Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by sanaa harrass.
Among millions of sufferers of chronic rhinosinusitis (CRS), the challenge is not only constantly coping with CRS-related symptoms, such as congested nose, sinus pain, and headaches, but also various complications, such as attention difficulties and possible depression. These complications suggest that neural activity in the central nervous system may be altered in those patients, leading to unexpected conditions, such as neurodegeneration in elderly patients.
- dementia
- nasal microbiome
- dysbiosis
- inflammation
- β-amyloid
- upper respiratory tract
- Neurodegenerative
- neurodegeneration
- bacteria
1. Introduction
Recently, the microbiome has been recognised as a human organ [1]. The altered gut microbiome has been well documented in several mental disorders and neurodegenerative disorders, and its potential as a therapeutic strategy has also been proposed in such conditions [2,3,4,5,6][2][3][4][5][6]. Similarly, the nasal cavity is a habitat for a diverse microbial community, which can also play an important role in human health. To date, the research in this area mainly focuses on conditions within the respiratory system, such as asthma and cystic fibrosis [7,8][7][8]. However, it also needs to be noted that the nasal cavity constitutes a very important route of entry for pathogens that can directly spread into the central nervous system, which may initiate or worsen neurodegenerative disorders, such as Alzheimer’s disease (AD) [9]. Nonetheless, insufficient attention has been given to the potential association between AD and chronic rhinosinusitis (CRS), a chronic condition of the nasal cavity that can alter the homeostasis of the local microbiome.
2. Nasal Microbiota
The nasal cavity is exposed to vast amounts of bacteria and viruses during breathing ~7000 L of air each day. The inhaled air can be loaded with different viruses and bacteria [13][10]. The physical function of the nasal epithelium is to form a barrier between the internal and external environments (7), by detecting and eliminating pathogens to prevent them from initiating infection in underlying cells locally and reaching the lower respiratory tract [14][11]. This is why the nasal epithelium has innate immune defences, such as lysozyme, lactoferrin, IgM and IgA, to fight against these pathogens [15][12]. However, the nasal cavity is also a house for resident microbial communities that play an important role in maintaining a healthy environment and prevent infection and inflammation [16][13]. For example, for opportunistic pathogens, nasal commensal bacteria can inhibit the infection and further spreading of such pathogens by depriving them of space and nutrients, as well as actively secreting toxic chemicals to prevent their thriving; on the other hand, dysbiosis can increase the susceptibility to certain external pathogen infections, e.g., influenza [17][14]. Even for the current pandemic pathogen SARS-CoV-2 that primarily enters the human body via the nasal cavity that is also the first site to get infected, it is suggested that the local response of the nasal commensal bacteria may affect local nasal mucosal barrier integrity for bacterial entry into the circulation, and regulate systemic immune response (e.g., activation of T regulatory cells and myeloid-derived suppressor cells) and subsequent disease severity [17][14]. Although there has been no successful clinical trial targeting nasal bacteria for disease treatment, healthy commensal bacterial compositions are important in preventing airborne bacterial and viral infections.
The nasal microbiome, like other body microbial niches, develops throughout the human life span. Before birth, foetuses are developed in a sterile uterus. During birth, the newborn gets the first contact with microorganisms from the vaginal canal during natural birth, or through skin contact in the case of caesarean section [18][15]. The nasal and nasopharyngeal microbiota start to shape after birth [19][16]. Several factors play a vital role in shaping the early microbiome, such as breastfeeding and respiration [20][17]. However, the diversity of neonatal microbiota remains low at birth [21][18]. This bacterial diversity increases over the first few months until the age of three. Afterwards, bacteria in the upper respiratory tract become more stable and resemble that of adults [22][19].
The nasal cavity has a diverse microbial community. The healthy nasal cavity is colonised with Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria on the phylum level, and Bifidobacterium, Corynebacterium, Staphylococcus, Streptococcus, Dolosigranulum and Moraxella on the genus level [23,24][20][21]. The nasal cavity may also harbour some pathogenic bacteria in a healthy status, such as Staphylococcus aureus and Hemophilus influenza [25][22]. However, these are opportunistic bacteria, therefore, only cause significant illness if a person becomes immunocompromised [26][23].
