Hormonal and growth factor alterations, related to an elevated food consumption and excessive adiposity, affect the regulation of genes involved in cellular processes including proliferation, differentiation and DNA repair, allowing cells to survive and proliferate despite the accumulation of mutations which lead to malignant transformation. The growth hormone/insulin growth factor-1 (GH/IGF-1)/ insulin pathway and its downstream effectors, in fact, are known to promote aging and/or age-related diseases, including cancer, in many model organisms. The restriction of nutrients is established to have strong effects on levels of hormones and growth factors, delaying the incidence of age-related diseases and prolonging lifespan.
- fasting
- growth hormones
- aging
- age-related disease
- DNA damage
- cancer prevention
1. Introduction
Higher protein intake increases the release of growth hormone releasing hormone, and consequently growth hormone release from the pituitary gland and IGF-1 release primarily from the liver [8][1]. High IGF-1 has been associated with elevated incidence of a number of cancers [8,9,10,11][1][2][3][4]. On the other hand, excessive carbohydrate and/or fat intake can result in excess adiposity, which is associated with high oxidative stress, inflammation, alterations in hormones and growth factors’ production, acquisition of insulin resistance and consequently hyperinsulinemia [12,13,14][5][6][7]. Overweight women are reported to frequently present insulin resistance and low plasma levels of sex hormone-binding globulin which lead, as a consequence, to an increase in total and free sex hormone levels [15][8]. Hyperinsulinemia, in fact, blocks the production of sex hormone-binding globulin by the liver and, moreover, is associated with an increased production of androgens which are reported, together with estrogens, to stimulate the development and growth of several cancers [16][9]. Furthermore, insulin sustains insulin like growth factor 1 (IGF-1) activity, partly through the reduction of IGF binding protein 1 (IGFBP-1) synthesis, and elevated GH-IGF-1 increases insulin levels and resistance [5,6,17][10][11][12]. Not surprisingly, both elevated concentrations of insulin and IGF-1 are associated with multiple cancer types, including breast, endometrium, pancreas and colon [3,4,7,18][13][14][15][16]. However, it is not clear whether insulin and IGF-1 may promote cancer directly by promoting growth and preventing apoptosis, or by accelerating the aging process, which is a key risk factor for many cancers.
2. Growth Genes, Longevity, and Cancer
2.1. Growth Genes Aging and DNA Damage in Yeast
Genome wide screen of deletion mutants obtained by transposon mutagenesis discovered Sch9-Tor pathway as perhaps the most potent pro-aging pathway in yeast [20][17]. SCH9, which was originally isolated as a suppressor of impaired Ras pathway [31][18], codes a serine-threonine protein kinase ortholog of mammalian S6K whose deletion increases resistance to multiple stresses and lifespan. The partial overlap with the PKA pathway is further confirmed by the phenotype reversion observed in sch9 deletion mutant after PKA hyperactivation. However, the overlap between these two pathways is not complete since the contemporary deletions of RAS2 and SCH9 has a greater effect on stress resistance as well as viability with respect to each single deletion. More comprehensive genome wide analysis performed using each of the aforementioned aging paradigms identified other genes whose deletion positively affected longevity. Deletion of genes involved in protein synthesis, such as protein component of ribosomal subunits, or genes involved in nuclear export of tRNA identified the transcription factor Gcn4 as another longevity regulator [32][19]. It must also be noted that these effects are not additive to Tor-Sch9 suggesting common aging regulatory pathways. Deletion of the tRNA wobbling regulator TRM9 was also identified as a lifespan regulator but its role was argued to be dependent on lower translation efficiency [33][20].
Moreover, the activation of transcription factors Msn2 and Msn4 in S. cerevisiae deficient in the Ras/cAMP/PKA signaling makes cells more resistant to stress, in part by inducing the expression of genes encoding for several heat shock proteins, catalase (Ctt1), and the DNA damage inducible gene DDR2 [20,35][17][21]. These results suggest that the effect of mutations in the Tor-Sch9/S6k and Ras/AC/PKA pathways is partly mediated by the regulation of antioxidant defenses and the reduction of oxidants. In fact, yeasts expressing constitutively active RAS2 oncogene present a lower resistance to oxidants and a decreased lifespan [36][22].
