Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 3 by Catherine Yang.
Diospyros kaki (persimmon) leaves have long been utilized as traditional medicine for the treatment of ischemic stroke, angina, and hypertension and as a healthy beverage and cosmetic for anti-aging. This study aimed to isolate as many compounds as possible from an ethanol extract of the persimmon leaves to identify the biologically active compounds. The antioxidative effect of the ethyl acetate layer from the ethanol extract of the persimmon leaves was demonstrated by 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay and online high-performance liquid chromatography-2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (HPLC-ABTS) analysis.
- Diospyros kaki
- persimmon leaves
- flavonoid
- antioxidant
1. Introduction
Diospyros kaki Thunb. (persimmon) belongs to the family of Ebenaceae and is widely distributed in Korea, China, and Japan. Its fruit is eaten fresh or dry, while the leaves have long been used as a traditional medicine to treat ischemic stroke, angina, hypertension, atherosclerosis, and infectious diseases [1]. Furthermore, its leaves have been utilized as healthy beverages and cosmetics due to their anti-aging properties and abilities to help prevent cholesterol and melanin accumulation [1]. Recent research has suggested that the extracts of the persimmon leaves possess a wide range of biological properties, including radical scavenging, neuroprotection, thrombosis inhibition, anti-atherosclerosis, and anti-allergy [2][3][4][5][6]. A previous phytochemical investigation suggested that various types of flavonoids and terpenoids are the main constituents [7], and several tannins, naphthoquinones, coumarins, ionones, and fatty acids were also reported [8][9][10][11][12].
Reactive oxygen species (ROS) are reactive molecules produced in biological systems, and the balance between the generation and elimination of ROS is well controlled in normal cellular physiology [13]. However, excessive generation of ROS causes oxidative damage, and in turn, aging and age-related diseases including cancer, diabetes, and Parkinson’s disease [14]. Hence, discovering antioxidants such as flavonoids and phenolic compounds could be a promising strategy to treat these diseases.
As part of our continuous project to find biologically active compounds [15], the antioxidative effect of the ethanol (EtOH) extract and solvent partitions from the persimmon leaves was evaluated using 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay and online high-performance liquid chromatography-2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (HPLC-ABTS) analysis. A phytochemical study on the persimmon leaves led to the isolation of one new flavonoid (1) and one new natural compound (3), along with 25 previously known compounds. The structures were characterized by the application of spectroscopic and spectrometric methods. All isolated compounds were rapidly screened for their antioxidative effects using online HPLC-ABTS. Furthermore, the quantitative analysis of all isolated compounds was performed in the present study.
2. Antioxidative Effect of the Persimmon Leaves
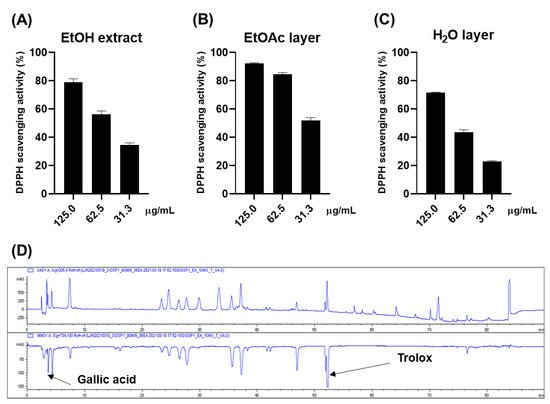
The antioxidative effect of the ethanol (EtOH) extract of persimmon leaves was evaluated for a preliminary screening through DPPH (Figure 1A). The 0.125 mg/mL of the extract scavenged approximately 80% of the DPPH radical, while 0.025 mg/mL of ascorbic acid made up 94% of the radical. The online HPLC-ABTS assay was carried out to rapidly ensure the reliability of these results (Figure 1D). Gallic acid and Trolox were used as internal standards. The chromatogram at 734 nm (negative peak) suggested that approximately nine constituents could have antioxidative activities. Gallic acid and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were used as internal standards to ensure the reliability of the results. It was inferred that most of these peaks were flavonoid derivatives such as flavonoid glycoside and flavanol, based on the dereplication analysis performed by comparing ultraviolet (UV) and mass spectra of the compounds with the published data. Bioassay-guided fractionation suggested that these antioxidative compounds were abundant in the ethyl acetate (EtOAc) layer, while the water (H2O) layer showed weak activity (Figure 1B,C).
