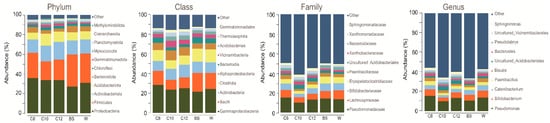
After processing the raw sequence files, we obtained a total of 273,217 reads, constituting 5347 ASVs. At the phylum level for the treated soil samples, we observed that Proteobacteria was the most dominant of the three treatments, C8 having 35.94%, C10 33.68%, and C12, 33.76%. Meanwhile, mono-cropped W showed a slightly lower percentage, although Proteobacteria also dominated it (30.87%). Mono-cropped BS, on the other hand, was dominated by the Firmicutes phylum at 32.50%. At the class level, all samples showed a high amount of γ-proteobacteria, with C8 showing 28.58%, C10 23.58%, and C12 25.00%, while the planted mono-cropped soil P showed 24.50%, and mono-cropped BS showed 21.70%. The family level was also checked, and similar results were obtained, with Pseudomonadaceae having the highest relative abundance for all samples (15.53%, 10.12%, 13.31%, 13.66%, and 14.24% for C8, C10, C12, W, and BS samples, respectively) (
Figure 2). Focusing on the top 10 taxa at the genus level, a high amount of Pseudomonas for all samples was observed. At the same time, we also see an increase
of Catenibacterium and
Bifidobacterium for W and BS samples. On the other hand, an increase in
Pseudolabrys and
Uncultured_Acidobacteriales was seen in the C10 and C12 samples.
Figure 2. Relative abundance of different samples. A represents the relative abundance of the phylum, class, and family, and genus level.
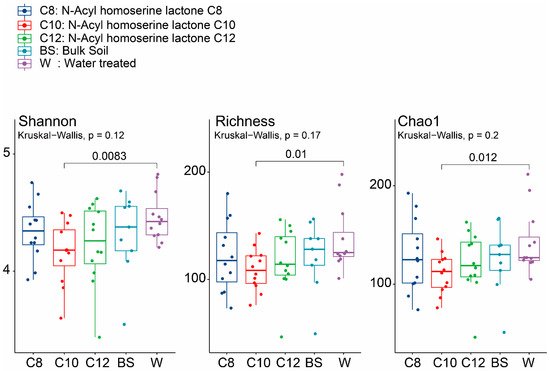
The diversity of the groups was explained through different alpha diversity indices, such as Shannon, Chao1, and richness. All three alpha diversity indices showed a similar trend in the AHL-treated soils, exhibiting a lower diversity than BS and mono-cropped W samples. Although no statistically significant differences were found among the different treatments (C8, C10, and C12), it is worth noting that the C10 sample, which gave the lowest diversity in the treated soils, was significantly different from P (
p < 0.05) (
Figure 3). We also checked the alpha diversity at other time points (2, 4, and 8 weeks). After two weeks of growth for all AHL-treated samples, we observed that the diversity was lower, while the opposite was seen in the W samples (
Supplementary Figure S1). Similar trends were observed for both C10 and C12 samples, which increased in diversity after 4 and 8 weeks of growth.
Figure 3. Total alpha diversity of soil microbial communities of the continuously mono-cropped treated with different moieties of acyl-homoserine lactones using Shannon, Richness, and Chao1 indices. The dark blue color shows the total alpha diversity for the C8 samples, while the red, green, light blue, and purple signify the C10, C12, BS, and W samples, respectively. The significance values were generated according to one-way ANOVA with Duncan’s multiple range test values (p < 0.05).
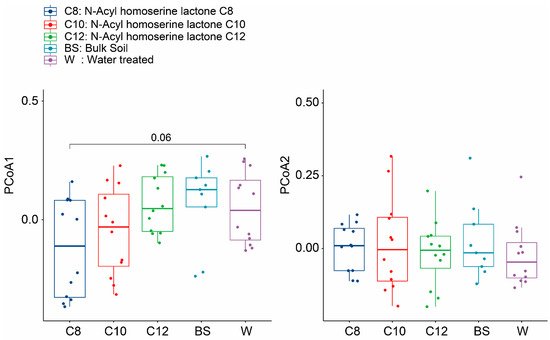
The total variation in the diversity of the treatment groups is shown by the PCoA using Bray–Curtis dissimilarity and NMDS (stress = 0.19) (
Supplementary Figure S1). There was an inconsistent pattern observed during the initial time points. However, we observed a shift in the soil microbiome between BS-, W-, and AHL-treated groups at different time points, especially after two weeks (
Figure 4). The AHL-treated groups were scattered during different time points but were seemingly clustered with W after 4 and 8 weeks of growth. Analysis using Adonis showed that the treatments were scattered and significantly different after two weeks of growth (
p < 0.05), while we found no significant difference after 4 and 8 weeks (
Table 2). Although the scattering observed was significant only after two weeks, this result still supports our theory that the addition of AHL changed the microbiome of ginseng grown in a continuously cropped manner.
