Tetrahydrofuran (THF) is widely used as a precursor for polymer syntheses and a versatile solvent in industries. THF is an environmental hazard and carcinogenic to humans. We characterized the THF degradation potential of a number of THF-degrading bacteria reported before and a new isolated filamentous fungus Pseudallescheria boydii ZM01. Two different microbial THF degradation pathways have been proposed here. In addition, The initial key metabolic intermediate 2-hydroxytetrahydrofuran was detected and identified by gas chromatography (GC) analyses for the first time during the THF degradation process.
- tetrahydrofuran
- Pseudallescheria boydii
- 2-hydroxytetrahydrofuran
- biodegradation
1. Introduction
Tetrahydrofuran (THF) is a colorless, highly soluble, and volatile heterocyclic compound that is widely used as an important basic feedstock for chemical syntheses and is an excellent solvent for lacquers and printing inks [1][2]. With the increasing consumption of THF, large amounts of industrial wastewater containing THF have been released into groundwater, rivers, and lakes [3][4]. Furthermore, increased attention has been paid to the carcinogenic effects of THF-polluted groundwater on human health after its toxicological effects were thoroughly demonstrated by a variety of assays in vivo. Various studies in female mice have demonstrated that THF can enhance tumor formation by inducing cell proliferation, can cause nervous system dysfunction, and can induce DNA damage [5][6][7]. Considering the advantages of microbial degradation, including the safety of disposal and cost effectiveness, the key to refractory organic pollutant treatment will be the exploitation of microbes from the natural environment with highly efficient pollutant-degrading activities [8][9]. THF is also classified as a refractory pollutant due to the high bond energy obstruction of C–O (360 KJ mol
−1
Rhodococcus
Pseudonocardia
Mycobacterium
Flavobacterium
Pseudomonas sp. have been reported to aerobically grow on THF [11][12][13][14][15]. Although many THF-degrading bacteria have been reported, most of them cannot utilize THF at concentrations higher than 35 mM, and the lag phase is prolonged with increased initial substrate concentrations. The widely reported THF-degrading strain
Rhodococcus ruber
R. aetherivorans
Pseudomonas oleovorans
Filamentous fungi are involved in multiple pathways associated with wastewater treatment, such as organic compound biodegradation, the formation of sludge flocs, settleability of the treated sludge, and sludge detoxification [18]. Filamentous fungi grow as thread-like hyphae and reproduce by sporulation [19]. The hyphae can immobilize sludge particles, resulting in the formation of pellets, which leads to microbial aggregation as natural flocculants [20][21]. Notably, filamentous fungi can also improve the dewaterability and filterability of sludge by modifying its porosity structure [22]. Many studies have shown that compounds that are highly toxic toward bacteria may be harmless to fungi, such as antibiotics [21]. In addition, the high contents of functional groups in the cell walls of fungi, such as amino, amide, and phosphate groups, promote the ability of fungi to absorb low concentrations of heavy metals as micronutrient sources and to resist heavy metal stress [23]. The ability of filamentous fungi to form mycelial networks, to undergo detoxification, and to maintain stress resistance and richness in terms of biodiversity enhances their potential application in bioremediation [24][25]. To date, only three fungi, including
Aureobasidium pullulans
Cordyceps sinensis
Graphium sp. ATCC 58400 [27] have been reported to grow on THF, and the THF degradation characteristics of fungi have rarely been evaluated. Thus, studies on the THF degradation characteristics of THF-degrading fungi is important for developing bioremediation technologies for THF-containing wastewater.
Two different microbial THF degradation pathways have been proposed in previous studies, but some metabolites remain speculative, such as 2-hydroxytetrahydrofuran. In the oxidation pathway, THF is inferred to be initially oxidized to 2-hydroxytetrahydrofuran, and 2-hydroxytetrahydrofuran is then oxidized to γ-butyrolactone before being transformed to 4-hydroxybutyrate. 2-hydroxytetrahydrofuran is a cyclic hemiacetal that can also autonomously form its tautomer 4-hydroxybutyraldehyde, which can be directly converted to 4-hydroxybutyrate. The metabolite 4-hydroxybutyrate, which is common to both metabolic pathways, is further oxidized to succinate and then thoroughly mineralized in the tricarboxylic acid cycle [17][28][29]. Several THF degradation intermediates in the proposed THF degradation pathway have been detected and verified in previous studies. Small amounts of γ-butyrolactone were detected as an intermediate in
Graphium
Mycobacterium vaccae
Rhodococcus sp. DTB after incubation with THF [27][29][30]. In addition, the derivatized product of 4-hydroxybutyrate was finally detected and confirmed by gas chromatography-mass spectrometry (GC–MS) in the resting cell reaction of
R. aetherivorans M8, which was initiated by the addition of 5 mM THF [17]. However, the initial key monooxygenation metabolite in both degradation pathways, 2-hydroxytetrahydrofuran, has not been detected and confirmed during the THF degradation process in all reported THF-degrading strains thus far [31][32].