Figure 1. DPPH scavenging effects of the EtOH extract (A), EtOAc layer (B), and H2O layer (C) H2O layer of the persimmon leaves; (D) online ABTS-HPLC chromatogram of the EtOH extract.
3. Phytochemical Investigation
To identify these antioxidative compounds, various chromatographic and spectroscopic methods were carried out for the isolation and structural characterization of the compounds. A new flavonoid (1) and a new natural compound (3) were obtained from the ethyl acetate layer of the ethanol extract, together with 25 previously reported compounds, namely kaempferol-3-O-β-2″-coumaroylglucoside (2) [16], (+)-catechin (4) [17], hyperoside (5) [17], isoquercitrin (6) [18], quercetin-3-O-β-2″-galloylgalactoside (7) [19], quercetin-3-O-β-2″-galloylglucoside (8) [20], trifolin (9) [18], astragalin (10) [18], kaempferol-3-O-β-2″-galloylgalactoside (11) [21], kaempferol-3-O-α-arabinoside (12) [22], kaempferol-3-O-β-2″-galloylglucoside (13) [23], quercetin-3-O-β-2″-coumaroylglucoside (14) [24], quercetin (15) [15], kaempferol (16) [25], (6S,9S)-roseoside (17) [26], scopoletin (18) [27], umbelliferone (19) [28], 1-(2,4-dihydroxy-6-methylphenyl)ethanone (20) [29], barbinervic acid (21) [30], diospyric acid B (22) [7], rotungenic acid (23) [31], pomolic acid (24) [32], siaresinolic acid (25) [33], oleanolic acid (26) [25], and ursolic acid (27) [25] by using spectroscopic and spectrometric and physical data in comparison with the published data and also with thin layer chromatography (TLC) analysis (Figure 2 and Figure 3). Among these, compounds 2, 16, 17, 19, 20, and 24 were firstly isolated from D. kaki.
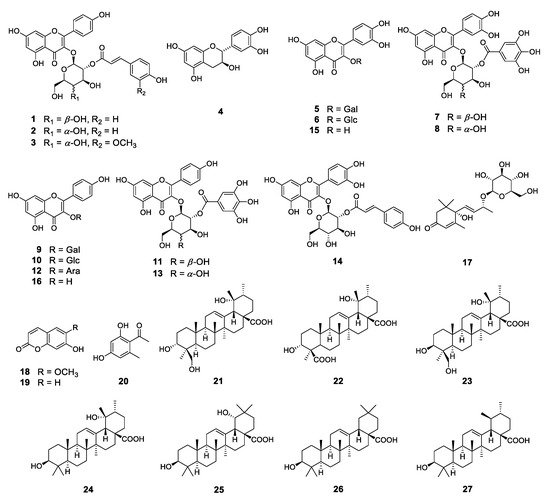

Figure 2. Structures of the compounds isolated from D. kaki leaves.
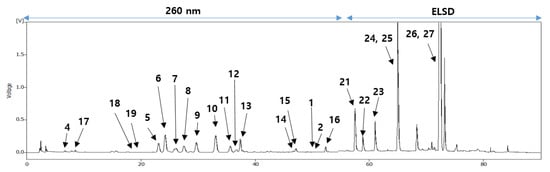

Figure 3. HPLC chromatogram of the EtOH extract from the persimmon leaves.