2.2. Growth Genes Aging and DNA Damage in Worms and Flies
The role of nutrient-response pathways was also examined in the worm Caenorabditis elegans and fruit fly Drosophila melanogaster. Studies of the nematode Caenorabditis elegans showed that a reduction of the insulin/insulin-like growth factor signaling pathway (IIS) and the consequent activation of the Forkhead FoxO transcription factor daf-16 which, similarly to Msn2/4 in yeast, regulates genes involved in the cellular stress response and detoxification of xenobiotics and free radicals, extends longevity [37,38,39][23][24][25]. The extension of lifespan in worms also requires the heat shock factor hsf-1, which regulates the expression of heat shock proteins [40][26]. As observed in yeast, inhibition of TOR-S6 kinase signaling can increase lifespan in worms; in particular, TOR pathway inhibition can activate the process of autophagy and alter the activity of other TOR targets, such as the hypoxia-inducible factor 1 (HIF-1) transcription factor, also independently shown to extend lifespan [39,41][25][27].
2.3. Growth Genes, Aging and Cancer in Mice
In summary, in mice there is very strong evidence for the link between high growth hormone and IGF-1 levels, DNA damage and cancer, likely mediated at least in part by the activation of AKT, TOR-S6K and PKA signaling, analogously to what is observed in yeast (Figure 1).
2.4. Growth Genes and Cancer in Humans
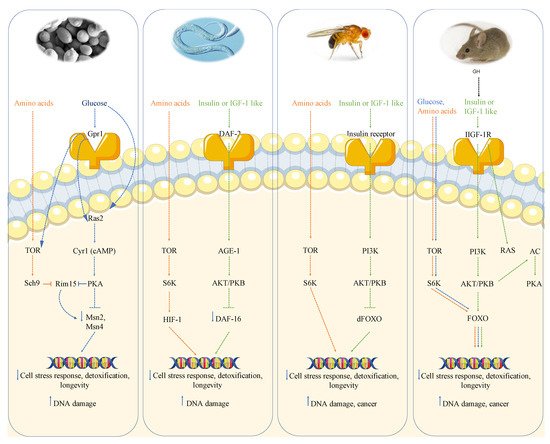
2.4. Growth Genes and Cancer in Humans
Alterations in GH-IGF-1 axis have also been studied also in humans. Notably, human cancers are frequently mutated in the IGF-1R (2.48% of all cancers) and in its downstream signaling proteins Ras (19% of all cancers) and Akt (1.8% of all cancers) [64,65,66,67][38][39][40][41]. In agreement with mouse studies, the modulation of the GH-IGF-1 pathway appears to have a key role in cancer prevention in humans. High levels of IGF-1 are, in fact, associated with an increased incidence of several cancers, including colorectal, prostate and breast cancers, while mutations in the human IGF-1R were found to protect against age-related disorders [9,68][2][42]. Recent evidence reports that centenarians most frequently present heterozygous mutations in the IGF-1R gene, associated with low IGF-1 serum levels and a higher IGF-1R activity compared to controls characterized by high IGF-1 serum levels [69][43]. The role of GH/IGF-1 axis activity on longevity and aging-related diseases in human is also supported by long-term studies of an Ecuadorian cohort affected by Laron syndrome (LS) which is characterized by GHR deficiency and consequently is responsible for a 90% reduction of the IGF-1 levels. Guevara-Aguirre et al., monitoring LS patients for more than 20 years, reported that the relation between pro-growth signaling pathways, oxidative stress, genomic instability and cellular damages shown in several model organisms is also observed in humans and human cells [6][11].