2. Detection and Identification of THF and Related Metabolites
The detection of the THF concentration was conducted as described in a previous study [33]. The THF concentration was measured by GC-2014C gas chromatography equipped with a flame ionization detector (FID) and an AOC-20i Auto injector (SHIMADZU, Shanghai, China). All samples were centrifuged at 10,000 rpm for 2 min, and the supernatant was subsequently collected after being filtered through a 0.45-μm filter for pretreatment. Samples at different time points corresponding to the growth curve were taken to detect the intermediate metabolites. The detection program was as follows: the initial temperature was set at 60 °C, gradually increased to 160 °C at a rate of 20 °C min
−1, and then held at 160 °C for 5 min. The reference standards, such as THF, 2-hydroxytetrahydrofuran, and γ-butyrolactone, were appropriately prepared at different concentrations to generate standard curves for use in intermediate identification and concentration calculations.
−1
3. Isolation and Identification of the THF-Degrading Fungus
A filamentous fungus capable of growing in MSM supplemented with THF as the sole carbon source was successfully isolated from activated sludge by enrichment cultivation and designated strain ZM01. Strain ZM01 could grow as white thread-like hyphae and could develop conidia growing directly on vegetative hyphae after cultivation for 7 d at 30 °C. Most conidia were usually brown, thick-walled, globose to subglobose. The shape of conidiogenous cells was cylindrical, and no yellow diffusible pigment was produced during the cultivation of strain ZM01 on PDA at 30 °C. The microscopic characteristics of strain ZM01 were consistent with the typical microscopic features of
A filamentous fungus capable of growing in MSM supplemented with THF as the sole carbon source was successfully isolated from activated sludge by enrichment cultivation and designated strain ZM01. Strain ZM01 could grow as white thread-like hyphae and could develop conidia growing directly on vegetative hyphae after cultivation for 7 d at 30 °C. Most conidia were usually brown, thick-walled, globose to subglobose. The shape of conidiogenous cells was cylindrical, and no yellow diffusible pigment was produced during the cultivation of strain ZM01 on PDA at 30 °C (Figure S1). The microscopic characteristics of strain ZM01 were consistent with the typical microscopic features ofPseudallescheria
sp. Based on phylogenetic analysis of the ITS region sequences (GenBank Accession No. MT754398) (Figure 1), strain ZM01 was observed to be closely related toP. boydii
(GenBank Accession No. AY213683) with a high degree of similarity (99%). Thus, on the basis of morphological characteristics and phylogenetic relationships analysis, we identified the isolated THF-degrading fungus asPseudallescheria boydii
strain ZM01. To the best of our knowledge, this is the first report showing that THF can be metabolized by aPseudallescheria
sp. strain.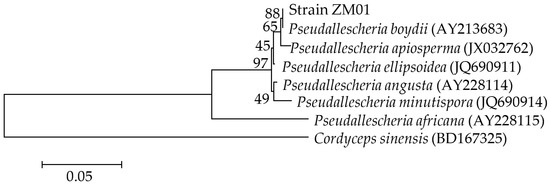
Figure 1.
Pseudallescheria
C. sinensis is used as the root. Numbers after the names of organisms are accession numbers of published sequences. Numbers adjacent to the branches indicate the bootstrap values based on 1000 replicates.