Compound 1 was obtained as a yellow powder, in which the molecular formula was established as C30H26O13 based on high-resolution mass spectrometry (HRMS) data. The UV spectrum exhibited absorption bands at 207 and 315 nm, indicating that compound 1 had a flavonol backbone. The 1H nuclear magnetic resonance (NMR) data (Table 1, Figure S1) showed a typical pattern of coumaroylated flavonol glycoside, showing two sets of AA′BB′-type signals (δH 8.00 (2H, d, J = 8.5 Hz, H-2′ and H-6′), 6.87 (2H, d, J = 8.5 Hz, H-3′ and H-5′)) in the B ring of kaempferol as well as the signals (δH 7.45 (2H, d, J = 8.5 Hz, H-2′′′ and H-6′′′), 6.81 (2H, d, J = 8.5 Hz, H-3′′′ and H-5′′′)) in the aromatic ring of the coumaroyl group. Two doublet signals (δH 7.65 (1H, d, J = 15.5 Hz, H-7′′′)) and δH 6.35 (1H, d, J = 16.0 Hz, H-8′′′) were observed, indicating trans-olefinic protons of the coumaroyl group. Additionaly, an anomeric proton signal (δH 5.57 (1H, d, J = 8.0 Hz, H-1′′)) was observed in the sugar region, suggesting the presence of the β-configurated cyclic sugar group. The 13C and distortionless enhancement by polarization transfer NMR data (Table 1, Figure S2) showed 30 resonances comprising two trans-olefinic carbons, ten aromatic carbons, and six glucosyl moiety carbons, and ten non-protonated carbons including two carbonyl carbons. In particular, the chemical shifts at C-2, C-3, and C-4 (δC 158.1, 134.9, and 179.2) were characteristic signals of flavonol 3-O-glycoside. Additionally, the carbonyl carbon signal at C-1′′′ (δC 168.7) and two trans-olefinic carbon signals at C-2′′′ and C-3′′′ (δC 146.9, 115.2) were typical chemical shifts of the coumaroyl group. The anomeric carbon signal at C-1′′(δC 100.4) and other signals for the glycosyl moiety from C-2′′ to C-6′′ (δC 74.3, 73.4, 70.5, 77.4, and 62.0) were observed. These one-dimensional (1D) NMR data were superimposable to those of kaempferol-3-O-β-2′′-coumaroylglucoside (2) [16]. However, the careful comparison of the 13C NMR data between the two compounds suggested that compound 1 had a galactose moiety, which was further demonstrated by the nuclear Overhauser enhancement spectroscopy (NOESY) NMR data (Figure S6). While the NOESY correlation between H-2′′ and H-4′′ was observed in compound 2, there was no correlation between these protons in compound 1. In general, interpreting 13C NMR and NOESY NMR data is an effective method to determine the type of glycosyl moiety. The location of the galactose moiety was deduced to be at C-3 according to the downfield shift of C-2 and C-4, as further evidenced by the heteronuclear multiple bond correlation (HMBC) between H-1′′ and C-3 (Figure S5). The position of the coumaroyl group was demonstrated to be at C-2′′ based on the downfield shift (δH 5.36 (1H, dd, J = 10.0, 8.0 Hz, H-2′′)) and the HMBC correlation between H-2′′ and C-1′′′. As a result, the structure of compound 1 was determined as kaempferol-3-O-β-2″-coumaroylgalactoside. Although compound 2 was previously isolated from various sources, including Quercus suber [16] and Allium porrum [34], compound 1 was isolated and structurally characterized for the first time.