3. Calorie Restriction (CR) and Cancer
Calorie restriction (CR), a dietary intervention that reduces calorie intake without inducing malnutrition, is the most studied intervention able to extend lifespan but also well established to postpone or even prevent age-related diseases, including cancer [75][44] (Table 1). Several studies showed that CR increases lifespan in multiple organisms including yeast, flies, worms, rodents and monkeys, protecting from disorders and decline in functions related to aging [22,30,76,77,78,79,80,81][35][45][46][47][48][49][50][51].
3
| Calorie Restriction (CR) |
Metabolic Adaptations | Molecular Adaptations | Cellular Adaptations |
| ↓ IGF-1 ↓ Insulin ↓ Oxidative stress ↓ Inflammation ↑ Cortisol |
↓ PI3K/Akt/S6K ↓ mTOR ↓ Ras/MAPK ↑ Nrf2 ↑ FOXO ↑ PTEN |
↓ Cell proliferation ↓ Oxidative damage ↑ DNA repair ↑ Genome instability |
4. Fasting and Fasting Mimicking Diets
Fasting methods (Table 2). Fasting can be performed for short-term frequent periods, called intermittent fasting (IF), or less frequent but longer periods, known as prolonged and periodic fasting (PF) [63,105][37][52]. There are multiple examples of IF diets, including: complete fasting every other day (also called alternate-day fasting ADF); 70% energy restriction every other day; time-restricted feeding (TRF), during which food intake is restricted to 6–12 h per day; and the 5:2 diet, which is achieved by consuming only 500–700 calories for two days a week [106,107,108,109,110][53][54][55][56][57]. Thus, IF interventions usually include a phase during which only water is consumed or calorie intake is extremely low, followed by a normal feeding phase which lasts between 12 and 72 h. PF periods, instead, in most cases refer to 2–5 days of water-only fasting or 4–7 days of a fasting mimicking diet (FMD), a diet designed to mimic the metabolic effects induced by fasting [111,112][58][59]. Differently from IF, PF does not need to occur at specific intervals and in most cases can be carried out only for few times per year [113][60]. All these types of fasting cause different degrees of metabolic changes, including the decrease in blood glucose levels, the reduction of glycogen stores, the decrease in circulating leptin levels and the mobilization of fatty acids accompanied by the generation of ketone bodies [63,113][37][60]. Moreover, fasting or FMD periods can lead to behavioral changes, including increased awareness, attention, mental acuity, vigilance and feelings of euphoria, thus lowering depressive symptoms [114,115][61][62].
| Type of Fasting | Schedule | Description | |
|---|---|---|---|
| Intermittent Fasting (IF) |
ADF | 24 h fast/ 24 h eating period | Water only fasting every other day |
| 5:2 | 2 days fast or very low calorie consumption (500–700 kcal)/ 5 days eating period | Alternation of 2 days of very low-calorie consumption with a 5 days ad libitum re-feeding period | |
| TRF | 12- to 18 h fast/ 6- to 12 h eating period | Food intake resctricted to 6–12 h per day | |
| Periodic Fasting (PF) |
Prolonged fasting | 2–5 days of water fast/ 7 days eating period (or longer) | Water only fasting period followed by an ad libitum re-feeding period |
| Prolonged FMD | 4- to 7 days FMD/ 10- to 25 days eating period | 30–50% of the normal caloric intake using a fasting mimicking diet for 4–7 days followed by an ad libitum re-feeding period |
Fasting can extend lifespan and protect from age-related disorders, including DNA damage or cancer, in different model organisms [63][37].