Similar to the intestine, changes in nasal microbiome homeostasis may play a significant role in disease progressions, such as CRS, allergic rhinitis, and asthma [27][24]. This dysbiosis is characterised by a reduced population of beneficial bacteria and the overgrowth of pathogens. Bacterial dysbiosis may start at early infancy or develop later in life. For example, Teo et al. observed nasopharyngeal bacteria of infants in their first year and showed that certain bacterial composition in the nasopharynx is a predictor of future development of asthma in these infants, with Streptococcus species specifically the main contributor to this outcome [28][25]. This study highlighted the importance of the nasal microbiome composition in infants as an indicator of future chronic pulmonary inflammatory disease. Another case-control study highlighted the role of the nasal microbiota in early life in the development of allergies in the upper respiratory tract in infants [29][26]. Nasal microbiota diversity is increased with age in healthy children, whereas diversity is decreased with age in children with rhinitis [29][26]. Thus, the nasal microbiome may play an important role in developing inflammatory diseases in the respiratory tract.
Moreover, aging plays a critical role in shifting the nasal microbiome in health and disease. In adulthood, the microbiota of the nasal cavity is distinct from the microbial community in other parts of the upper respiratory tract; the microbial composition remains relatively constant throughout adulthood [30][27]. However, alteration in the nasal microbiota has been observed in middle-aged individuals. In healthy adults aged 40–65 years, the microbiota is altered and dominated by Staphylococcus, Cutibacterium and Corynebacterium [31][28]. The bacterial composition changes again in people aged 65 years and over, and is dominated by oropharyngeal bacteria [32,33][29][30]. The spread of bacteria from the distinct niche of the oropharynx upwards to the nasopharyngeal region can be due to the weakening of the immune system with aging (immunosenescence), leading to increased pro-inflammatory markers, lowered ability to manage immune stress, and the loss of bacterial niches and decreased bacterial diversity [32][29].
3. CRS, Nasal Microbiota and Their Influence on Neurological Health
CRS is a chronic inflammatory disease of the nasal cavity and sinuses that lasts for more than three months [34][31]. CRS is a debilitating disease that negatively affects life quality and poses an economic burden on the community. The disease is characterised by persistent inflammation of the nasal cavity and paranasal sinuses that results in symptoms of nasal obstruction, rhinorrhea, facial pain, headache and loss of smell (Figure 1) [35][32]. CRS is affected by three major factors: altered epithelial barrier and immunity, chronic inflammation, and nasal microbial dysbiosis (Figure 1) [36][33].
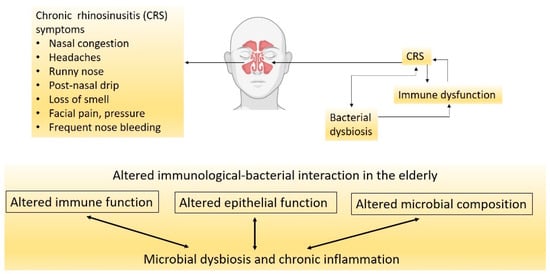

Figure 1. CRS is a multifactorial disease with the exact triggers not fully understood. Factors that cause bacterial dysbiosis could worsen with age by promoting immunological dysfunction.
Although limited studies are available, lower nasal microbial diversity has been found in patients with CRS [37][34]. At the phylum level, there is a decrease in Actinobacteria and Firmicutes and an increase in Proteobacteria numbers [38][35]. In terms of the species, there is an increase in opportunistic pathogenic bacteria, such as S. aureus and Corynebacterium tuberculosteriaticum [38][35]. Although only a few studies have investigated the nasal microbiome in the elderly with CRS, some evidence suggests immunological mechanisms leading to bacterial dysbiosis in this age group. Epithelial cell integrity and regeneration are impaired in adults with CRS and even to a greater extent in the elderly. For example, S100 family proteins involved in epithelial proliferation, repair and defence against pathogens are reduced with aging [39][36]. Moreover, the epithelial changes are involved in reduced mucociliary clearance in the healthy population aged 40 years and over, along with microtubules disarrangement [40,41][37][38]. Another study showed that mucociliary clearance has been significantly diminished in people older than 60 years with diabetes and hypertension, independent of smoking [42][39]. Similarly, a mouse study showed that mucociliary clearance was diminished in the upper and lower airways of elderly mice compared with young mice [43][40]. Moreover, elderly people with upper respiratory tract allergies have also presented with thinner nasal mucosa, which can be due to reduced blood flow to the nasal cavity and reduced mucus production [44][41]. Biopsy samples from the elderly also showed reduced thickness of the epithelium and basement membrane resulting in increased volume of the nasal cavity [45][42]. All these changes in the epithelium with aging can lead to less pathogen clearance, nasal dysbiosis and hence, the translocation of oropharyngeal microbiota to the nasal cavity.