Table 1. 1H and 13C NMR data of compounds 1 and 3 in methanol-d4
| Number of Carbon |
1 | 3 | ||
|---|---|---|---|---|
| δH Multi (J in Hz) | δC | δH Multi (J in Hz) | δC | |
| 2 | 158.1 | 158.6 | ||
| 134.9 | 134.8 | |||
| 4 | 179.2 | 179.0 | ||
| 5 | 163.1 | 163.0 | ||
| 6 | 6.16 d (1.5) | 101.2 | 6.11 d (2.0) | 100.6 |
| 7 | 167.7 | 168.3 | ||
| 8 | 6.34 s | 95.1 | 6.29 d (2.0) | 95.2 |
| 9 | 158.5 | 158.1 | ||
| 10 | 105.3 | 105.2 | ||
| 1′ | 122.7 | 122.8 | ||
| 2′,6′ | 8.00 d (8.5) | 132.1 | 7.98 d (9.0) | 132.1 |
| 3′,5′ | 6.87 d (8.5) | 116.3 | 6.88 d (9.0) | 116.2 |
| 4′ | 161.6 | 161.6 | ||
| 1′′ | 5.57 d (8.0) | 100.4 | 5.64 d (8.0) | 100.7 |
| 2′′ | 5.36 dd (10.0, 8.0) | 74.3 | 5.03 dd (9.0, 8.0) | 75.8 |
| 3′′ | 3.75 dd (10.5, 3.5) | 73.4 | 3.64 t (9.0) | 76.3 |
| 4′′ | 3.89 d (3.5) | 70.5 | 3.41 t (10.0) | 71.5 |
| 5′′ | 3.55 t (6.0) | 77.4 | 3.29 m | 78.8 |
| 6′′ | 3.67 m | 62.0 | 3.78 dd (12.0, 2.0) | 62.5 |
| 3.61 m | ||||
| 1′′′ | 127.2 | 127.8 | ||
| 2′′′ | 7.45 d (8.5) | 131.2 | 7.18 d (1.5) | 111.7 |
| 3′′′ | 6.81 d (8.5) | 116.8 | 149.4 | |
| 4′′′ | 161.3 | 150.7 | ||
| 5′′′ | 6.81 d (8.5) | 116.8 | 6.81 d (8.5) | 116.5 |
| 6′′′ | 7.45 d (8.5) | 131.2 | 7.07 dd (8.5, 1.5) | 124.1 |
| 7′′′ | 7.65 d (15.5) | 146.9 | 7.66 d (16.0) | 147.2 |
| 8′′′ | 6.35 d (16.0) | 115.2 | 6.37 d (16.0) | 115.5 |
| 9′′′ | 168.7 | 168.4 | ||
| 3′′′-OCH3 | 3.91 s | 56.4 | ||
Compound 3 was isolated as a yellow powder, and the molecular formula was established as C31H28O14 by analyzing HRMS data. The UV spectrum showed the UV absorption at 210 and 327 nm due to the same aglycone with compounds 1 and 2. The 1H NMR data (Table 1, Figure S10) were similar to those of compound 2, but compound 3 had a feruloyl group instead of the coumaroyl group, as evidenced by the presence of an additional methoxy group (δH 3.91 (3H, s, 3′′′-OCH3)). Additionally, an anomeric proton signal (δH 5.64 (1H, d, J = 8.0 Hz, H-1′′)) was observed, indicating that the glycosyl linkage was a β-configuration, and the downfield-shifted signal (5.03 (1H, t, J = 8.5 Hz, H-2′′)) was shown in the sugar region, as with compound 1. The 13C NMR data (Table 1, Figure S11) showed 31 resonances comprising two trans-olefinic carbons, ten quarternary carbons, ten aromatic carbons, six glucosyl moiety carbons, and one methoxy carbon, and ten non-protonated carbons, including two carbonyl carbons corresponding to kaempferol, feruloyl, and glucose groups. In particular, carbon signals from C-2′′ to C-6′′ (δC 75.8, 76.3, 71.5, 78.8 and 62.5) suggested the presence of a glucose moiety. The locations of the glucose moiety and feruloyl group were assigned by the long-range HMBC correlations between H-1′′ and C-3 (δC 134.8) and H-2′′ and C-1′′′ (δC 168.4) (Figure S14). The position of an additional methoxy group was determined by the key correlation between 3′′′-OCH3 and C-3′′′ (δC 149.4). The above results suggested the structure of compound 3 as kaempferol-3-O-β-2”-feruloylglucoside. To the best of our knowledge, compound 3 was only reported as a product of the hydrolysis of 3-O-β-(2-O-feruloyl)-glucosyl-7,4′-di-O-β-glucosylkaempferol, isolated from Allium tuberosum [35]. Therefore, the structure of 3 was elucidated as a new natural compound.
