Bone tissue engineering (BTE) is a process of combining live osteoblast progenitors with a biocompatible scaffold to produce a biological substitute that can integrate into host bone tissue and recover its function. Mesenchymal stem cells (MSCs) are the most researched post-natal stem cells because they have self-renewal properties and a multi-differentiation capacity that can give rise to various cell lineages, including osteoblasts. BTE technology utilizes a combination of MSCs and biodegradable scaffold material, which provides a suitable environment for functional bone recovery and has been developed as a therapeutic approach to bone regeneration.
- bone tissue engineering
- MSCs
- osteoblasts
- scaffolds
1. Introduction
2. MSCs Derived from Embryonic Stem Cells and Induced Pluripotent Stem Cells for Bone Tissue Regeneration
Functional bone tissue engineering generally involves the use of osteoprogenitors derived from MSCs and seeded onto a scaffold to predictably restore the lost architecture and function of bone tissue. MSCs have been isolated from adult tissues such as adipose tissue, bone marrow, and dental tissues, are widely used in regenerative medicine, including BTE, and, thus, have both research and clinical applications. However, MSCs cannot be isolated from patients with systemic disorders such as cardiovascular disease, diabetes, inflammatory bone disease, or advanced aging-related issues. Embryonic stem cell (ESCs)- or Induced pluripotent stem cell (iPSCs)-derived MSCs may be potential cell sources for the clinical trial of BTE [17][15]. A better understanding of cell fate decisions and differentiation processes during osteoblast development may help to generate functional progenitor cells for tissue restoration. Over the years, technologies involving the osteoblast differentiation of ESCs and iPSCs have been significantly improved, and several studies have demonstrated the successful production of MSCs derived from ESCs/iPSCs for use in BTE therapies [18,19,20][16][17][18] (Figure 1).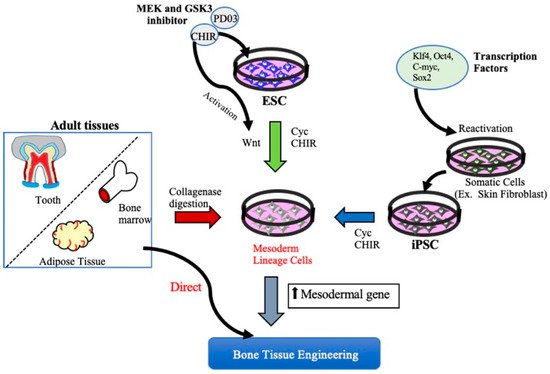
3. MSCs for Bone Regeneration
3.1. Characterization of MSCs
Surface markers are currently being used to identify MSCs for quality control assurance prior to cell preparation, based on ‘good manufacturing practice,’ which is required for investor-mediated clinical developments. Hence, the characterization of MSCs based on surface marker analysis is an essential criterion for the clinical application of BTE methodologies. According to the International Society of Cell Therapy (ISCT) criteria, MSCs express a cluster of differentiation (CD) surface markers such as CD90, CD105, and CD73, but do not express CD11b, CD14, CD19, CD34, CD45, or human leukocyte antigen (HLA)-DR [32,33,34][22][23][24].3.2. Clinical Translation of MSC-Based Bone Regeneration
The basic concept behind a scaffold is to mimic the structure and function of the extracellular matrix (ECM) in tissues. The ECM provides both structural and mechanical stability and regulates some of the core cellular functions [42,43,44][25][26][27]. The basic role of scaffolds in BTE is to mimic the ECM of the native bone tissue and provide a functional three-dimensional space for the adhesion, migration, proliferation, and differentiation of osteoblast progenitors in which bone growth can occur [38,45,46,47][28][29][30][31]. An ideal scaffold for BTE should substitute for both the structure and function of the ECM and thus be capable of regenerating the lost bone tissue when seeded in conjunction with osteoblast progenitors. BTE innovations have led to the development of new biomaterials that resemble the 3D bone structure, in terms of mechanical properties as well as osteoconductive, osteoinductive, and osteogenic features [48,49][32][33]. Traditional bone repair approaches mainly focus on the use of bone grafts from autologous, allogeneic, and xenogeneic sources; however, complications such as donor-site morbidity and host immune rejection limit the application of these tissues [50][34]. The promise of BTE has principally involved overcoming these problems. The aims of BTE are to regenerate and restore the function of lost bone tissue using combinations of osteoblast progenitors and synthetic biomaterial scaffolds. Over the past decade, the use of synthetic biomaterials to enhance bone regeneration has significantly developed because of their capacity to mimic the natural environment of the extracellular matrix. The synthetic scaffold biomaterials predominantly used in BTE include calcium phosphate ceramics, biodegradable polymers, and composites, and the combination of ceramics and polymer scaffolds aims to utilize the properties of both materials [47,50,51][31][34][35].