In addition to physiological changes, immune responses are also altered in the elderly. For example, Cho et al. observed an age-dependent loss of immune function in the upper respiratory tract of CRS patients [39][36]. They showed that the eosinophilic inflammatory marker in the nasal cavity was significantly lower in CRS patients aged 60 years and over compared to that in younger patients, although eosinophilic infiltration was the same between the two groups [39][36]. The authors attributed this feature to eosinophils being less active in the older group. This suggests that there is an age-dependent loss in the function of the immune system in CRS patients. Thus, impaired immune response to new and probably existing pathogens in the elderly may be an important factor in their higher susceptibility to infections, the persistence of CRS, and probably the development of other inflammatory diseases in this population.
The interaction between the epithelium and bacterial residents may be a determinant of homeostasis or local inflammation. The sinonasal epithelium contains complex innate and adaptive immune pathways that drive inflammatory responses to pathogens in order to protect the host from exogenous or resident pathogen infections [46][43]. Any inappropriate activation or lack of inhibition of the immune system can lead to chronic inflammation [47][44]. Because bacterial pathogens are often observed in CRS, it is speculated that bacterial dysbiosis plays a vital role in initiating or contributing to chronic nasal inflammation [10][45]. Due to the proximity of the nasal cavity to the brain, inflammatory diseases in the nasal cavity, such as acute and chronic sinusitis, can initiate a wide range of neurological complications, including epidural abscess, meningitis, brain abscess, venous sinus thrombosis and orbital cellulitis [48][46]. These neural infections have common consequences, such as permanent visual changes and epilepsy [48][46]. The nasal-induced neural infections in the central nervous system suggest that the inflammatory environment in the nasal cavity can affect the brain. Hence, it is hypothesised that the inflammatory milieu in the nasal cavity can also lead to the initiation and/or the development of certain neurodegenerative diseases, such as AD.
4. CRS and AD—How Close Are They?
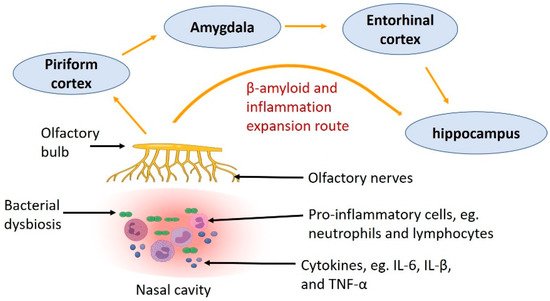
Researchers have investigated the correlation between dementia and CRS. A recent retrospective study following patients from 2006 to 2019 found that in patients with CRS, the risk of dementia was also increased [71][47]. This study followed patients with mild cognitive impairment with or without CRS for the study period, which found that patients with mild cognitive impairment and CRS were more likely to develop dementia than those with mild cognitive impairment but without CRS [71][47]. Similarly, a case-control Taiwanese study included 8768 dementia patients and confirmed significantly higher odds of CRS in the dementia population [72][48]. The authors suggested that CRS is associated with other comorbidities, such as stroke and vasculopathy, leading to an increased risk of vascular dementia. In addition, several other studies have also found a correlation between CRS and cognitive impairment [73,74,75][49][50][51]. Some studies, however, failed to find a correlation between AD and CRS [76][52]. This may be due to the variation in how CRS was diagnosed. In some cases, CRS was self-diagnosed, and even with clinical examination, it is hard to differentiate between CRS and rhinitis. Thus, computed tomography (CT) scans are needed to confirm the diagnosis, which is not routinely performed in every patient [77][53]. Another reason why studies failed to find a correlation between CRS and dementia is that dementia is a disease manifested in old age, and CRS symptoms can be improved with aging due to the alterations in the immune system. For example, Holmes et al. showed that the burden of CRS is higher in patients under 39 years of age than elderly patients [78][54]. Another study found that in CRS patients, the nasal epithelial barrier function is worsened with aging [39][36]. This reduced inflammatory burden of