The use of Microfiltered Water Dispensers (MWDs) is increasing in offices, companies, or commercial facilities, as a response to plastic pollution. Despite their widespread use, poor data are available about the water quality and pathogens developed. Starting from a high contamination found in MWDs, a Water Safety Plan (WSP) was implemented on 57 MWDs to improve the water quality. To assess the effectiveness of WSP during the period 2017–2021, the environmental monitoring of heterotrophic plate counts (HPCs) at 36 °C and 22 °C, Enterococcus spp., Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, and Clostridium perfringens, as prescribed by Italian regulation for drinking water, was conducted. A high level of HPCs at both temperatures was observed, over the regulation limit; contrarily, the pathogenic bacteria were absent. The contamination found was studied with respect to the temperature threshold of 25 °C, suggested by directives. No significant differences were found between samples above and below the threshold, while a significant decrease over the years was observed for HPCs at 36 °C (p = 0.0000000001) and 22 °C (p = 0.000006). The WSP implementation resulted in a 43.09% decrease for HPCs at 36 °C and a 24.26% decrease for HPCs at 22 °C. Moreover, during the SARS-CoV-2 pandemic, the WSP contributed to limit the microbial contamination, preserving the MWDs’ functionality and hygienic conditions and the drinking water quality.
1. Introduction
It is known that the quality of drinking water is an important public health issue, and the Microfiltered Water Dispensers (MWDs) are often adopted as devices able to improve water organoleptic characteristics and are easy to use and to maintain
[1]. MWDs are devices directly connected to a municipal water supply and they often may include a CO
2 system for sparkling water or cooling systems for cold water. An activated carbon filtration system incorporated in the device improves the treatment of drinking water; sometimes an Ag
+ coating may be present. The activated carbon filter is used for a bacteriostatic effect and to reduce tastes and odors or remove organic and inorganic contaminants
[2][3]. Furthermore, the use of activated carbon filters could amplify the microbiological contamination at point of use, especially in the presence of water stagnation
[4][5]. The filtration system is sometimes associated with an UV lamp, installed inside MWDs or immediately before the water is dispensed, at the output nozzle. The UV lamp is able to remove airborne or surface microorganisms
[6][7].
For these reasons, an adequate maintenance and disinfection protocol should be implemented on MWDs. Several studies have discussed the use of oxidants’ agents, such as peracetic acid and hydrogen peroxide, to reduce the microbiological contamination and to assure the quality of resulting water
[5][8]. The disinfection products need to be certified as food-grade products to preserve the health of consumers; at the same time, it is recommended to avoid the continuous use of aggressive disinfectants, such as chlorine disinfectants, that could damage the devices, often characterized by plastic material causing a decay of the material, which can dissolve into the water flow and reach the consumers. Another aspect regards the continuous disinfection treatment that can lead to a bacterial species’ selection with consequent increase in bacterial resistance and inefficient disinfection procedures
[9][10].
However, concerns have been raised about the quality of the water dispensed from MWDs due to the non-compliant maintenance procedures that could lead to a potential risk of uncontrolled bacterial contamination and the consequence of waterborne infectious diseases, especially as regards the sensitive and immunocompromised population
[1]. Multiple studies show that the contamination of MWDs is caused by missed or improper cleaning.
Pseudomonas aeruginosa (P. aeruginosa) is considered an opportunistic pathogen, and its occurrence in drinking water can pose a serious health risk to children and immunocompromised individuals
[11].
Such findings indicate that the companies and customers should be informed about the proper use and correct maintenance of MWDs, and this helps to keep them free from microbiological contamination
[12].
In order to avoid or to reduce the risk of acquiring water-related infections, World Health Organization (WHO) Guidelines for Drinking-Water Quality and European Directive and subsequent amendments on the quality of water intended for human consumption have been introduced in some countries
[13][14][15][16][17]. In Italy, established criteria exist for human consumption of water, including water coming from MWDs
[7][18].