3.2.2. Preclinical Studies of BTE in a Large Animal Model Using MSC/Scaffold Combinations
To translate the clinical use of MSCs combined with scaffolds for BTE, large animal model systems that closely resemble human physiology are required. A number of preclinical studies conducted using MSCs with varying combinations of biomaterials in critical bone defect models are listed in Table 1.| Author | Experiment Animal | Type and Size of Defect | Experimental Transplant Groups | Post-Transplant Follow up Period |
Outcome |
|---|
| NCT Number | Brief Title | Phase | Conditions | Interventions | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Probst et al., 2020 [34] | Mini pigs | Critical mandibular defect (3 × 1 × 2 cm) | 3D TCP-PLGA scaffold seeded with osteogenic differentiated Porcine ADSCs (pADSCs). | 12 weeks | pADSCs seeded TCP-PLGA scaffold constructs significantly improved bone regenerations compared to empty scaffold. | ||||||
| NCT04297813 | Efficacy in Alveolar Bone Regeneration With Autologous MSCs and Biomaterial in Comparison to Autologous Bone Grafting | Phase I | • Alveolar Bone Atrophy | Autologous MSCs and a biomaterial, biphasic Calcium Phosphate (BCP). | Wang et al., 2019 [36] | Rhesus Monkeys | Critical alveolar bone defect (10 × 10 × 5 mm) | 3D-Bioactive glass (BG) + BMP/chitosan (CS) + BMMSCs | 12 weeks | BMP/CS nanoparticles loaded on 3D-BG scaffold promoted bone regeneration ability in vivo, and preload of BMMSCs promote this ability further. | |
| Humerus, Tibia and Femur Critical sized defects | Combination of HA granules (Bongros®-HA, Bioalpha, Seungnam, Korea), BMP2 and BMMSCs mixed with Plasma solution. | Autologous Bone marrow harvested from posterior Iliac crestal bone | None | Dramatic improvement of bone regeneration compared to preoperative radiographs. | |||||||
| NCT03325504 | A Comparative Study of 2 Doses of BM Autologous H- MSC+Biomaterial vs. Iliac Crest AutoGraft for Bone Healing in Non-Union | Phase III | • Non Union Fracture | Culture-expanded autologous BMMSC combined with biphasic calcium phosphate (BCP) biomaterial granules | Hsieh et al., 2019 [37] | Gjerde et al., 2018 [54domestic Ds-Red pigs | Calvarias defect (8 mm in diameter and 2 mm in depth) | Hemostatic gelatin sponge scaffold seeded with EGFP pig BMMSCs | ] | Severe mandibular ridge resorption. | Expanded, autologous MSCs with biphasic calcium phosphate (MBCP+ |
| NCT02803177 | 1, 2, 3 and 4 weeks | Osteoid formation in the scaffolds transplanted with seeded BMMSCs was significantly higher than the control group. | |||||||||
| Cell Therapy by Autologous BMC for Large Bone Defect Repair | TM; Biomatlante, France) | Bone marrow cells from the posterior iliac crest | Shi et al., 2019 [38] | Minipigs | Maxillary Intraosseous circular defects (12 mm in diameter and 5 mm in depth) | Bio-Oss/autogenous (Pig Gingival MSCs) pGMSCs (2 × 106)/SB431542 (TGF-β signalling inhibitor). | 8 weeks | pGMSCs treated with a TGF- β signaling inhibitor successfully repair minipig severe maxillofacial bone defects. | |||
| None | MSCs successfully induce significant new bone formation | Qiu et al., 2018 [39] | Minipigs | Lateral femoral condyle defect (8 mm in diameter and 10 mm in depth) | Calcium phosphate cement (CPC) scaffold seeded with autologous BMMSCs plus autologous PRP (CPC-BMSC-PRP, 1 × 106 cells/scaffold) | 6 and 12 weeks | CPC scaffold co-delivered BMMSCs-PRP promoted scaffold resorption and doubled bone regeneration in large defects than control groups | ||||
| Zhang et al., 2017 [40] | Minipigs | Non-healing full thickness cranial defects (2 cm width × 3 cm length × 0.5 cm depth) | IMC (intrafibrillarly-mineralized collagen) scaffold seeded with 1 × 106 PDLSCs cells | 12 weeks | Compared with HA, IMC-seeded PDLSCs achieved a significantly higher extent of new bone formation, with the normal architecture of natural bones and blood vessels. | ||||||