CRS in the aging population may make it harder to be recognised by the patients themselves, and subsequently identified and diagnosed by the clinicians. Even with impaired epithelial integrity in elderly patients, a bacterial infection in the sinuses cannot spread to the brain because of the blood-brain barrier; however, the inflammation can spread through the olfactory bulb and the olfactory neural system to reach the brain where the blood-brain barrier is lacking. Chronic inflammation is present in both CRS and AD, which may bridge CRS and the risk of neurodegeneration causing dementia [79][55]. It is well known that increased brain inflammation causes cognitive decline even before the onset of AD neuropathology, i.e., Aβ aggregation and tau hyperphosphorylation [80][56]. Inflammatory cytokines from active microglia and astrocytes impair cortical function and reduce hippocampal volume, which leads to memory and learning impairments [81][57]. Moreover, human brains with Aβ aggregations have microglial activation and increased pro-inflammatory cytokine production; increased circulating levels of inflammatory proteins, such as C-reactive protein, are also correlated with the presence of dementia [82,83][58][59]. During the early stages of AD, microglia and astrocytes are activated and able to clear Aβ, but the chronic activation of those cells has detrimental effects due to the secretion of inflammatory mediators tumour necrosis factor-α (TNF-α) and interleukin (IL)-6 [84][60]. These inflammatory mediators play crucial roles in the neurodegenerative process due to less Aβ clearance and increased accumulation. Interestingly, the role of inflammation in AD has been supported by a human study showing that AD risk was lowered by anti-inflammatory medications [85][61]. In CRS, the immune system is dysregulated, which may be the driving force for inflammation. The innate immune system is suppressed with decreased immunoglobulin J chain, antileukoproteinase, tertiary lymphoid structure and surfactant protein-A [86][62]. On the other hand, immune cells, such as eosinophils and basophils, are increased in patients with CRS [87][63]. Activation of these inflammatory cells leads to the recruitment of more cells, the polarisation of Th2 cells, and the production of inflammatory cytokines, such as IL-13, IL-5 and IL-4 [88][64]. Moreover, inflammation disrupts nasal epithelial cell regeneration through the inhibition of neural progenitor cell proliferation, which may aggravate CRS [89][65]. Such inflammatory response due to CRS can further affect the development of AD (Figure 2). A meta-analysis shows that dementia is associated with increased circulating inflammatory mediators, e.g., IL-6, IL-12, IL-18, TNF-α, IL-1β and transforming growth factor-β (TGF-β) [90][66]. IL-1β, IL-6, TNF-α and TGF-β are also elevated in the mucosa of patients with CRS [91,92,93,94][67][68][69][70]. However, it is unclear if increased cytokines in the nasal cavity can directly affect neural integrity in the central nervous system that leads to neurodegeneration.
Figure 2. The route showing how inflammation caused by nasal bacterial dysbiosis and Aβ deposition in the olfactory nerve in CRS spread to other parts of the central nervous system, through the olfactory bulb to the piriform cortex, amygdala, entorhinal cortex and hippocampus.
References
- Baquero, F.; Nombela, C. The microbiome as a human organ. Clin. Microbiol. Infect. 2012, 18 (Suppl. 4), 2–4.
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256.
- Hsieh, T.-H.; Kuo, C.-W.; Hsieh, K.-H.; Shieh, M.-J.; Peng, C.-W.; Chen, Y.-C.; Chang, Y.-L.; Huang, Y.-Z.; Chen, C.-C.; Chang, P.-K.; et al. Probiotics Alleviate the Progressive Deterioration of Motor Functions in a Mouse Model of Parkinson’s Disease. Brain Sci. 2020, 10, 206.
- Morshedi, M.; Hashemi, R.; Moazzen, S.; Sahebkar, A.; Hosseinifard, E.-S. Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: A systematic review. J. Neuroinflammation 2019, 16, 231.
- Yang, Z.; Li, J.; Gui, X.; Shi, X.; Bao, Z.; Han, H.; Li, M.D. Updated review of research on the gut microbiota and their relation to depression in animals and human beings. Mol. Psychiatry 2020, 25, 2759–2772.
