not only serve as a supportive function but can also form a more complex layered structure that is similar to the skin [34][36]. Most tumors, especially cancers from the epithelium, are divided into epithelial and mesenchymal layers. CAFs are mainly located in the mesenchyme and interact with tumor cells. Theoretically, by mixing CAFs with a gel and allowing it to solidify, the surface layer of CAFs can be inoculated with a layer of tumor cells, which results in a hierarchical structure similar to that of epithelial tumors in vivo. To support this notion, Chantravekin et al. seeded epithelial cells on the surface of a gel containing ameloblastoma-associated fibroblasts, and the surface became white because of the multiplication of epithelial cells [40][44]. Similarly, since cells in the top layer are usually exposed to the air and only receive growth support from the gel containing CAFs below, which simulates the actual TME, Horie et al. exposed the gels to the air by placing them on a mesh in new plates with a growth medium for 5 days. Invasion and nodular epithelial structures were observed in the CAF layer [41][57], indicating that CAFs enhanced the invasion of tumor cells and might induce the differentiation of lung cancer cells into mucinous cells.
4. Nanomaterials Targeting CAFs
Accumulating evidence suggests that the response to anti-tumor therapies highly depends on the features of the tumor cells and the TME [42][72]. As described above, CAFs, as the major cells in the TME, can secrete a variety of soluble cytokines and/or exosomes to support tumor survival, promote metastasis, and enable tumor cells to escape immune attacks [43][73]. Therapies targeting CAFs might improve the efficacy of anti-tumor treatment and reduce therapeutic resistance [44][74]. However, the ECM and fluid pressure formed by CAFs create a physical barrier that restricts the entry of drugs [45][46][75,76]. Recently, a variety of materials have been successfully prepared to target CAFs for anti-cancer treatment, and have shown therapeutic effects [47][48][77,78]. These data have shown that nanoparticles or drug delivery systems designed to target the functions of CAFs exhibit promising therapeutic effects. Although different nanosystems considerably vary in their composition and mechanism, they mainly consist of three parts: targeting ligands, drug-carrying systems, and cargo ( Figure 3 ).
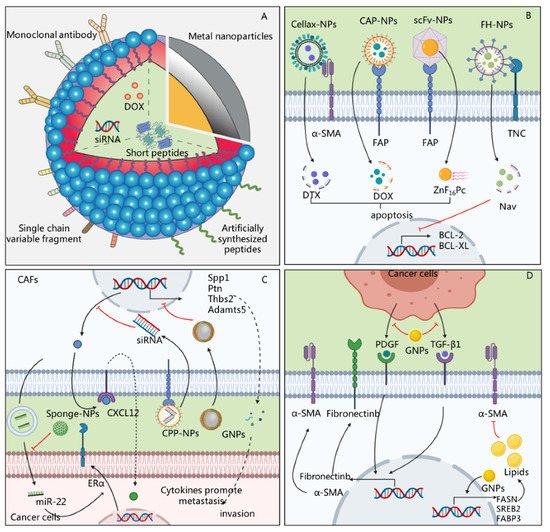
A variety of drugs or small molecular agents can be carried by nanosystems and enter CAFs to exert therapeutic effects. At present, nanosystems can carry a variety of cargo, including chemotherapeutic drugs, nucleic acids, and short peptides, to directly kill CAFs or regulate their functions [49][50][51][12,92,93]. Cytotoxic drugs are the most commonly carried drugs [52][53][81,82]. Insoluble drugs carried by nanosystems can penetrate the barrier formed by CAFs, which can then be eliminated to further promote the entry of drugs and exert anti-tumor effects. For instance, Zhu et al. used glycolipid-based polymeric micelles (GLPMs) to deliver telmisartan and doxorubicin to breast cancer cells, which significantly reduced the α-SMA + CAF population, attenuated the solid stress in the tumor and the tumor vessel pressure, and inhibited tumor growth in vivo [54][84]. However, chemotherapeutic drugs can also have potential side effects. FAP is expressed not only on the surface of CAFs but also in multipotent bone marrow stem cells, and some therapeutic approaches, such as immunotherapy designed to bind FAP, lead to bone marrow suppression [55][56][15,96]. In order to reduce the occurrence of adverse reactions, photodynamic therapy (PDT) can be used for the targeted removal of CAFs. Zinc hexafluorophthalocyanine (ZnF 16Pc) is one of the most commonly used photosensitizers. It can enter CAFs to generate 1O2, which has cell-killing effects [57][97]. In another study, Li et al. injected a nanosystem containing ZnF16Pc into tumor-bearing mice and irradiated the tumor with a 671 nm laser, which significantly reduced the positive staining of α-SMA and the synthesis of ECM [58][86].
However, the massive death or depletion of CAFs in the stroma does not always result in the expected therapeutic effect. Interestingly, the extensive depletion of CAFs caused an increase in molecules of the damage response program (DRP), such as Wnt 16, which increased the secretion of inflammatory factors and reduced the effectiveness of chemotherapy [14]. Therefore, another strategy for CAF-oriented nanotherapy could be based on regulating CAF-related functions or reducing their activity. Nucleic acids, peptides, and other small-molecule drugs carried by nanosystems have exhibited potential anti-tumor effects by regulating the function of CAFs. For instance, Gao et al. prepared cyclic RGD (cRGD)-miR-22-sponge nanoparticles to neutralize miR-22 in the exosomes of breast cancer CAFs, and they found that a decrease in miR-22 enhanced the therapeutic effect of tamoxifen [43][73]. In addition, metal sodium nanoparticles, such as gold nanoparticles (GNPs), can regulate the function of CAFs without relying on drugs, making it a potential method of anti-tumor therapy ( Figure 3 ). For instance, GNPs induced the up-regulated expression of fatty acid synthesis genes in CAFs, including fatty acid synthetase (FASN), sterol regulatory element-binding protein 2 (SREBP2), and fatty acid-binding protein 3 (FABP3) genes, and increased the lipid content in cells, thereby transforming CAFs into cells with a static phenotype with a high fat content and low proliferation [59][80].
In summary, a variety of nanomaterials, including biocompatible polymers, liposomes, and ferritin, can be used to deliver drugs and facilitate their entry into tumor tissues. The modification of targeting ligands allows them to specifically bind to CAFs and exert an anti-tumor effect. In order to reduce the occurrence of adverse reactions or treatment failures, the two main alternative treatment strategies are photodynamic therapy, to locally eliminate CAFs, or the delivery of small-molecule targeted drugs, to regulate the function of CAFs.