| Scarano et al., 2017 [41] | Minipigs | Critical-size circular defects (5 mm diameter; 5 mm thickness) in the mandibular body | Bone porcine block (BPB) scaffold seeded with 100 ul cell suspension of BMMSCs | 12 weeks | BPB when used as a scaffold induce bone regeneration and further benefit from the addition of BMMSCs in the tissue-engineered constructs. | ||||||
| Lin et al., 2015 [42] | Minipigs | Massive segmental bone defects (30 mm in length) at the mid-diaphysis of femora | Transduced pig ADSCs loaded onto PLGA scaffold | 2, 4, 8 and 12 weeks | ADSCs/scaffold constructs successfully healed massive segmental bone defects at the mid-diaphysis of femora in minipigs significantly than control group. | ||||||
| Cao et al., 2015 [43] | Mini pigs | Calvarial bone defects (3 cm × 1.8 cm oval defect) | BMMSCs pretreated with 75 μg/mL aspirin for 24 h seeded onto hydroxyapatite/tricalcium phosphate (HA/TCP) | 6 months | BMMSCs pretreated with aspirin have a greater capacity to repair calvarial bone defects in a mini swine model | ||||||
| Fan et al., 2014 [44] | Rhesus monkeys | Segmental tibial defects (20 mm in length) | Autologous prevascularized BMMSCs (5 × 106)-β-TCP constructs | 4, 8 and 12 weeks |
Significantly higher amount of neo-vascularization and radiographic grading score in prevascularized BMMSCs-β-TCP constructs |
3.2.3. Gene Therapy for Bone Regeneration
Gene therapy is another promising approach for enhancing bone regeneration. Today, the advancement in life-sciences technology allows gene transfer technology to fabricate a tissue-engineered scaffold to accommodate the growth of genetically modified cells and the endogenous synthesis of desired gene products in a controlled manner. Gene therapy allows for the transfer of genetic material in the precise anatomic location of target cells, allowing the transgene expression from the cells with the currently available techniques [76][45]. Gene transfer can be performed by several ex vivo and in vivo delivery techniques and by either using viral (transduction) or non-viral (transfection) vectors [76,77][45][46]. Since this review is focused on the use of combined cell scaffold constructs for bone regeneration, we mainly discuss the ex vivo delivery method, which requires isolation of target cells and transfer of the desired gene to express the respective protein in vitro and then seeded onto the biocompatible carrier material to obtain cell-scaffold construct for bone tissue engineering applications. The two standard methods of ex vivo delivery include viral and non-viral, it being said that each type has its advantages and disadvantages. Viral vectors demonstrate high transfection efficiency with immunogenicity and toxicity, raising an issue of safety. In contrast, non-viral vectors usually consist of plasmid or related DNA, which are non-immunogenic and high safety but with low transfection efficiency [77,78][46][47]. Another promising approach is the sequential delivery of exogenous genes to promote the osteogenesis of stem cells. For example, genes that are expressed early and in the final stages of osteogenesis are different. Hence, delivering required osteogenic genes at specific time intervals into target cells induces efficient osteogenic differentiation. A recent study by Kim et al. demonstrated an effective sequential delivery of runt-related transcription factor 2 (RUNX2) and osterix genes induced conversion of human MSCs into pre-osteoblasts and subsequent delivery of activating transcription factor 4 (ATF4) gene triggered further osteogenesis. Differentiation of MSCs into desired mature cells can be regulated by the delivery time of specific osteogenic genes mimicking the natural process of bone remodeling [78,79][47][48].