Compound 11 was isolated as a yellow powder. The 1H NMR data (Figure S19) displayed a set of AA′BB′-type signals (δH 8.06 (2H, d, J = 9.0 Hz, H-2′, H-6′), 6.87 (2H, d, J = 9.0 Hz, H-3′, H-5′)) in the B ring of kaempferol and a singlet signal at δH 7.02 (2H, s, H-3′′′, H-7′′′) of a galloyl moiety in aromatic region, which is a characteristic signal of galloylated flavonol. An anomeric proton signal (δH 5.78 (1H, d, J = 8.0 Hz, H-1′′)) indicated that the glycosyl linkage was a β-configuration. Furthermore, a downfield shifted proton signal (5.27 (1H, t, J = 9.5 Hz, H-2′′)) suggested that the galloyl group was attached at the hydroxyl group of C-2′′ because this shift could be attributed to the anisotropic influence of the O-galloyl moiety [21]. The 13C NMR data (Figure S20) exhibited 26 resonances, indicating galloylated flavonol glycoside. The carbon signals from C-2′′ to C-6′′ (δC 71.1, 72.7, 68.2, 76.0, and 60.1) suggested the presence of a galactose moiety. Therefore, the structure of compound 11 was confirmed as kaempferol-3-O-β-2′′-galloylgalactoside. Although compound 11 was previously isolated from various sources, including D. kaki [21][36], only the 1H NMR and MS data were previously reported. Thus, the 13C NMR data was reported for the first time in this study.
4. Discussion
Phytochemical investigations to identify biologically active compounds in persimmon leaves have been widely carried out. So far, a considerable number of triterpenoids and flavonoids, including kaempferol and quercetin derivatives, have been reported from D. kaki [1]. In this study, we obtained 27 compounds, including sixteen flavonoids, one ionone, two coumarins, seven triterpenoids, and one acetophenone. Of these, compound 1 was found to be a new flavonoid and compound 2 was firstly isolated from D. kaki. Additionally, kaempferol-3-O-β-2′′-feruloylglucoside (3) was only reported as a hydrolyzed product of 3-O-β-(2-O-feruloyl)-glucosyl-7,4′-di-O-β-glucosylkaempferol (3), isolated from Allium tuberosum [35]. Compound 3 was not only obtained directly from a natural source for the first time but has also not been reported in D. kaki previously. Furthermore, kaempferol-3-O-β-2′′-galloylgalactoside (11) has been previously reported in many sources, including D. kaki, but only the 1H NMR and MS have been reported due to the lack of detailed research. Hence, the 13C NMR data was reported for the first time here. Until now, there have been few studies that demonstrated the antioxidative abilities of extracts or fractions of persimmon leaves [37][38]. Most studies used rapid assay methods such as DPPH or ABTS assays. In particular, in the previous paper, 200 μg/mL of flavonoid-rich fraction exhibited 68.73% inhibition of DPPH radical. Aside from this result, however, this fraction also showed superoxide anion radical scavenging, hydroxyl radical scavenging, and metal chelating activities [38]. Although we did not evaluate these assays, bioassay-guided isolation was carried out because the ethanol extract and ethyl acetate fraction in the present study showed comparable DPPH radical scavenging activity. Additionally, despite previous results, only a few studies to identify biologically active compounds have been carried out. A few secoiridoids and lignans showed radical scavenging activities [39]. In the case of flavonoids, there have been several reports that quercetin, kaempferol, and their glycosides have antioxidative properties [40]. Antioxidative properties of galloylated kaempferol glycoside and galloylated quercetin glycoside obtained from other sources have been reported [41]. As yet, there have been no reports that each of these compounds derived from the persimmon leaves has antioxidative effects, except that a mixture of these compounds exhibited an antioxidative effect [21]. Additionally, so far, simultaneous determination of only a few triterpenoids or flavonoids has been carried out for the quantitative analysis of these compounds [42][43]. However, the present study suggests a method for simultaneous determination of most components in the persimmon leaves.References
- Xie, C.; Xie, Z.; Xu, X.; Yang, D. Persimmon (Diospyros kaki L.) leaves: A review on traditional uses, phytochemistry and pharmacological properties. J. Ethnopharmacol. 2015, 163, 229–240.