References
- Chen, J.M.; Zhou, Y.Y.; Chen, D.Z.; Jin, X.J. A newly isolated strain capable of effectively degrading tetrahydrofuran and its performance in a continuous flow system. Bioresour Technol. 2010, 101, 6461–6467. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, C.; Li, H.; Lei, A. Direct functionalization of tetrahydrofuran and 1,4-dioxane: Nickel-catalyzed oxidative C(sp3)-H arylation. Angew. Chem. Int. Ed. Engl. 2013, 52, 4453–4456. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, C.; Mohr, T.K.; Field, J.A. Quantitative determination of 1, 4-dioxane and tetrahydrofuran in groundwater by solid phase extraction GC/MS/MS. Environ. Sci. Technol. 2006, 40, 7305–7311. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Guan, J.; Tang, P.; Jiao, H.; Lin, C.; Wang, J.; Lu, Z.; Min, H.; Gao, H. Assessment of toxicity of tetrahydrofuran on the microbial community in activated sludge. Bioresour. Technol. 2010, 101, 5213–5221. [Google Scholar] [CrossRef]
- Gamer, A.O.; Jaeckh, R.; Leibold, E.; Kaufmann, W.; Gembardt, C.; Bahnemann, R.; van Ravenzwaay, B. Investigations on cell proliferation and enzyme induction in male rat kidney and female mouse liver caused by tetrahydrofuran. Toxicol. Sci. 2002, 70, 140–149. [Google Scholar] [CrossRef]
- Hermida, S.A.S.; Possari, E.P.M.; Souza, D.B.; Campos, I.P.D.; Gomes, O.F.; Di Mascio, P.; Medeiros, M.H.G.; Loureiro, A.P.M. 2′-Deoxyguanosine, 2′-deoxycytidine, and 2′-deoxyadenosine adducts resulting from the reaction of tetrahydrofuran with DNA bases. Chem. Res. Toxicol. 2006, 19, 927–936. [Google Scholar] [CrossRef]
- Malley, L.A.; Christoph, G.R.; Stadler, J.C.; Hansen, J.F.; Biesemeier, J.A.; Jasti, S.L. Acute and subchronic neurotoxicological evaluation of tetrahydrofuran by inhalation in rats. Drug Chem. Toxicol. 2001, 24, 201–219. [Google Scholar] [CrossRef]
- Asgher, M.; Bhatti, H.N.; Ashraf, M.; Legge, R.L. Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system. Biodegradation 2008, 19, 771–783. [Google Scholar] [CrossRef]
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Chen, D.Z.; Zhu, R.Y.; Chen, J.M. Substrate interactions during the biodegradation of BTEX and THF mixtures by Pseudomonas oleovorans DT4. Bioresour. Technol. 2011, 102, 6644–6649. [Google Scholar] [CrossRef]
- Sei, K.; Miyagaki, K.; Kakinoki, T.; Fukugasako, K.; Inoue, D.; Ike, M. Isolation and characterization of bacterial strains that have high ability to degrade 1,4-dioxane as a sole carbon and energy source. Biodegradation 2013, 24, 665–674. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Mathieu, J.; Yang, Y.; Yu, P.F.; da Silva, M.L.B.; Alvarez, P.J.J. 1,4-Dioxane biodegradation by Mycobacterium dioxanotrophicus PH-06 is associated with a group-6 soluble di-iron monooxygenase. Environ. Sci. Tech. Let. 2017, 4, 494–499. [Google Scholar] [CrossRef]
- Daye, K.J.; Groff, J.C.; Kirpekar, A.C.; Mazumder, R. High efficiency degradation of tetrahydrofuran (THF) using a membrane bioreactor: Identification of THF-degrading cultures of Pseudonocardia sp. strain M1 and Rhodococcus ruber isolate M2. J. Ind. Microbiol. Biot. 2003, 30, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.Z.; Ko, K.; Ramsay, J.A. Biodegradation of 1,4-dioxane by a Flavobacterium. Biodegradation 2011, 22, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Tsunoda, T.; Sawada, K.; Yamamoto, N.; Saito, Y.; Sei, K.; Ike, M. 1,4-Dioxane degradation potential of members of the genera Pseudonocardia and Rhodococcus. Biodegradation 2016, 27, 277–286. [Google Scholar] [CrossRef]
- Yao, Y.L.; Lv, Z.M.; Min, H.; Lv, Z.H.; Jiao, H.P. Isolation, identification and characterization of a novel Rhodococcus sp. strain in biodegradation of tetrahydrofuran and its medium optimization using sequential statistics-based experimental designs. Bioresour. Technol. 2009, 100, 2762–2769. [Google Scholar] [CrossRef]