2. Microfiltered Water Dispensers’ Characteristics
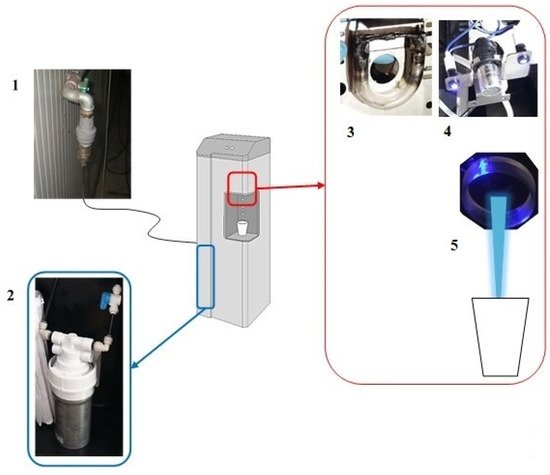
The MWDs involved in the study are considered “point of use”, meaning that they are connected to a main potable water network (
Figure 1).
Figure 1. Schematic representation of Microfiltered Water Dispensers (MWDs) from our previous study (Girolamini et al. 2019): 1: Input from municipal water, 2: Activated carbon filter, 3: UV Lamp at water supply point, 4: UV lamp, without glass, and water supply point (output), 5: Water supply nozzle (output).
A dedicated pipeline containing a removable filter with a pore size of 1 mm was used to remove particulate matter that could be delivered from the municipal water. The main water flowed through an internal activated carbon filter with a pore size of 0.5 µm, which reduced tastes, odors, and various organic and inorganic substances (e.g., clays, chlorine, etc.), as well as potentially disinfectant chemicals used in municipal water. The MWDs showed an approximate capacity of 20–28 L/h and were provided by a single, low-pressure UV lamp at the output water supply point. The lamp consisted of a small, glass tube encircled with a power of 1.5 W. The MWDs provided water at room temperature, sparkling water (by carbon dioxide gas cylinder), and hot water, via a circular supply point (nozzle). The water was supplied by a single nozzle located at the base of the dispenser, which had a capacity of 30 cL. The technical characteristics of MWDs were provided in the manufacturer’s manuals and conformed to the Italian directives
[7].
The MWDs involved in this study were installed in several industrial sites (e.g., coffee break areas, offices, recreational points, etc.) and the intended use was especially to provide drinking water in common or private spaces, not catering use, as admitted. Moreover, in Italy, it is mandatory to notify the presence and use of MWDs to the Public Health Authorities
[19] only when these devices are in use in hotels, canteens, restaurants, and cafés; therefore, their presence in other facilities often is underestimated, with a lack of authority control.
3. Studies of Water Dispenser
Considering the contamination found in the previous study
[20], in relation to HPCs at 36 °C and 22 °C,
P. aeruginosa and other pathogenic bacteria,
Table 1 displays the status of MWDs’ contamination at the beginning of the study (2017) and the mean contamination found during the period of the study.
Table 1. MWDs’ mean contamination ± standard deviation (cfu/mL ± SD) found at the beginning of the WSP implemented in 2017 until today (2021), with respect to mean contamination found in the previous study (Girolamini et al., 2019), from 2015 to 2017.
| MWDs’ Mean Contamination ± Standard Deviation (cfu/mL ± SD) |
| Microbiological Parameters |
Girolamini et al., 2019 | [20] |
(2015–2017) |
Present Study (2017–2021) |
| 2017 |
2018 |
2019 |
2020 |
2021 |
| HPCs 36 °C |
1852 ± 0.67 |
1014.99 ± 2637.63 |
321.67 ± 802.31 |
39.05 ± 86.37 |
124.83 ± 766.07 |
30.02 ± 77.62 |
| HPCs 22 °C |
2437 ± 0.56 |
686.25 ± 1952.55 |
453.58 ± 1442.75 |
69.78 ± 374.89 |
161.50 ± 776.76 |
91.75 ± 469.23 |
| P. aeruginosa |
37.4 ± 0.81 |
9.48 ± 36.86 |
20.81 ± 59.50 |
2.18 ± 20.63 |
5.04 ± 27.58 |
0.67 ± 5.03 |
| Enterococcus spp. |
3.3 ± 0.20 |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
| E. coli |
| S. aureus |
| C. perfringens |
n.d. |
In detail, a total of 477 water samples were collected from 57 MWDs and analyzed for HPCs at 36 °C and 22 °C and for pathogenic bacteria,
Enterococcus spp.,
P. aeruginosa,
Escherichia Coli (
E. coli),
Staphilococcus aureus (
S. aureus), and
Clostridium perfringens (
C. perfringens).
The overall results showed that the samples were more contaminated by HPCs at 36 °C and 22 °C; instead,
P. aeruginosa was detected in 44/477 (9.2%) samples. Total and fecal coliforms,
E. coli,
S. aureus, and
C. perfringens were not detected in any of the investigated samples. The MWDs’ mean contamination found, shown in
Table 1, displayed a continuous decrease during the years of the study. The HPCs at 36 °C and 22 °C data were studied to understand the trend of bacteriological contamination. The analysis described below was not performed for other pathogenic parameters found, due to the sample size of data being too small for statistical reporting and for other microbiological parameters never detected in MWDs during the period.