- Bei, W.; Zang, L.; Guo, J.; Peng, W.; Xu, A.; Good, D.A.; Hu, Y.; Wu, W.; Hu, D.; Zhu, X. Neuroprotective effects of a standardized flavonoid extract from Diospyros kaki leaves. J. Ethnopharmacol. 2009, 126, 134–142.
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575.
- Sa, Y.S.; Kim, S.-J.; Choi, H.-S. The anticoagulant fraction from the leaves of Diospyros kaki L. has an antithrombotic activity. Arch. Pharmacal Res. 2005, 28, 667–674.
- Zhang, K.; Zhang, Y.; Zhang, M.; Gu, L.; Liu, Z.; Jia, J.; Chen, X. Effects of phospholipid complexes of total flavonoids from Persimmon (Diospyros kaki L.) leaves on experimental atherosclerosis rats. J. Ethnopharmacol. 2016, 191, 245–253.
- Kotani, M.; Matsumoto, M.; Fujita, A.; Higa, S.; Wang, W.; Suemura, M.; Kishimoto, T.; Tanaka, T. Persimmon leaf extract and astragalin inhibit development of dermatitis and IgE elevation in NC/Nga mice. J. Allergy Clin. Immunol. 2000, 106, 159–166.
- Thuong, P.T.; Lee, C.H.; Dao, T.T.; Nguyen, P.H.; Kim, W.G.; Lee, S.J.; Oh, W.K. Triterpenoids from the leaves of Diospyros kaki (persimmon) and their inhibitory effects on protein tyrosine phosphatase 1B. J. Nat. Prod. 2008, 71, 1775–1778.
- Matsuo, T.; Ito, S. The chemical structure of kaki-tannin from immature fruit of the persimmon (Diospyros kaki L.). Agric. Biol. Chem. 1978, 42, 1637–1643.
- Bawazeer, S.; Rauf, A. In vivo anti-inflammatory, analgesic, and sedative studies of the extract and naphthoquinone isolated from Diospyros kaki (persimmon). ACS Omega 2021, 6, 9852–9856.
- Yoshimura, M.; Mochizuki, A.; Amakura, Y. Identification of phenolic constituents and inhibitory activity of persimmon calyx and shiteito against tumor cell proliferation. Chem. Pharm. Bull. 2021, 69, 32–39.
- Wang, L.; Xu, M.L.; Rasmussen, S.K.; Wang, M.-H. Vomifoliol 9-O-α-arabinofuranosyl (1→ 6)-β-D-glucopyranoside from the leaves of Diospyros Kaki stimulates the glucose uptake in HepG2 and 3T3-L1 cells. Carbohydr. Res. 2011, 346, 1212–1216.
- Hitaka, Y.; Nakano, A.; Tsukigawa, K.; Manabe, H.; Nakamura, H.; Nakano, D.; Kinjo, J.; Nohara, T.; Maeda, H. Characterization of carotenoid fatty acid esters from the peels of the persimmon Diospyros kaki. Chem. Pharm. Bull. 2013, 61, 666–669.
- Simpson, D.S.; Oliver, P.L. ROS generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 2020, 9, 743.
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 477.
- Kwon, J.; Hwang, H.; Selvaraj, B.; Lee, J.H.; Park, W.; Ryu, S.M.; Lee, D.; Park, J.-S.; Kim, H.S.; Lee, J.W. Phenolic constituents isolated from Senna tora sprouts and their neuroprotective effects against glutamate-induced oxidative stress in HT22 and R28 cells. Bioorganic Chem. 2021, 114, 105112.