3.2.4. Clinical Trials of MSCs for BTE
Over the past decade, a greater understanding has emerged with regard to the capabilities of MSCs to promote bone tissue regeneration, with numerous preclinical and clinical studies now underway. To identify the current potential combination of cell-scaffold constructs or tissue-engineered substitutes for bone tissue regeneration, we found twenty clinical trials. Nine are published (Table 2), and others are listed in the ClinicalTrails.gov database (Table 3). These trials have highlighted the importance of using cell-based therapy with various scaffolds to treat bone tissue regeneration in a real clinical setting. From the twenty identified clinical studies listed in Table 2 and Table 3, the majority report the use of BMMSCs, reflecting the fact that they are the most accepted cell source and the current gold standard in most clinical trials for treating bone disease, including nonunion fractures of long bones and craniofacial bone defects. However, in a few clinical trials, researchers have used umbilical cord (UC)- MSCs [85][49], BMMSCs [86][50], and adipose-derived MSCs as allogeneic cell sources to prepare the tissue-engineered constructs for regeneration of critical bone defects (NCT02307). Ceramic-based scaffolds are the primary choice in the majority of clinical trials, indicating their high clinical relevance. From the clinical trials listed in Table 2 and Table 3, most studies used a combination of BMMSCs with calcium-phosphate ceramics such as hydroxyapatite [85[49][51][52],87,88], β-TCP [86,89][50][53] (NCT02803177, NCT02153372), biphasic calcium phosphate, a combination of hydroxyapatite and β-TCP [90][54] (NCT04297813, NCT03325504, NCT01842477). Although most of these clinical trials used a simple combination of calcium-phosphate ceramics with BMMSCs, in a few studies, however, additional factors were included to facilitate enhanced bone regeneration. For example, Dilogo et al. added growth factor BMP2 along with cell scaffold constructs to enhance bone regeneration [85,88][49][52]. Similarly, researchers used BMMSCs mixed with BMP2 and loaded them on to 3-dimensional tissue-engineered collagen scaffold (NCT01958502) in another clinical trial. However, a clinical study by Baba et al. used polylactic scaffold seeded BMMSCs mixed with platelet-rich plasma solution and an additional 5000 units of human thrombin dissolved in 10% calcium chloride [91][55].| Author | Type and Size of Defect | Transplant Groups | Origin of Cell Source | Pre-Transplant Incubation | Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dilogo et al., 2020 [49] |
Nonunion fractures of Humerus/tibia with critical size bone defects | Combination of HA Bongros®-HA, Daewoong), BMP2, UC-MSCs with demineralized bone matrix | Allogeneic Umbilical Cord MSCs (UC-MSCs) | None | Allogeneic UC-MSCs can be used safely to treat the critical sized bone defects of long bones. | |||||
| Dilogo et al., 2019 [52] | ||||||||||
| Phase II | • Humerus Fracture Displaced Proximal | Baba et al., 2016 [55] | Intrabony Periodontal defect. Probing depth >4 mm | The mixture of BMMSCs and PRP, combined with human thrombin dissolved in 10% calcium chloride perfused in a 3D woven-fabric composed of poly-L-lactic acid resin fibers (MSCs/PRP-3D woven Fabric) | Autologous Bone marrow harvested from posterior Iliac crestal bone | Induced under Osteogenic Medium | BMMSCs/PRP-3D woven Fabric constructs showed efficient regeneration of the periodontal tissue including alveolar bone. | |||
| Morrison et al., 2018 [50] | Cranial defects with less than 80 mm diameter | Allogeneic mesenchymal stromal cells (MSCs) on a ceramic carrier (ChronOS granules, synthes, and polymer scaffold, |
Allogenic BMMSCs from 18–25 years aged donors | None | Allogeneic MSCs can be safely used for bone regeneration. | |||||
| Autologous Bone Marrow-derived Mononuclear Cells (BMC) seeded onto ß-TCP | Kaigler et al., 2015 [53] | Severe Bone Atrophy of upper Jaw | Combination of BMMSCs and β-TCP (Cerasorb, Curasan AG, Germany) | Autologous Bone marrow harvested from posterior Iliac crestal bone | None | Higher density of regenerated bone with MSCs+ β-TCP group was observed than control group. | ||||
| NCT02307435 | Marcacci et al., 2007 [51] | Humerus, Tibia and ulnar Critical sized defects | Combination of invitro expanded BMMSCs seeded with porous hydroxy apatite scaffolds (Finblock, FinCeramica Srl, Faenza, Italy) | Autologous Bone marrow harvested from posterior Iliac crestal bone | None | Significant healing of the CSDs. Attained long term durability of bone regeneration. | ||||
| Allogenic Mesenchymal Stem Cell for Bone Defect or Non Union Fracture | Early Phase I | • Non Union Fracture, Metaphyseal Fibrous Defect | Allogeneic MSCs from umbilical cord/bone marrow/adipose combined and HA-CaSo4 | |||||||