45. Fasting Mimicking Diet, Hormones and Cancer Prevention
Several studies indicate that PF is a much more viable strategy than a continuous CR, for cancer prevention and treatment in humans because: (1) it cause a much more extreme set of metabolic changes than CR, including IGF-1, insulin, leptin and glucose decreases, which can be combined with standard of care drugs to promote strong anticancer effects and cancer-free survival; (2) it stimulates anticancer immune responses; (3) it prevents muscle loss; (4) it is amenable to combination with standard cancer treatments but also cancer preventions since it is only conducted for several days periodically and does not require dietary changes between periodic fasting cycles. Preliminary clinical data report that 48 h of fasting are necessary to obtain relevant clinical effects in oncology, such as preventing DNA damages induced by chemotherapy in healthy tissues and improve quality of life to cancer patients [136,137,138][63][64][65]. However, most patients undergoing water-only fasting during cancer treatment had difficulties with sustaining water fasting and reported side effects such as headache, nausea, light-headedness, anemia and weakness [139][66]. Thus, water-only fasting and intermittent fasting which are expected to be repeated every other day or twice a week, remain a challenging option for the majority of population, especially frail and older subjects and cancer patients. FMD is a plant-based caloric-restricted alimentary regimen (typically between 300 and 1100 kcal per day) characterized by low proteins and sugars and relatively high unsaturated fats. It was developed to mimic many of the metabolic effects induced by water-only fasting but with reduced nutritional risk and burden [112,138,140,141,142][59][65][67][68][69].56. Alternative Interventions to Reduce Age-Related Diseases Risk Factors
Aging is frequently associated with impaired glucose tolerance and hyperinsulinemia, due to an increase in insulin secretion as a result of high glucose levels [146][70]. A decline in glucose tolerance is often associated with an increased risk of developing atherosclerosis or non-insulin dependent diabetes mellitus (NIDDM) [147,148][71][72]. Endurance exercise training reduces insulin levels both during fasting and feeding periods. Several studies showed that individuals who practice exercise periodically have improved glucose tolerance and responsiveness to insulin [149,150,151,152,153][73][74][75][76][77]. Seals et al., showed that regular exercise prevented the decline in glucose tolerance and hyperinsulinemia development in older people [152][76]. Moreover, exercise training was reported to normalize glucose tolerance by reducing insulin resistance in patients with mild NIDDM or impaired glucose tolerance (IGT) [154][78]. Furthermore, consuming a low-calorie and low-protein vegan diet, composed of unprocessed and uncooked plant-derived foods, for at least two years, or performing endurance exercise are associated with a decrease in cardiometabolic risk [155][79]. In particular, they reduced the plasma concentrations of lipids, lipoproteins, glucose, insulin, C-reactive protein (CRP) and systolic and diastolic blood pressure [155][79].67. Conclusions
Studies in simple organisms and mice, demonstrate the link between nutrients and particularly protein intake, growth factors, DNA damage and cancer. The effect of growth factors on DNA damage and cancer is mediated, at least in part, by oxidative stress and damage, but in part also by the inhibition of apoptosis. The reduced activity of growth factors and the lowering of oxidation and DNA damage not only decreases cancer but also extends longevity, since aging is the most important factor promoting cancer. Calorie restriction is a powerful anti-aging intervention, but it also forces the organism into an extremely low nourishment state, which may not constitute malnourishment in the short-term but which may do so long-term. Interventions such as IF and PF are emerging as alternatives to CR, with some of them being able to minimize side effects and burden while maximizing efficacy. Studies on PF have also pointed to 2 key processes absent or low in CR and IF: (a) a pronounced breakdown process both at the intracellular (autophagy etc) and cellular (apoptosis) levels requiring 2 or more days and associated with a high ketogenic state, (b) a rebuilding/regeneration process involving stem and progenitor cells in multiple system and associated with the return from PF to normal feeding (re-feeding). The FMD developed and studied by our laboratories is emerging as a viable and effective intervention in the longevity and cancer prevention fields, since it does not require chronic treatment, it does not cause malnourishment or loss of muscle mass and may be effective when performed only a few times a year for 5 days. In the future years it will be important to continue to test different nutritional interventions with the potential to extend the health span and prevent cancer, with a focus on those that are safe and feasible for long-term use in humans.
References
- Bartke, A.; Sun, L.Y.; Longo, V. Somatotropic signaling: Trade-offs between growth, reproductive development, and longevity. Physiol. Rev. 2013, 93, 571–598.