The positive samples were analyzed with respect to the reference values prescribed by the Italian regulations.
Regarding HPCs at 36 °C and 22 °C, the following microbiological profiles were recorded: a total of 187/477 (39.2%) samples, with a range of contamination of 21–14,940 cfu/mL, and 94/477 (19.7%) samples, with a range of contamination of 104–10,960 cfu/mL, exceeded the regulation limit values of 20 cfu/mL and 100 cfu/mL, respectively, for HPCs at 36 °C and 22 °C.
The contamination found was studied also in relation to the temperature measured, considering the temperature as one of the factors that could influence the bacterial growth. According to the Guidelines for Drinking-Water Quality, the reference water temperature was between the range of 12–20 °C, with a limit not to exceed set at 25 °C
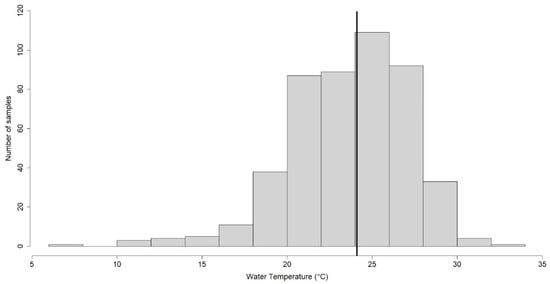
[14][16][17][21]. In particular, in our study, 292/477 (61.2%) samples showed temperature values ≤ 25° C and 185/477 (38.8%) samples showed values > 25° C. The samples showed a minimum value measured of 7.2 °C and a maximum of 32.4 °C, with a median value of 24.1 °C.
Figure 2 shows how most samples were centered around the median value of 24.1 °C, a temperature value close to the reference limit threshold of 25 °C
[17].
Figure 2. Temperature-based distribution, with respect to the interval between 7.2 °C and 32.4 °C and the median value of 24.1 °C (bold row).
The HPCs’ contamination found (for both 36 °C and 22 °C) were separated into two groups based on the temperature values above and below the 25 °C temperature threshold, and compared with each other. In both cases there was no significant difference between the groups with values above and below 25 °C, with a
p-value (
p) = 0.56 and
p = 0.18 for HPCs at 36 °C and 22 °C, respectively.
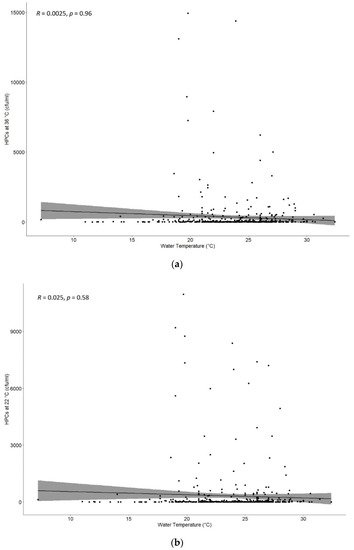
Moreover, data were evaluated to find a possible correlation between HPCs at 36 °C and 22 °C and the temperature of samples measured, as shown in
Figure 3a,b. There was no significant correlation with a
p = 0.96 and
p = 0.58 for HPCs at 36 °C and 22 °C, respectively.
Figure 3. (a) Correlation between heterotrophic plate counts (HPCs) at 36 °C and the temperatures detected during the drinking water monitoring program. There was no significant correlation with a p-value = 0.96. (b) Correlation between heterotrophic plate counts (HPCs) at 22 °C and the temperatures detected during the drinking water monitoring program. There was no significant correlation with a p-value = 0.58.
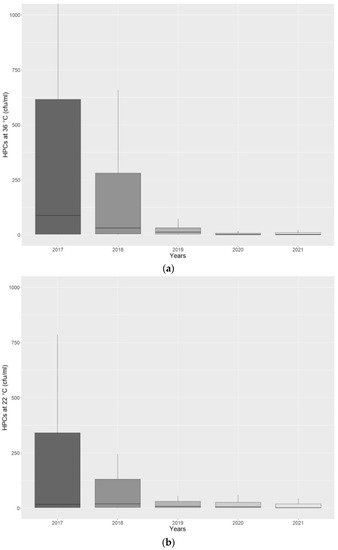
Considering the period of analysis, from 2017 to 2021, to assess the compliance of monitoring and maintenance measures undertaken, researchers studied the microbiological contamination trend of HPCs at 36 °C and 22 °C (
Figure 4a,b).