| NCT02153372 | Cell Therapy by Bone Marrow- derived Mononuclear Cells (BMC) for Large Bone Defect Repair: Phase-I Clinical Trial | Phase I | • Humerus Fracture Displaced Proximal | Autologous Bone Marrow-derived Mononuclear Cells (BMC) seeded onto ß-TCP | ||||||
| NCT01958502 | Evaluation the Treatment of Nonunion of Long Bone Fracture of Lower Extremities (Femur and Tibia) Using Mononuclear Stem Cells from the Iliac Wing Within a 3-D Tissue Engineered Scaffold | Phase II | • Nonunion of Fracture | BMMSCs with BMP2 within a 3-D tissue engineered collagen scaffold | ||||||
| NCT01842477 | Evaluation of Efficacy and Safety of Autologous MSCs Combined to Biomaterials to Enhance Bone Healing | Phase I/II | • Delayed Union After Fracture of Humerus, Tibial or Femur | BMMScs mixed with biphasic calciulm granules | Bajada et al., 2007 [56] | Tibial non-union | Combination of invitro expanded BMMSCs seeded with calcium sulphate pellets (Stimulan, Biocomposites Ltd., Keele, United Kingdom) | Autologous Bone marrow harvested from posterior Iliac crestal bone | None | Clinical and radiological healing of nonunion was observed |
| NCT00250302 | Autologous Implantation of Mesenchymal Stem Cells for the Treatment of Distal Tibial Fractures | Phase I/II | • Tibial Fracture | BMMSCs loaded onto a carrier and implanted locally at the defect site | Morishita et al., 2006 [57] | Tibial/femur massive defects | Attachment of invitro expanded BMMSCs-HA granules | Autologous Bone marrow harvested from posterior Iliac crestal bone | Induced under Osteogenic Medium | |
| NCT00557635 | Good integration of BMMSCs-HA constructs to the host bone and increased radiographic density of the defect area. |
| Osseous Setting Improvement With Co-implantation of Osseous Matrix and Mesenchymal Progenitors Cells From Autologous Bone Marrow | ||||
| Phase II | ||||
| • Tibia or Femur Pseudo-arthrosis | ||||
| Injection of an osseous matrix (osteopure) combined with MSC progenitors from autologous bone marrow. | ||||
| NCT02177565 | Autologous Stem Cell Therapy for Fracture Non-union Healing | Not available | • Non-union of Fractures | Autologous BMSCs combined with carrier material |
| NCT01435434 | Mononucleotide Autologous Stem Cells and Demineralized Bone Matrix in the Treatment of Non Union/Delayed Fractures | Not available | • Non Union/Delayed Fractures | Injection of Autologous Stem Cells and Demineralized Bone Matrix |
4. Osteoblast-Based Bone Tissue Regeneration
In addition to the efforts to increase the bone-forming ability of MSCs as a cell source for bone tissue engineering, the use of osteoblasts that are capable of proliferating before maturing, and that can synthesize and deposit bone extracellular matrix components such as osteocalcin (OCN) and bone sialoprotein (BSP), provides a potential alternative BTE cell source for the treatment of large bone defects. However, since BTE is generally approached using a combination of osteoblasts induced from MSCs on biodegradable scaffolds, the resulting bone forming efficacy will be dependent on the differentiation potential of MSCs into osteoblasts. This could hamper the progress of BTE for treating large bone defects. There are two major mechanisms underlying skeletal development, intramembranous and endochondral ossification. In intramembranous ossification, osteoblast lineage cells, i.e., immature osteoblasts, are formed directly from condensed mesenchymal tissue. Endochondral ossification, by contrast, involves the production of osteochondral progenitors from MSCs that give rise to hyperchondrocytes which activate perichondrial cells to differentiate into immature osteoblasts. From the perspective of BTE, the formation of immature osteoblasts is the convergence point for both types of ossification.5. Conclusions
References
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma 2018, 32, S7–S11.
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362.
- Yuan, H.; Xing, K.; Hsu, H.-Y. Trinity of Three-Dimensional (3D) Scaffold, Vibration, and 3D Printing on Cell Culture Application: A Systematic Review and Indicating Future Direction. Bioengineering 2018, 5, 57.
- Plotkin, L.I.; Bellido, T. Osteocytic signalling pathways as therapeutic targets for bone fragility. Nat. Rev. Endocrinol. 2016, 12, 593–605.
- Wang, T.; Yang, X.; Qi, X.; Jiang, C. Osteoinduction and proliferation of bone-marrow stromal cells in three-dimensional poly (ε-caprolactone)/ hydroxyapatite/collagen scaffolds. J. Transl. Med. 2015, 13, 152.
- Bhumiratana, S.; Bernhard, J.C.; Alfi, D.M.; Yeager, K.; Eton, R.E.; Bova, J.; Shah, F.; Gimble, J.M.; Lopez, M.J.; Eisig, S.B.; et al. Tissue-engineered autologous grafts for facial bone reconstruction. Sci. Transl. Med. 2016, 8, 343ra83.
- Chamieh, F.; Collignon, A.-M.; Coyac, B.R.; Lesieur, J.; Ribes, S.; Sadoine, J.; Llorens, A.; Nicoletti, A.; Letourneur, D.; Colombier, M.-L.; et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci. Rep. 2016, 6, 38814.