- Romussi, G.; Bignardi, G.; Pizza, C.; De Tommasi, N. Constituents of cupuliferae, XIII: New and revised structures of acylated flavonoids from Quercus Suber L. Arch. Der Pharm. 1991, 324, 519–524.
- Li, H.-Z.; Song, H.-J.; Li, H.-M.; Pan, Y.-Y.; Li, R.-T. Characterization of phenolic compounds from Rhododendron alutaceum. Arch. Pharmacal Res. 2012, 35, 1887–1893.
- Jung, M.; Choi, J.; Chae, H.-S.; Cho, J.Y.; Kim, Y.-D.; Htwe, K.M.; Lee, W.-S.; Chin, Y.-W.; Kim, J.; Yoon, K.D. Flavonoids from Symplocos racemosa. Molecules 2015, 20, 358–365.
- Xu, J.; Wang, X.; Yue, J.; Sun, Y.; Zhang, X.; Zhao, Y. Polyphenols from acorn leaves (Quercus liaotungensis) protect pancreatic beta cells and their inhibitory activity against α-glucosidase and protein tyrosine phosphatase 1B. Molecules 2018, 23, 2167.
- Botirov, E.K. Flavonoids and phenolcarboxylic acids from Lamium album. Chem. Nat. Compd. 2019, 55, 1159–1160.
- Kawakami, K.; Shibukura, Y.; Kanno, T.; Furuki, T.; Aketa, S.; Hirayama, M. Identification of 2′′-galloylated flavonol 3-O-glycosides accumulating in developing leaves of Persimmon. Phytochem. Anal. 2011, 22, 403–410.
- Suktap, C.; Lee, H.K.; Amnuaypol, S.; Suttisri, R.; Sukrong, S. Wound healing effect of flavonoid glycosides from Afgekia mahidolae BL Burtt & Chermsir. Leaves. Rec. Nat. Prod. 2018, 12, 391–396.
- Isobe, T.; Ito, N.; Noda, Y. Minor flavonoids of Polygonum nodosum. Phytochemistry 1980, 19, 1877.
- Wan, C.; Yuan, T.; Cirello, A.L.; Seeram, N.P. Antioxidant and α-glucosidase inhibitory phenolics isolated from highbush blueberry flowers. Food Chem. 2012, 135, 1929–1937.
- Liao, C.-R.; Kuo, Y.-H.; Ho, Y.-L.; Wang, C.-Y.; Yang, C.-S.; Lin, C.-W.; Chang, Y.-S. Studies on cytotoxic constituents from the leaves of Elaeagnus oldhamii Maxim. in non-small cell lung cancer A549 cells. Molecules 2014, 19, 9515–9534.
- Wu, Z.-G.; Wei, W.; Xu, H.-Y.; Zheng, L.-L.; Ma, C.-M.; Wang, Y.-C. Constituents from the leaves of Tetraena mongolica and their protective activity in HEK 293t cells damaged by CdCl2. J. Nat. Prod. 2019, 82, 2707–2712.
- Kang, Y.-F.; Liu, C.-M.; Kao, C.-L.; Chen, C.-Y. Antioxidant and anticancer constituents from the leaves of Liriodendron tulipifera. Molecules 2014, 19, 4234–4245.
- Kwon, J.; Hiep, N.T.; Kim, D.-W.; Hong, S.; Guo, Y.; Hwang, B.Y.; Lee, H.J.; Mar, W.; Lee, D. Chemical constituents isolated from the root bark of Cudrania tricuspidata and their potential neuroprotective effects. J. Nat. Prod. 2016, 79, 1938–1951.
- Liu, Q.; Mu, Y.; An, Q.; Xun, J.; Ma, J.; Wu, W.; Xu, M.; Xu, J.; Han, L.; Huang, X. Total synthesis and anti-inflammatory evaluation of violacin A and its analogues. Bioorganic Chem. 2020, 94, 103420.