- Pollak, M.N. Insulin-like growth factors and neoplasia. Novartis Found. Symp. 2004, 262, 84–98.
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417.
- Anisimov, V.N.; Bartke, A. The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. Crit. Rev. Oncol. Hematol. 2013, 87, 201–223.
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867.
- Kaaks, R.; Lukanova, A. Effects of weight control and physical activity in cancer prevention: Role of endogenous hormone metabolism. Ann. N. Y. Acad. Sci. 2002, 963, 268–281.
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556.
- Berrino, F.; Bellati, C.; Secreto, G.; Camerini, E.; Pala, V.; Panico, S.; Allegro, G.; Kaaks, R. Reducing bioavailable sex hormones through a comprehensive change in diet: The diet and androgens (DIANA) randomized trial. Cancer Epidemiol. Biomark. Prev. 2001, 10, 25–33.
- Pugeat, M.; Crave, J.C.; Elmidani, M.; Nicolas, M.H.; Garoscio-Cholet, M.; Lejeune, H.; Dechaud, H.; Tourniaire, J. Pathophysiology of sex hormone binding globulin (SHBG): Relation to insulin. J. Steroid Biochem. Mol. Biol. 1991, 40, 841–849.
- Guevara-Aguirre, J.; Rosenbloom, A.L.; Balasubramanian, P.; Teran, E.; Guevara-Aguirre, M.; Guevara, C.; Procel, P.; Alfaras, I.; De Cabo, R.; Di Biase, S.; et al. GH Receptor Deficiency in Ecuadorian Adults Is Associated With Obesity and Enhanced Insulin Sensitivity. J. Clin. Endocrinol. Metab. 2015, 100, 2589–2596.
- Guevara-Aguirre, J.; Balasubramanian, P.; Guevara-Aguirre, M.; Wei, M.; Madia, F.; Cheng, C.W.; Hwang, D.; Martin-Montalvo, A.; Saavedra, J.; Ingles, S.; et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med. 2011, 3, 70ra13.
- Guevara-Aguirre, J.; Teran, E.; Lescano, D.; Guevara, A.; Guevara, C.; Longo, V.; Gavilanes, A.W.D. Growth hormone receptor deficiency in humans associates to obesity, increased body fat percentage, a healthy brain and a coordinated insulin sensitivity. Growth Horm. IGF Res. 2020, 51, 58–64.
- Kaaks, R. Nutrition, hormones, and breast cancer: Is insulin the missing link? Cancer Causes Control 1996, 7, 605–625.
- Gunter, M.J.; Hoover, D.R.; Yu, H.; Wassertheil-Smoller, S.; Rohan, T.E.; Manson, J.E.; Li, J.; Ho, G.Y.; Xue, X.; Anderson, G.L.; et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst. 2009, 101, 48–60.
- Giovannucci, E. Insulin and colon cancer. Cancer Causes Control 1995, 6, 164–179.
- Weiderpass, E.; Partanen, T.; Kaaks, R.; Vainio, H.; Porta, M.; Kauppinen, T.; Ojajarvi, A.; Boffetta, P.; Malats, N. Occurrence, trends and environment etiology of pancreatic cancer. Scand. J. Work Environ. Health 1998, 24, 165–174.
- Fabrizio, P.; Pozza, F.; Pletcher, S.D.; Gendron, C.M.; Longo, V.D. Regulation of longevity and stress resistance by Sch9 in yeast. Science 2001, 292, 288–290.
- Toda, T.; Cameron, S.; Sass, P.; Wigler, M. SCH9, a gene of Saccharomyces cerevisiae that encodes a protein distinct from, but functionally and structurally related to, cAMP-dependent protein kinase catalytic subunits. Genes Dev. 1988, 2, 517–527.
- McCormick, M.A.; Delaney, J.R.; Tsuchiya, M.; Tsuchiyama, S.; Shemorry, A.; Sim, S.; Chou, A.C.; Ahmed, U.; Carr, D.; Murakami, C.J.; et al. A Comprehensive Analysis of Replicative Lifespan in 4698 Single-Gene Deletion Strains Uncovers Conserved Mechanisms of Aging. Cell Metab. 2015, 22, 895–906.