- Haj, J.; Haj Khalil, T.; Falah, M.; Zussman, E.; Srouji, S. An ECM-Mimicking, Mesenchymal Stem Cell-Embedded Hybrid Scaffold for Bone Regeneration. BioMed Res. Int. 2017, 2017, 1–12.
- Kjærgaard, K.; Dreyer, C.H.; Ditzel, N.; Andreasen, C.M.; Chen, L.; Sheikh, S.P.; Overgaard, S.; Ding, M. Bone Formation by Sheep Stem Cells in an Ectopic Mouse Model: Comparison of Adipose, and Bone Marrow Derived Cells and Identification of Donor-Derived Bone by Antibody Staining. Stem Cells Int. 2016, 2016, 1–10.
- Nie, L.; Yang, X.; Duan, L.; Huang, E.; Pengfei, Z.; Luo, W.; Zhang, Y.; Zeng, X.; Qiu, Y.; Cai, T.; et al. The healing of alveolar bone defects with novel bio-implants composed of Ad-BMP9-transfected rDFCs and CHA scaffolds. Sci. Rep. 2017, 7, 6373.
- Lin, Y.; Umebayashi, M.; Abdallah, M.-N.; Dong, G.; Roskies, M.G.; Zhao, Y.F.; Murshed, M.; Zhang, Z.; Tran, S.D. Combination of polyetherketoneketone scaffold and human mesenchymal stem cells from temporomandibular joint synovial fluid enhances bone regeneration. Sci. Rep. 2019, 9, 472.
- Medhat, D.; Rodríguez, C.I.; Infante, A. Immunomodulatory Effects of MSCs in Bone Healing. Int. J. Mol. Sci. 2019, 20, 5467.
- Jayakumar, P.; Di Silvio, L. Osteoblasts in bone tissue engineering. Proc. Inst. Mech. Eng. 2010, 224, 1415–1440.
- Aino, M.; Nishida, E.; Fujieda, Y.; Orimoto, A.; Mitani, A.; Noguchi, T.; Makino, H.; Murakami, S.; Umezawa, A.; Yoneda, T.; et al. Isolation and characterization of the human immature osteoblast culture system from the alveolar bones of aged donors for bone regeneration therapy. Expert Opin. Biol. Ther. 2014, 14, 1731–1744.
- Marolt Presen, D.; Traweger, A.; Gimona, M.; Redl, H. Mesenchymal Stromal Cell-Based Bone Regeneration Therapies: From Cell Transplantation and Tissue Engineering to Therapeutic Secretomes and Extracellular Vesicles. Front. Bioeng. Biotechnol. 2019, 7, 352.
- Kanke, K.; Masaki, H.; Saito, T.; Komiyama, Y.; Hojo, H.; Nakauchi, H.; Lichtler, A.C.; Takato, T.; Chung, U.; Ohba, S. Stepwise Differentiation of Pluripotent Stem Cells into Osteoblasts Using Four Small Molecules under Serum-free and Feeder-free Conditions. Stem Cell Rep. 2014, 2, 751–760.
- Marolt, D.; Campos, I.M.; Bhumiratana, S.; Koren, A.; Petridis, P.; Zhang, G.; Spitalnik, P.F.; Grayson, W.L.; Vunjak-Novakovic, G. Engineering bone tissue from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2012, 109, 8705–8709.
- Zhu, H.; Kimura, T.; Swami, S.; Wu, J.Y. Pluripotent stem cells as a source of osteoblasts for bone tissue regeneration. Biomaterials 2019, 196, 31–45.
- Morikawa, S.; Mabuchi, Y.; Niibe, K.; Suzuki, S.; Nagoshi, N.; Sunabori, T.; Shimmura, S.; Nagai, Y.; Nakagawa, T.; Okano, H.; et al. Development of mesenchymal stem cells partially originate from the neural crest. Biochem. Biophys. Res. Commun. 2009, 379, 1114–1119.
- Caplan, A.I. Adult mesenchymal stem cells and the NO pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 2695–2696.
- Caplan, A.I. Adult Mesenchymal Stem Cells: When, Where, and How. Stem Cells Int. 2015, 2015, 1–6.
- Sousa, B.R.; Parreira, R.C.; Fonseca, E.A.; Amaya, M.J.; Tonelli, F.M.; Lacerda, S.M.; Lalwani, P.; Santos, A.K.; Gomes, K.N.; Ulrich, H.; et al. Human adult stem cells from diverse origins: An overview from multiparametric immunophenotyping to clinical applications. Cytometry A 2014, 85, 43–77.
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A 2018, 93, 19–31.