- Collins, S.M. A role for the gut microbiota in IBS. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 497–505.
- Fazlollahi, M.; Lee, T.D.; Andrade, J.; Oguntuyo, K.; Chun, Y.; Grishina, G.; Grishin, A.; Bunyavanich, S. The nasal microbiome in asthma. J. Allergy Clin. Immunol. 2018, 142, 834–843.e2.
- Boutin, S.; Graeber, S.Y.; Weitnauer, M.; Panitz, J.; Stahl, M.; Clausznitzer, D.; Kaderali, L.; Einarsson, G.; Tunney, M.M.; Elborn, J.S.; et al. Comparison of microbiomes from different niches of upper and lower airways in children and adolescents with cystic fibrosis. PLoS ONE 2015, 10, e0116029.
- Dando, S.J.; Mackay-Sim, A.; Norton, R.; Currie, B.J.; St John, J.A.; Ekberg, J.A.; Batzloff, M.; Ulett, G.C.; Beacham, I.R. Pathogens penetrating the central nervous system: Infection pathways and the cellular and molecular mechanisms of invasion. Clin. Microbiol. Rev. 2014, 27, 691–726.
- Andualem, Z.; Gizaw, Z.; Bogale, L.; Dagne, H. Indoor bacterial load and its correlation to physical indoor air quality parameters in public primary schools. Multidiscip. Respir. Med. 2019, 14, 2.
- Jones, N. The nose and paranasal sinuses physiology and anatomy. Adv. Drug Deliv. Rev. 2001, 51, 5–19.
- Mellert, T.K.; Getchell, M.L.; Sparks, L.; Getchell, T.V. Characterization of the immune barrier in human olfactory mucosa. Otolaryngol. Head Neck Surg. 1992, 106, 181–188.
- Koskinen, K.; Reichert, J.L.; Hoier, S.; Schachenreiter, J.; Duller, S.; Moissl-Eichinger, C.; Schöpf, V. The nasal microbiome mirrors and potentially shapes olfactory function. Sci. Rep. 2018, 8, 1296.
- Di Stadio, A.; Costantini, C.; Renga, G.; Pariano, M.; Ricci, G.; Romani, L. The Microbiota/Host Immune System Interaction in the Nose to Protect from COVID-19. Life 2020, 10, 345.
- Jakobsson, H.E.; Abrahamsson, T.R.; Jenmalm, M.C.; Harris, K.; Quince, C.; Jernberg, C.; Björkstén, B.; Engstrand, L.; Andersson, A.F. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut 2014, 63, 559–566.
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial community variation in human body habitats across space and time. Science 2009, 326, 1694–1697.
- Biesbroek, G.; Bosch, A.A.; Wang, X.; Keijser, B.J.; Veenhoven, R.H.; Sanders, E.A.; Bogaert, D. The impact of breastfeeding on nasopharyngeal microbial communities in infants. Am. J. Respir. Crit. Care Med. 2014, 190, 298–308.
- Gritz, E.C.; Bhandari, V. The human neonatal gut microbiome: A brief review. Front. Pediatr. 2015, 3, 17.
- Mueller, N.T.; Bakacs, E.; Combellick, J.; Grigoryan, Z.; Dominguez-Bello, M.G. The infant microbiome development: Mom matters. Trends Mol. Med. 2015, 21, 109–117.
- Bassis, C.M.; Tang, A.L.; Young, V.B.; Pynnonen, M.A. The nasal cavity microbiota of healthy adults. Microbiome 2014, 2, 27.
- Stearns, J.C.; Davidson, C.J.; McKeon, S.; Whelan, F.J.; Fontes, M.E.; Schryvers, A.B.; Bowdish, D.M.; Kellner, J.D.; Surette, M.G. Culture and molecular-based profiles show shifts in bacterial communities of the upper respiratory tract that occur with age. ISME J. 2015, 9, 1246–1259.
- Dimitri-Pinheiro, S.; Soares, R.; Barata, P. The Microbiome of the Nose—Friend or Foe? Allergy Rhinol. 2020, 11, 2152656720911605.