- Su, B.-N.; Kang, Y.-H.; Pinos, R.E.; Santarsiero, B.D.; Mesecar, A.D.; Soejarto, D.D.; Fong, H.H.; Pezzuto, J.M.; Kinghorn, A.D. Isolation and absolute stereochemistry of coussaric acid, a new bioactive triterpenoid from the stems of Coussarea brevicaulis. Phytochemistry 2003, 64, 293–302.
- Nakatani, M.; Miyazaki, Y.; Iwashita, T.; Naoki, H.; Hase, T. Triterpenes from Ilex rotunda fruits. Phytochemistry 1989, 28, 1479–1482.
- Lee, T.H.; Juang, S.H.; Hsu, F.L.; Wu, C.Y. Triterpene acids from the leaves of Planchonella duclitan (Blanco) Bakhuizan. J. Chin. Chem. Soc. 2005, 52, 1275–1280.
- Mimaki, Y.; Fukushima, M.; Yokosuka, A.; Sashida, Y.; Furuya, S.; Sakagami, H. Triterpene glycosides from the roots of Sanguisorba officinalis. Phytochemistry 2001, 57, 773–779.
- Fattorusso, E.; Lanzotti, V.; Taglialatela-Scafati, O.; Cicala, C. The flavonoids of leek, Allium porrum. Phytochemistry 2001, 57, 565–569.
- Yoshida, T.; Saito, T.; Kadoya, S. New acylated flavonol glucosides in Allium tuberosum Rottler. Chem. Pharm. Bull. 1987, 35, 97–107.
- Zehl, M.; Braunberger, C.; Conrad, J.; Crnogorac, M.; Krasteva, S.; Vogler, B.; Beifuss, U.; Krenn, L. Identification and quantification of flavonoids and ellagic acid derivatives in therapeutically important Drosera species by LC–DAD, LC–NMR, NMR, and LC–MS. Anal. Bioanal. Chem. 2011, 400, 2565–2576.
- Hossain, A.; Moon, H.K.; Kim, J.-K. Antioxidant properties of Korean major persimmon (Diospyros kaki) leaves. Food Sci. Biotechnol. 2018, 27, 177–184.
- Sun, L.; Zhang, J.; Lu, X.; Zhang, L.; Zhang, Y. Evaluation to the antioxidant activity of total flavonoids extract from persimmon (Diospyros kaki L.) leaves. Food Chem. Toxicol. 2011, 49, 2689–2696.
- Huang, S.-W.; Qiao, J.-W.; Sun, X.; Gao, P.-Y.; Li, L.-Z.; Liu, Q.-B.; Sun, B.; Wu, D.-L.; Song, S.-J. Secoiridoids and lignans from the leaves of Diospyros kaki Thunb. with antioxidant and neuroprotective activities. J. Funct. Foods 2016, 24, 183–195.
- Loizzo, M.R.; Said, A.; Tundis, R.; Hawas, U.W.; Rashed, K.; Menichini, F.; Frega, N.G.; Menichini, F. Antioxidant and antiproliferative activity of Diospyros lotus L. extract and isolated compounds. Plant Foods Hum. Nutr. 2009, 64, 264.
- Masuda, T.; Iritani, K.; Yonemori, S.; Oyama, Y.; Takeda, Y. Isolation and antioxidant activity of galloyl flavonol glycosides from the seashore plant, Pemphis acidula. Biosci. Biotechnol. Biochem. 2001, 65, 1302–1309.
- Fan, J.-P.; He, C.-H. Simultaneous quantification of three major bioactive triterpene acids in the leaves of Diospyros kaki by high-performance liquid chromatography method. J. Pharm. Biomed. Anal. 2006, 41, 950–956.
- Peng, L.; Zhao, M.; Li, H. Method development and validation for simultaneous determination of six flavonoids in rat eyes after oral administration of Diospyros kaki leaves extract by UPLC-MS/MS. Chem. Pharm. Bull. 2020, 69, 218–221.
More