- Nugraha, A.P.; Rantam, F.A.; Narmada, I.B.; Ernawati, D.S.; Ihsan, I.S. Gingival-Derived Mesenchymal Stem Cell from Rabbit (Oryctolagus cuniculus): Isolation, Culture, and Characterization. Eur. J. Dent. 2021, 15, 332–339.
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479.
- Yu, X.; Tang, X.; Gohil, S.V.; Laurencin, C.T. Biomaterials for bone regenerative engineering. Adv. Healthc. Mater. 2015, 4, 1268–1285.
- Kim, Y.; Ko, H.; Kwon, I.K.; Shin, K. Extracellular matrix revisited: Roles in tissue engineering. Int. Neurourol. J. 2016, 20, S23.
- Vasanthan, J.; Gurusamy, N.; Rajasingh, S.; Sigamani, V.; Kirankumar, S.; Thomas, E.L.; Rajasingh, J. Role of Human Mesenchymal Stem Cells in Regenerative Therapy. Cells 2020, 10, 54.
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568.
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12, 6.
- Alizadeh-Osgouei, M.; Li, Y.; Wen, C. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact. Mater. 2019, 4, 22–36.
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 1–33.
- Girón, J.; Kerstner, E.; Medeiros, T.; Oliveira, L.; Machado, G.; Malfatti, C.; Pranke, P. Biomaterials for bone regeneration: An orthopedic and dentistry overview. Braz. J. Med. Biol. Res. 2021, 54, e11055.
- Probst, F.A.; Fliefel, R.; Burian, E.; Probst, M.; Eddicks, M.; Cornelsen, M.; Riedl, C.; Seitz, H.; Aszódi, A.; Schieker, M.; et al. Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate- poly(D,L-lactide-co-glycolide) scaffolds. Sci. Rep. 2020, 10, 2062.
- Amin, A.M.M.; Ewais, E.M.M. Bioceramic Scaffolds. In Scaffolds in Tissue Engineering Materials, Technologies and Clinical Applications; Baino, F., Ed.; InTech: London, UK, 2017.
- Wang, L.; Xu, W.; Chen, Y.; Wang, J. Alveolar bone repair of rhesus monkeys by using BMP-2 gene and mesenchymal stem cells loaded three-dimensional printed bioglass scaffold. Sci. Rep. 2019, 9, 18175.
- Hsieh, M.-K.; Wu, C.-J.; Su, X.-C.; Chen, Y.-C.; Tsai, T.-T.; Niu, C.-C.; Lai, P.-L.; Wu, S.-C. Bone regeneration in Ds-Red pig calvarial defect using allogenic transplantation of EGFP-pMSCs—A comparison of host cells and seeding cells in the scaffold ed G Papaccio. PLoS ONE 2019, 14, e0215499.
- Shi, A.; Heinayati, A.; Bao, D.; Liu, H.; Ding, X.; Tong, X.; Wang, L.; Wang, B.; Qin, H. Small molecule inhibitor of TGF-β signaling enables robust osteogenesis of autologous GMSCs to successfully repair minipig severe maxillofacial bone defects. Stem Cell Res. Ther. 2019, 10, 172.
- Qiu, G.; Shi, Z.; Xu, H.H.K.; Yang, B.; Weir, M.D.; Li, G.; Song, Y.; Wang, J.; Hu, K.; Wang, P.; et al. Bone regeneration in minipigs via calcium phosphate cement scaffold delivering autologous bone marrow mesenchymal stem cells and platelet-rich plasma. J. Tissue Eng. Regen. Med. 2018, 12, e937–e948.
- Zhang, C.; Yan, B.; Cui, Z.; Cui, S.; Zhang, T.; Wang, X.; Liu, D.; Yang, R.; Jiang, N.; Zhou, Y.; et al. Bone regeneration in minipigs by intrafibrillarly-mineralized collagen loaded with autologous periodontal ligament stem cells. Sci. Rep. 2017, 7, 10519.
- Scarano, A.; Crincoli, V.; Di Benedetto, A.; Cozzolino, V.; Lorusso, F.; Podaliri Vulpiani, M.; Grano, M.; Kalemaj, Z.; Mori, G.; Grassi, F.R. Bone Regeneration Induced by Bone Porcine Block with Bone Marrow Stromal Stem Cells in a Minipig Model of Mandibular “Critical Size” Defect. Stem Cells Int. 2017, 2017, 9082869.
- Lin, C.-Y.; Wang, Y.-H.; Li, K.-C.; Sung, L.-Y.; Yeh, C.-L.; Lin, K.-J.; Yen, T.-C.; Chang, Y.-H.; Hu, Y.-C. Healing of massive segmental femoral bone defects in minipigs by allogenic ASCs engineered with FLPo/Frt-based baculovirus vectors. Biomaterials 2015, 50, 98–106.