- Fabrizio, P.; Hoon, S.; Shamalnasab, M.; Galbani, A.; Wei, M.; Giaever, G.; Nislow, C.; Longo, V.D. Genome-wide screen in Saccharomyces cerevisiae identifies vacuolar protein sorting, autophagy, biosynthetic, and tRNA methylation genes involved in life span regulation. PLoS Genet. 2010, 6, e1001024.
- Thevelein, J.M.; de Winde, J.H. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 1999, 33, 904–918.
- Hlavata, L.; Aguilaniu, H.; Pichova, A.; Nystrom, T. The oncogenic RAS2(val19) mutation locks respiration, independently of PKA, in a mode prone to generate ROS. EMBO J. 2003, 22, 3337–3345.
- Johnson, T.E. Increased life-span of age-1 mutants in Caenorhabditis elegans and lower Gompertz rate of aging. Science 1990, 249, 908–912.
- Kenyon, C.; Chang, J.; Gensch, E.; Rudner, A.; Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature 1993, 366, 461–464.
- Hansen, M.; Chandra, A.; Mitic, L.L.; Onken, B.; Driscoll, M.; Kenyon, C. A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet. 2008, 4, e24.
- Johnson, T.E. Caenorhabditis elegans 2007: The premier model for the study of aging. Exp. Gerontol. 2008, 43, 1–4.
- Mehta, R.; Steinkraus, K.A.; Sutphin, G.L.; Ramos, F.J.; Shamieh, L.S.; Huh, A.; Davis, C.; Chandler-Brown, D.; Kaeberlein, M. Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science 2009, 324, 1196–1198.
- LaFever, L.; Drummond-Barbosa, D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science 2005, 309, 1071–1073.
- Clancy, D.J.; Gems, D.; Harshman, L.G.; Oldham, S.; Stocker, H.; Hafen, E.; Leevers, S.J.; Partridge, L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 2001, 292, 104–106.
- Tatar, M.; Kopelman, A.; Epstein, D.; Tu, M.P.; Yin, C.M.; Garofalo, R.S. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 2001, 292, 107–110.
- Igaki, T.; Pagliarini, R.A.; Xu, T. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr. Biol. 2006, 16, 1139–1146.
- Bjedov, I.; Toivonen, J.M.; Kerr, F.; Slack, C.; Jacobson, J.; Foley, A.; Partridge, L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010, 11, 35–46.
- Orr, W.C.; Sohal, R.S. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 1994, 263, 1128–1130.
- Lin, Y.J.; Seroude, L.; Benzer, S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science 1998, 282, 943–946.
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—from yeast to humans. Science 2010, 328, 321–326.
- Honda, Y.; Honda, S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999, 13, 1385–1393.
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192.
- Rodriguez-Viciana, P.; Tetsu, O.; Oda, K.; Okada, J.; Rauen, K.; McCormick, F. Cancer targets in the Ras pathway. Cold Spring Harb. Symp. Quant. Biol. 2005, 70, 461–467.
- Yoeli-Lerner, M.; Toker, A. Akt/PKB signaling in cancer: A function in cell motility and invasion. Cell Cycle 2006, 5, 603–605.
- Prior, I.A.; Hood, F.E.; Hartley, J.L. The Frequency of Ras Mutations in Cancer. Cancer Res. 2020, 80, 2969–2974.
- Consortium, A.P.G. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831.
- Renehan, A.G.; Zwahlen, M.; Minder, C.; O’Dwyer, S.T.; Shalet, S.M.; Egger, M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet 2004, 363, 1346–1353.
- Suh, Y.; Atzmon, G.; Cho, M.O.; Hwang, D.; Liu, B.; Leahy, D.J.; Barzilai, N.; Cohen, P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc. Natl. Acad. Sci. USA 2008, 105, 3438–3442.