- Riccardi, N.; Rotulo, G.A.; Castagnola, E. Definition of Opportunistic Infections in Immunocompromised Children on the Basis of Etiologies and Clinical Features: A Summary for Practical Purposes. Curr. Pediatr. Rev. 2019, 15, 197–206.
- Mahdavinia, M.; Keshavarzian, A.; Tobin, M.C.; Landay, A.L.; Schleimer, R.P. A comprehensive review of the nasal microbiome in chronic rhinosinusitis (CRS). Clin. Exp. Allergy 2016, 46, 21–41.
- Cope, E.K.; Goldberg, A.N.; Pletcher, S.D.; Lynch, S.V. Compositionally and functionally distinct sinus microbiota in chronic rhinosinusitis patients have immunological and clinically divergent consequences. Microbiome 2017, 5, 53.
- Ta, L.D.H.; Yap, G.C.; Tay, C.J.X.; Lim, A.S.M.; Huang, C.-H.; Chu, C.W.; De Sessions, P.F.; Shek, L.P.; Goh, A.; Van Bever, H.P.S.; et al. Establishment of the nasal microbiota in the first 18 months of life: Correlation with early-onset rhinitis and wheezing. J. Allergy Clin. Immunol. 2018, 142, 86–95.
- Bomar, L.; Brugger, S.D.; Lemon, K.P. Bacterial microbiota of the nasal passages across the span of human life. Curr. Opin. Microbiol. 2018, 41, 8–14.
- Ramakrishnan, V.R.; Feazel, L.M.; Gitomer, S.A.; Ir, D.; Robertson, C.E.; Frank, D.N. The microbiome of the middle meatus in healthy adults. PLoS ONE 2013, 8, e85507.
- Whelan, F.J.; Verschoor, C.P.; Stearns, J.C.; Rossi, L.; Luinstra, K.; Loeb, M.; Smieja, M.; Johnstone, J.; Surette, M.G.; Bowdish, D.M. The loss of topography in the microbial communities of the upper respiratory tract in the elderly. Ann. Am. Thorac. Soc. 2014, 11, 513–521.
- Schenck, L.P.; Surette, M.G.; Bowdish, D.M. Composition and immunological significance of the upper respiratory tract microbiota. FEBS Lett. 2016, 590, 3705–3720.
- Desrosiers, M.; Evans, G.A.; Keith, P.K.; Wright, E.D.; Kaplan, A.; Bouchard, J.; Ciavarella, A.; Doyle, P.W.; Javer, A.R.; Leith, E.S.; et al. Canadian clinical practice guidelines for acute and chronic rhinosinusitis. Allergy Asthma Clin. Immunol. 2011, 7, 2.
- Sedaghat, A.R. Chronic Rhinosinusitis. Am. Fam Physician 2017, 96, 500–506.
- Hamilos, D.L.M.D. Drivers of chronic rhinosinusitis: Inflammation versus infection. J. Allergy Clin. Immunol. 2015, 136, 1454–1459.
- Stevens, W.W.; Lee, R.J.; Schleimer, R.P.; Cohen, N.A. Chronic rhinosinusitis pathogenesis. J. Allergy Clin. Immunol. 2015, 136, 1442–1453.
- Abreu, N.A.; Nagalingam, N.A.; Song, Y.; Roediger, F.C.; Pletcher, S.D.; Goldberg, A.N.; Lynch, S.V. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci. Transl. Med. 2012, 4, 151ra124.
- Cho, S.H.; Hong, S.J.; Han, B.; Lee, S.H.; Suh, L.; Norton, J.; Lin, D.; Conley, D.B.; Chandra, R.; Kern, R.C.; et al. Age-related differences in the pathogenesis of chronic rhinosinusitis. J. Allergy Clin. Immunol. 2012, 129, 858–860.e2.
- Busse, P.J.; Mathur, S.K. Age-related changes in immune function: Effect on airway inflammation. J. Allergy Clin. Immunol. 2010, 126, 690–699.
- Ho, J.C.; Chan, K.N.; Hu, W.H.; Lam, W.K.; Ling, Z.; Tipoe, G.L.; June, S.U.N.; Leung, R.; Tsang, K.W. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am. J. Respir. Crit. Care Med. 2001, 163, 983–988.