- Cao, Y.; Xiong, J.; Mei, S.; Wang, F.; Zhao, Z.; Wang, S.; Liu, Y. Aspirin promotes bone marrow mesenchymal stem cell-based calvarial bone regeneration in mini swine. Stem Cell Res. Ther. 2015, 6, 210.
- Fan, H.; Zeng, X.; Wang, X.; Zhu, R.; Pei, G. Efficacy of prevascularization for segmental bone defect repair using β-tricalcium phosphate scaffold in rhesus monkey. Biomaterials 2014, 35, 7407–7415.
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66.
- Evans, C. Gene therapy for the regeneration of bone. Injury 2011, 42, 599–604.
- Franceschi, R.T.; Yang, S.; Rutherford, R.B.; Krebsbach, P.H.; Zhao, M.; Wang, D. Gene Therapy Approaches for Bone Regeneration. Cells Tissues Organs 2004, 176, 95–108.
- Kim, H.J.; Park, J.S.; Yi, S.W.; Oh, H.J.; Kim, J.-H.; Park, K.-H. Sequential transfection of RUNX2/SP7 and ATF4 coated onto dexamethasone-loaded nanospheres enhances osteogenesis. Sci. Rep. 2018, 8, 1447.
- Dilogo, I.H.; Rahmatika, D.; Pawitan, J.A.; Liem, I.K.; Kurniawati, T.; Kispa, T.; Mujadid, F. Allogeneic umbilical cord-derived mesenchymal stem cells for treating critical-sized bone defects: A translational study. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 265–273.
- Morrison, D.A.; Kop, A.M.; Nilasaroya, A.; Sturm, M.; Shaw, K.; Honeybul, S. Cranial reconstruction using allogeneic mesenchymal stromal cells: A phase 1 first-in-human trial. J. Tissue Eng. Regen. Med. 2018, 12, 341–348.
- Marcacci, M.; Kon, E.; Moukhachev, V.; Lavroukov, A.; Kutepov, S.; Quarto, R.; Mastrogiacomo, M.; Cancedda, R. Stem Cells Associated with Macroporous Bioceramics for Long Bone Repair: 6- to 7-Year Outcome of a Pilot Clinical Study. Tissue Eng. 2007, 13, 947–955.
- Dilogo, I.H.; Phedy, P.; Kholinne, E.; Djaja, Y.P.; Fiolin, J.; Kusnadi, Y.; Yulisa, N.D. Autologous mesenchymal stem cell implantation, hydroxyapatite, bone morphogenetic protein-2, and internal fixation for treating critical-sized defects: A translational study. Int. Orthop. 2019, 43, 1509–1519.
- Kaigler, D.; Avila-Ortiz, G.; Travan, S.; Taut, A.D.; Padial-Molina, M.; Rudek, I.; Wang, F.; Lanis, A.; Giannobile, W.V. Bone Engineering of Maxillary Sinus Bone Deficiencies Using Enriched CD90+ Stem Cell Therapy: A Randomized Clinical Trial: Stem cell therapy for advanced maxillary bone defects. J. Bone Miner. Res. 2015, 30, 1206–1216.
- Gjerde, C.; Mustafa, K.; Hellem, S.; Rojewski, M.; Gjengedal, H.; Yassin, M.A.; Feng, X.; Skaale, S.; Berge, T.; Rosen, A.; et al. Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res. Ther. 2018, 9, 213.
- Baba, S.; Yamada, Y.; Komuro, A.; Yotsui, Y.; Umeda, M.; Shimuzutani, K.; Nakamura, S. Phase I/II Trial of Autologous Bone Marrow Stem Cell Transplantation with a Three-Dimensional Woven-Fabric Scaffold for Periodontitis. Stem Cells Int. 2016, 2016, 1–7.
- Bajada, S.; Harrison, P.E.; Ashton, B.A.; Cassar-Pullicino, V.N.; Ashammakhi, N.; Richardson, J.B. Successful treatment of refractory tibial nonunion using calcium sulphate and bone marrow stromal cell implantation. J. Bone Jt. Surg. Br. 2007, 89-B, 1382–1386.
- Morishita, T.; Honoki, K.; Ohgushi, H.; Kotobuki, N.; Matsushima, A.; Takakura, Y. Tissue Engineering Approach to the Treatment of Bone Tumors: Three Cases of Cultured Bone Grafts Derived From Patients’ Mesenchymal Stem Cells. Artif. Organs 2006, 30, 115–118.