Figure 4. (a) Boxplot graph showing a decreasing trend for heterotrophic plate counts (HPCs) at 36 °C observed during the period of analysis, from 2017 to 2021. Outliers are not displayed. (b) Boxplot graph showing a decreasing trend for heterotrophic plate counts (HPCs) at 22 °C observed during the period of analysis, from 2017 to 2021. Outliers are not displayed.
Figure 4a,b show how the continuous water quality monitoring and the MWDs’ maintenance, according to International Regulations and Guidelines, led to a decrease in bacterial contamination. The same contamination trend was observed in terms of percentage of positive samples that exceeded the regulation limits. We found 68/109 (62.4%) for HPCs at 36 °C and 36/109 (33.0%) for HPCs at 22 °C positive samples that exceeded the regulation limits in 2017, compared to 11/57 (19.3%) and 5/57 (8.8%) in 2021.
The value of mean contamination ± SD found were 1014.99 ± 2637.63 cfu/mL and 686.25 ± 1952.55 cfu/mL for HPCs at 36 °C and 22 °C, respectively, in 2017, compared to 30.02 ± 77.62 cfu/mL and 91.75 ± 469.23 cfu/mL for HPCs at 36 °C and 22 °C, respectively, in 2021.
These data are supported by the statistical analysis applied on HPCs at 36 °C and 22 °C detected in 2017 compared to 2021, when it was possible to assess a significant decrease of HPCs at 36 °C (
p = 0.0000000001) and 22 °C (
p = 0.000006) in 2021 compared to 2017.
The WSP implementation resulted in a 43.09% decrease for HPCs at 36 °C and 24.26% decrease for HPCs at 22 °C.
Finally, in order to verify the impact of the lockdown period, due to the global SARS-CoV-2 pandemic that occurred during 2020, which also affected the industrial company involved in this study, causing the closure of MWDs’ devices, we focused on the analysis of the data, both for HPCs at 36 °C and 22 °C, comparing the contamination found in the pre-COVID (year 2019) and post-COVID (year 2021) periods.
The extended period of inactivity did not affect the MDWs’ contamination, as evidenced by a significant decreasing trend of HPCs’ parameters, with a
p-value of 0.000005 for 36 °C and 0.001 for 22 °C, respectively. Moreover, a decreasing number of positive samples over the directive limits was observed: 31/94 (33.0%) for HPCs at 36 °C and 9/94 (9.6%) for HPCs at 22 °C in 2019 compared to 11/57 (19.3%) and 5/57 (8.8%) in 2021.
Regarding the contamination found, it was possible to evaluate again a decrease, such as 39.05 ± 86.37 cfu/mL and 69.78 ± 347.89 cfu/mL for HPCs at 36 °C and 22 °C, respectively, in 2019 compared to 30.02 ± 77.62 cfu/mL and 91.75 ± 469.23 cfu/mL for HPCs at 36 °C and 22 °C, respectively, in 2021.
4. Conclusions
The facts prove the long-term effectiveness of the WSP followed by the company and the consumers, in addition to the role of a multidisciplinary approach established to assure the drinking water quality, according to the International and National directives. In addition, researchers suggests a need to implement more rigorous microbiological monitoring to apply to MWDs’ environment, underestimated to date, despite the large diffusion of these devices in the communities.
Lack of awareness and knowledge on these issues, in addition to missing systematical control by users as well as by public health authorities, can lead to a high risk to human health, especially in hospitals and, in general, in all health-care settings, where the use of MWDs is already widespread and the presence of immunocompromised people could increase the risk of infections.
Moreover, these findings assume a relevant importance even across new scenarios: the higher request for plastic reduction, the introduction, starting from January 2021, of the new European Directive 2184/2020 on the quality of water intended for human consumption focused to implement a WSP approach in all facilities, and the introduction of new microbiological parameters in order to assure the water quality, such as, for example,
Legionella spp.
[22]. Considering the habitat, the pathogenic role, and the impact on public health demonstrated for these bacteria, the sanitation and maintenance procedures of MWDs will play a relevant role to preserve the water quality.