- Tajima, T.; Hayashida, N.; Matsumura, R.; Omura, A.; Nakashimada, Y.; Kato, J. Isolation and characterization of tetrahydrofuran-degrading Rhodococcus aetherivorans strain M8. Process. Biochem. 2012, 47, 1665–1669. [Google Scholar] [CrossRef]
- Niu, L.H.; Li, Y.; Xu, L.L.; Wang, P.F.; Zhang, W.L.; Wang, C.; Cai, W.; Wang, L.Q. Ignored fungal community in activated sludge wastewater treatment plants: Diversity and altitudinal characteristics. Environ. Sci. Pollut. Res. 2017, 24, 4185–4193. [Google Scholar] [CrossRef]
- Stajich, J.E.; Berbee, M.L.; Blackwell, M.; Hibbett, D.S.; James, T.Y.; Spatafora, J.W.; Taylor, J.W. The Fungi. Curr. Biol. 2009, 19, 840–845. [Google Scholar] [CrossRef]
- Li, X.J.; Li, Y.; Zhao, X.D.; Zhang, X.L.; Zhao, Q.; Wang, X.; Li, Y.T. Restructured fungal community diversity and biological interactions promote metolachlor biodegradation in soil microbial fuel cells. Chemosphere 2019, 221, 735–749. [Google Scholar] [CrossRef]
- More, T.T.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Potential use of filamentous fungi for wastewater sludge treatment. Bioresour. Technol. 2010, 101, 7691–7700. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Zheng, G.Y.; Zhou, L.X. Degradation of slime extracellular polymeric substances and inhibited sludge flocs destruction contribute to sludge dewaterability enhancement during fungal treatment of sludge using filamentous fungus Mucor sp. GY-1. Bioresour. Technol. 2015, 192, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Das, B.K.; Roy, A.; Koschorreck, M.; Mandal, S.M.; Wendt-Potthoff, K.; Bhattacharya, J. Occurrence and role of algae and fungi in acid mine drainage environment with special reference to metals and sulfate immobilization. Water Res. 2009, 43, 883–894. [Google Scholar] [PubMed]
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Dijksterhuis, J. Fungal spores: Highly variable and stress-resistant vehicles for distribution and spoilage. Food Microbiol. 2019, 81, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Nakamiya, K.; Hashimoto, S.; Ito, H.; Edmonds, J.S.; Morita, M. Degradation of 1,4-dioxane and cyclic ethers by an isolated fungus. Appl. Environ. Microbiol. 2005, 71, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Skinner, K.; Cuiffetti, L.; Hyman, M. Metabolism and cometabolism of cyclic ethers by a filamentous fungus, a Graphium sp. Appl. Environ. Microbiol. 2009, 75, 5514–5522. [Google Scholar] [CrossRef]
- Trippe, K.M.; Wolpert, T.J.; Hyman, M.R.; Ciuffetti, L.M. RNAi silencing of a cytochrome P450 monoxygenase disrupts the ability of a filamentous fungus, Graphium sp., to grow on short-chain gaseous alkanes and ethers. Biodegradation 2014, 25, 137–151. [Google Scholar] [CrossRef]
- Moreno-Horn, M.; Garbe, L.A.; Tressl, R.; Gorisch, H. Transient accumulation of gamma-butyrolactone during degradation of bis(4-chloro-n-butyl) ether by diethylether-grown Rhodococcus sp. strain DTB. Appl. Microbiol. Biotechnol. 2005, 69, 335–340. [Google Scholar] [CrossRef]
- Kohlweyer, U.; Thiemer, B.; Schrader, T.; Andreesen, J.R. Tetrahydrofuran degradation by a newly isolated culture of Pseudonocardia sp. strain K1. FEMS Microbiol. Lett. 2000, 186, 301–306. [Google Scholar] [CrossRef]
- Kim, Y.M.; Jeon, J.R.; Murugesan, K.; Kim, E.J.; Chang, Y.S. Biodegradation of 1,4-dioxane and transformation of related cyclic compounds by a newly isolated Mycobacterium sp. PH-06. Biodegradation 2009, 20, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Sales, C.M.; Grostern, A.; Parales, J.V.; Parales, R.E.; Alvarez-Cohen, L. Oxidation of the cyclic ethers 1,4-dioxane and tetrahydrofuran by a monooxygenase in two Pseudonocardia species. Appl. Environ. Microbiol. 2013, 79, 7702–7708. [Google Scholar] [CrossRef]