- Proença de Oliveira-Maul, J.; Barbosa de Carvalho, H.; Goto, D.M.; Maia, R.M.; Fló, C.; Barnabé, V.; Franco, D.R.; Benabou, S.; Perracini, M.R.; Jacob-Filho, W.; et al. Aging, diabetes, and hypertension are associated with decreased nasal mucociliary clearance. Chest 2013, 143, 1091–1097.
- Grubb, B.R.; Livraghi-Butrico, A.; Rogers, T.D.; Yin, W.; Button, B.; Ostrowski, L.E. Reduced mucociliary clearance in old mice is associated with a decrease in Muc5b mucin. Am. J. Physiology. Lung Cell. Mol. Physiol. 2016, 310, L860–L867.
- Baptist, A.P.; Nyenhuis, S. Rhinitis in the Elderly. Immunol. Allergy Clin. N. Am. 2016, 36, 343–357.
- Loftus, P.A.; Wise, S.K.; Nieto, D.; Panella, N.; Aiken, A.; DelGaudio, J.M. Intranasal volume increases with age: Computed tomography volumetric analysis in adults. Laryngoscope 2016, 126, 2212–2215.
- Ramanathan, M., Jr.; Lane, A.P. Innate immunity of the sinonasal cavity and its role in chronic rhinosinusitis. Otolaryngol. Head Neck Surg. 2007, 136, 348–356.
- Lane, A.P. The role of innate immunity in the pathogenesis of chronic rhinosinusitis. Curr. Allergy Asthma Rep. 2009, 9, 205–212.
- Lee, S.; Lane, A.P. Chronic rhinosinusitis as a multifactorial inflammatory disorder. Curr. Infect. Dis. Rep. 2011, 13, 159–168.
- Ziegler, A.; Patadia, M.; Stankiewicz, J. Neurological Complications of Acute and Chronic Sinusitis. Curr. Neurol. Neurosci. Rep. 2018, 18, 5.
- Jung, H.J.; Lee, J.Y.; Choi, Y.S.; Choi, H.G.; Wee, J.H. Chronic rhinosinusitis and progression of cognitive impairment in dementia. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2021, 138, 147–151.
- Chung, S.-D.; Hung, S.-H.; Lin, H.-C.; Kang, J.-H. Dementia is associated with chronic rhinosinusitis: A population-based case-controlled study. Am. J. Rhinol. Allergy 2015, 29, 44–47.
- Tarasidis, G.S.; DeConde, A.S.; Mace, J.C.; Ashby, S.; Smith, T.L.; Orlandi, R.R.; Alt, J.A. Cognitive dysfunction associated with pain and quality of life in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2015, 5, 1004–1009.
- Matsui, T.; Arai, H.; Nakajo, M.; Maruyama, M.; Ebihara, S.; Sasaki, H.; Yoshida, Y.-i. Role of Chronic Sinusitis in Cognitive Functioning in the Elderly. J. Am. Geriatr. Soc. 2003, 51, 1818–1819.
- Jafari, A.; de Lima Xavier, L.; Bernstein, J.D.; Simonyan, K.; Bleier, B.S. Association of Sinonasal Inflammation With Functional Brain Connectivity. JAMA Otolaryngol.-Head Neck Surg. 2021, 147, 534–543.
- Yasue, M.; Sugiura, S.; Uchida, Y.; Otake, H.; Teranishi, M.; Sakurai, T.; Toba, K.; Shimokata, H.; Ando, F.; Otsuka, R.; et al. Prevalence of Sinusitis Detected by Magnetic Resonance Imaging in Subjects with Dementia or Alzheimer’s Disease. Curr. Alzheimer Res. 2015, 12, 1006–1011.
- Brook, C.D.; Kuperstock, J.E.; Rubin, S.J.; Ryan, M.W.; Platt, M.P. The Association of Allergic Sensitization with Radiographic Sinus Opacification. Am. J. Rhinol. Allergy 2017, 31, 12–15.
- Lal, D.; Keim, P.; Delisle, J.; Barker, B.; Rank, M.A.; Chia, N.; Schupp, J.M.; Gillece, J.D.; Cope, E.K. Mapping and comparing bacterial microbiota in the sinonasal cavity of healthy, allergic rhinitis, and chronic rhinosinusitis subjects. Int. Forum Allergy Rhinol. 2017, 7, 561–569.