- Hursting, S.D.; Lavigne, J.A.; Berrigan, D.; Perkins, S.N.; Barrett, J.C. Calorie restriction, aging, and cancer prevention: Mechanisms of action and applicability to humans. Annu. Rev. Med. 2003, 54, 131–152.
- Wei, M.; Fabrizio, P.; Hu, J.; Ge, H.; Cheng, C.; Li, L.; Longo, V.D. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008, 4, e13.
- Barrows, C.H., Jr.; Kokkonen, G. The effect of various dietary restricted regimes on biochemical variables in the mouse. Growth 1978, 42, 71–85.
- Weindruch, R.; Walford, R.L.; Fligiel, S.; Guthrie, D. The retardation of aging in mice by dietary restriction: Longevity, cancer, immunity and lifetime energy intake. J. Nutr. 1986, 116, 641–654.
- Lane, M.A.; Mattison, J.; Ingram, D.K.; Roth, G.S. Caloric restriction and aging in primates: Relevance to humans and possible CR mimetics. Microsc. Res. Tech. 2002, 59, 335–338.
- Anderson, R.M.; Shanmuganayagam, D.; Weindruch, R. Caloric restriction and aging: Studies in mice and monkeys. Toxicol. Pathol. 2009, 37, 47–51.
- Grandison, R.C.; Piper, M.D.; Partridge, L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 2009, 462, 1061–1064.
- Colman, R.J.; Beasley, T.M.; Kemnitz, J.W.; Johnson, S.C.; Weindruch, R.; Anderson, R.M. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat. Commun. 2014, 5, 3557.
- Trepanowski, J.F.; Canale, R.E.; Marshall, K.E.; Kabir, M.M.; Bloomer, R.J. Impact of caloric and dietary restriction regimens on markers of health and longevity in humans and animals: A summary of available findings. Nutr. J. 2011, 10, 107.
- Bruce-Keller, A.J.; Umberger, G.; McFall, R.; Mattson, M.P. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann. Neurol. 1999, 45, 8–15.
- Johnson, J.B.; Summer, W.; Cutler, R.G.; Martin, B.; Hyun, D.H.; Dixit, V.D.; Pearson, M.; Nassar, M.; Telljohann, R.; Maudsley, S.; et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med. 2007, 42, 665–674.
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes. 2011, 35, 714–727.
- Regmi, P.; Chaudhary, R.; Page, A.J.; Hutchison, A.T.; Vincent, A.D.; Liu, B.; Heilbronn, L. Early or delayed time-restricted feeding prevents metabolic impact of obesity in mice. J. Endocrinol. 2021, 248, 75–86.
- Chaix, A.; Lin, T.; Le, H.D.; Chang, M.W.; Panda, S. Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab. 2019, 29, 303–319.e304.
- Raffaghello, L.; Lee, C.; Safdie, F.M.; Wei, M.; Madia, F.; Bianchi, G.; Longo, V.D. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc. Natl. Acad. Sci. USA 2008, 105, 8215–8220.
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99.
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059.
- Fond, G.; Macgregor, A.; Leboyer, M.; Michalsen, A. Fasting in mood disorders: Neurobiology and effectiveness. A review of the literature. Psychiatry Res. 2013, 209, 253–258.
- Maniaci, G.; La Cascia, C.; Giammanco, A.; Ferraro, L.; Chianetta, R.; Di Peri, R.; Sardella, Z.; Citarrella, R.; Mannella, Y.; Larcan, S.; et al. Efficacy of a fasting-mimicking diet in functional therapy for depression: A randomised controlled pilot trial. J. Clin. Psychol. 2020, 76, 1807–1817.
- Safdie, F.M.; Dorff, T.; Quinn, D.; Fontana, L.; Wei, M.; Lee, C.; Cohen, P.; Longo, V.D. Fasting and cancer treatment in humans: A case series report. Aging 2009, 1, 988–1007.