Figure 4. Boxplot of the Bray–Curtis dissimilarity for different samples across different sampling time points. The first plot shows the difference along the PCoA1 plane, while the second plot shows the difference along the PCoA2 plane. The dark blue color shows the total alpha diversity for the C8 samples, while the red, green, light blue, and purple colors signify the C10, C12, BS, and W samples, respectively. The significance values were generated according to one-way ANOVA with Duncan’s multiple range test values (p < 0.05).
Table 2. PERMANOVA analysis using Adonis during the overall and different sampling points using Bray–Curtis dissimilarity distance values between N-acyl homoserine lactone treatments (C8, C10, C12) and the continuously mono-cropped ginseng soil (W).
| |
|
DF |
Sums of Sqs |
Means Sqs |
F. Model |
R2 |
Pr (>F) |
| Overall |
Treatment |
4 |
1.8139 |
0.45346 |
1.1343 |
0.08025 |
0.021 * |
| |
Residuals |
52 |
20.7888 |
0.39978 |
|
0.91975 |
|
| |
Total |
56 |
22.6026 |
|
|
1.00000 |
|
| 2 weeks |
Treatment |
4 |
1.9865 |
0.49661 |
1.2304 |
0.32983 |
0.00 ** |
| |
Residuals |
10 |
4.0362 |
0.40362 |
|
0.67017 |
|
| |
Total |
14 |
6.0226 |
|
|
1.00000 |
|
| 4 weeks |
Treatment |
4 |
1.2441 |
0.31103 |
0.98797 |
0.30512 |
0.539 |
| |
Residuals |
9 |
2.8334 |
0.31482 |
|
0.69488 |
|
| |
Total |
13 |
4.0775 |
|
|
1.00000 |
|
| 8 weeks |
Treatment |
4 |
1.5079 |
0.37697 |
1.0388 |
0.34185 |
0.27 |
| |
Residuals |
8 |
2.9030 |
0.36288 |
|
0.65815 |
|
| |
Total |
12 |
4.4109 |
|
|
1.00000 |
|
PERMANOVA analysis using Adonis during the overall and different sampling points using Bray–Curtis dissimilarity distance values between N-acyl homoserine lactone treatments (C8, C10, C12) and the continuously mono-cropped ginseng soil (W).
3. Current Insights
A major challenge in the cultivation of ginseng plants is replanting, as it poses a considerable amount of loss in crops. This continuous monocropping is affected by the dynamic changes in soil microorganisms
[15][30]. This study aimed to change the microbial community of soil used in monocropping through AHL treatment. A number of studies involving the addition of AHLs proved that it has positive effects on plant performance and beneficial plant responses
[16][31]. The effect may vary depending on the length of side chains present in AHLs. Schenk and Schikora
[17][32] showed that the use of AHLs having long side chains activated the oxylipin signaling pathway and priming for induced resistance. This was demonstrated by the involvement of defense hormones through AHL priming by inducing resistance in salicylic acid accumulation in tomatoes
[18][33]. While Liu et al.
[19][34] observed that AHLs with short side chains stimulate root length and antioxidative capacities in barley leaves
[20][35]. A review done by Shrestha and Schikora
[21][15] explains that there are ‘AHL-primable’ and ‘AHL-non-primable’ types depending on the response of plants through AHL priming. It has yet to be seen whether ginseng is a possible ‘AHL-primable’ crop, although our phenotypic results proved to be an excellent example of good ginseng growth when treated with AHLs, as can be determined by the data presented in
Figure 1 and
Table 1.
Observation of the microbiome composition of samples through 16S rRNA amplicon sequencing revealed that at the class level, all samples showed a high amount of γ-proteobacteria. The treated samples were more enriched by γ-proteobacteria, which corroborates our treatment since the signaling molecules used were for AI-1 mediated signaling.
At the genus level, we found that Pseudomonas was dominant for all samples, although BS and W showed a higher amount of
Catenibacterium and
Bifidobacterium. Surprisingly, these genera, including
Blautia, are more commonly found in the intestinal microbiomes of humans
[22][36]. Although these genera are already present in soil
[23][37], it is still unknown how they affect plant health. On the other hand, we observed an increase in
Pseudolabrys which is included in the phylum proteobacteria
[24][38] and Uncultured_Acidobacteriales in both C10 and C12 samples linked to promoting plant growth
[25][39]. Moreover, a study by Cipriano et al.
[26][40] and Chen et al.
[27][41] showed that there was an increase in abundance of
Pseudolabrys when a plant growth promoting bacteria was used for inoculation. It may be possible that, instead of plant growth promoting bacteria, the addition of AHLs helped in Pseudolabrys enrichment.