- Wee, J.H.; Yoo, D.M.; Byun, S.H.; Hong, S.J.; Park, M.W.; Choi, H.G. Association between neurodegenerative dementia and chronic rhinosinusitis: A nested case-control study using a national health screening cohort. Medicine 2020, 99, e22141.
- Daulatzai, M.A. Quintessential Risk Factors: Their Role in Promoting Cognitive Dysfunction and Alzheimer’s Disease. Neurochem. Res. 2012, 37, 2627–2658.
- Ziehn, M.O.; Avedisian, A.A.; Tiwari-Woodruff, S.; Voskuhl, R.R. Hippocampal CA1 atrophy and synaptic loss during experimental autoimmune encephalomyelitis, EAE. Lab. Investig. 2010, 90, 774–786.
- Wang, W.-Y.; Tan, M.-S.; Yu, J.-T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136.
- Yarchoan, M.; Louneva, N.; Xie, S.X.; Swenson, F.J.; Hu, W.; Soares, H.; Trojanowski, J.Q.; Lee, V.M.Y.; Kling, M.A.; Shaw, L.M.; et al. Association of plasma C-reactive protein levels with the diagnosis of Alzheimer’s disease. J. Neurol. Sci. 2013, 333, 9–12.
- Kaur, D.; Sharma, V.; Deshmukh, R. Activation of microglia and astrocytes: A roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology 2019, 27, 663–677.
- Broe, G.A.; Grayson, D.A.; Creasey, H.M.; Waite, L.M.; Casey, B.J.; Bennett, H.P.; Brooks, W.S.; Halliday, G.M. Anti-inflammatory Drugs Protect Against Alzheimer Disease at Low Doses. Arch. Neurol. 2000, 57, 1586–1591.
- Brandtzaeg, P. Cells Producing Immunoglobulins and other Immune Factors in Human Nasal Mucosa. In Protides of the Biological Fluids; Peeters, H., Ed.; Elsevier: Amsterdam, The Netherland, 1985; Volume 32, pp. 363–366.
- Brescia, G.; Barion, U.; Zanotti, C.; Giacomelli, L.; Martini, A.; Marioni, G. The prognostic role of serum eosinophil and basophil levels in sinonasal polyposis. Int. Forum Allergy Rhinol. 2017, 7, 261–267.
- Hulse, K.E.; Stevens, W.W.; Tan, B.K.; Schleimer, R.P. Pathogenesis of nasal polyposis. Clin. Exp. Allergy 2015, 45, 328–346.
- Turner, J.H.; Liang, K.L.; May, L.; Lane, A.P. Tumor necrosis factor alpha inhibits olfactory regeneration in a transgenic model of chronic rhinosinusitis-associated olfactory loss. Am. J. Rhinol. Allergy 2010, 24, 336–340.
- Swardfager, W.; Lanctôt, K.; Rothenburg, L.; Wong, A.; Cappell, J.; Herrmann, N. A meta-analysis of cytokines in Alzheimer’s disease. Biol. Psychiatry 2010, 68, 930–941.
- Lennard, C.M.; Mann, E.A.; Sun, L.L.; Chang, A.S.; Bolger, W.E. Interleukin-1 beta, interleukin-5, interleukin-6, interleukin-8, and tumor necrosis factor-alpha in chronic sinusitis: Response to systemic corticosteroids. Am. J. Rhinol. 2000, 14, 367–373.
- Scheckenbach, K.; Wagenmann, M. Cytokine Patterns and Endotypes in Acute and Chronic Rhinosinusitis. Curr. Allergy Asthma Rep. 2016, 16, 3.
- Kou, W.; Hu, G.H.; Yao, H.B.; Wang, X.; Shen, Y.; Kang, H.Y.; Hong, S.L. Regulation of Transforming Growth Factor-β1 Activation and Expression in the Tissue Remodeling Involved in Chronic Rhinosinusitis. ORL 2012, 74, 172–178.
- von Bernhardi, R.; Cornejo, F.; Parada, G.E.; Eugenín, J. Role of TGFβ signaling in the pathogenesis of Alzheimer’s disease. Front. Cell. Neurosci. 2015, 9, 426.
More