- de Groot, S.; Vreeswijk, M.P.; Welters, M.J.; Gravesteijn, G.; Boei, J.J.; Jochems, A.; Houtsma, D.; Putter, H.; van der Hoeven, J.J.; Nortier, J.W.; et al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: A randomized pilot study. BMC Cancer 2015, 15, 652.
- Bauersfeld, S.P.; Kessler, C.S.; Wischnewsky, M.; Jaensch, A.; Steckhan, N.; Stange, R.; Kunz, B.; Bruckner, B.; Sehouli, J.; Michalsen, A. The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: A randomized cross-over pilot study. BMC Cancer 2018, 18, 476.
- Dorff, T.B.; Groshen, S.; Garcia, A.; Shah, M.; Tsao-Wei, D.; Pham, H.; Cheng, C.W.; Brandhorst, S.; Cohen, P.; Wei, M.; et al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer 2016, 16, 360.
- Wei, M.; Brandhorst, S.; Shelehchi, M.; Mirzaei, H.; Cheng, C.W.; Budniak, J.; Groshen, S.; Mack, W.J.; Guen, E.; Di Biase, S.; et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 2017, 9.
- Caffa, I.; Spagnolo, V.; Vernieri, C.; Valdemarin, F.; Becherini, P.; Wei, M.; Brandhorst, S.; Zucal, C.; Driehuis, E.; Ferrando, L.; et al. Author Correction: Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 2020, 588, E33.
- de Groot, S.; Lugtenberg, R.T.; Cohen, D.; Welters, M.J.P.; Ehsan, I.; Vreeswijk, M.P.G.; Smit, V.; de Graaf, H.; Heijns, J.B.; Portielje, J.E.A.; et al. Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 DIRECT trial. Nat. Commun. 2020, 11, 3083.
- Davidson, M.B. The effect of aging on carbohydrate metabolism: A review of the English literature and a practical approach to the diagnosis of diabetes mellitus in the elderly. Metabolism 1979, 28, 688–705.
- Pyorala, K. Relationship of glucose tolerance and plasma insulin to the incidence of coronary heart disease: Results from two population studies in Finland. Diabetes Care 1979, 2, 131–141.
- Stout, R.W. The relationship of abnormal circulating insulin levels to atherosclerosis. Atherosclerosis 1977, 27, 1–13.
- Bjorntorp, P.; De Jounge, K.; Sjostrom, L.; Sullivan, L. The effect of physical training on insulin production in obesity. Metabolism 1970, 19, 631–638.
- LeBlanc, J.; Nadeau, A.; Richard, D.; Tremblay, A. Studies on the sparing effect of exercise on insulin requirements in human subjects. Metabolism 1981, 30, 1119–1124.
- Heath, G.W.; Gavin, J.R., 3rd; Hinderliter, J.M.; Hagberg, J.M.; Bloomfield, S.A.; Holloszy, J.O. Effects of exercise and lack of exercise on glucose tolerance and insulin sensitivity. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 512–517.
- Seals, D.R.; Hagberg, J.M.; Allen, W.K.; Hurley, B.F.; Dalsky, G.P.; Ehsani, A.A.; Holloszy, J.O. Glucose tolerance in young and older athletes and sedentary men. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 56, 1521–1525.
- De Groote, E.; Britto, F.A.; Balan, E.; Warnier, G.; Thissen, J.P.; Nielens, H.; Sylow, L.; Deldicque, L. Effect of hypoxic exercise on glucose tolerance in healthy and prediabetic adults. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E43–E54.
- Holloszy, J.O.; Schultz, J.; Kusnierkiewicz, J.; Hagberg, J.M.; Ehsani, A.A. Effects of exercise on glucose tolerance and insulin resistance. Brief review and some preliminary results. Acta Med. Scand. Suppl. 1986, 711, 55–65.
- Fontana, L.; Meyer, T.E.; Klein, S.; Holloszy, J.O. Long-term low-calorie low-protein vegan diet and endurance exercise are associated with low cardiometabolic risk. Rejuvenation Res. 2007, 10, 225–234.
