Microbial transglutaminase (mTG) is a heavily used food additive and its industrial transamidated complexes usage is rising rapidly. It was classified as a processing aid and was granted the GRAS (generally recognized as safe) definition, thus escaping full and thorough toxic and safety evaluations. Despite the manufacturers claims, mTG or its cross-linked compounds are immunogenic, pathogenic, proinflammatory, allergenic and toxic, and pose a risk to public health.
- microbial transglutaminase
- gluten
- celiac disease
- autoimmune disease
- neurodegenerative disease
- cross-linking
- posttranslational modification of proteins
- side effects
- safety
1. Introduction
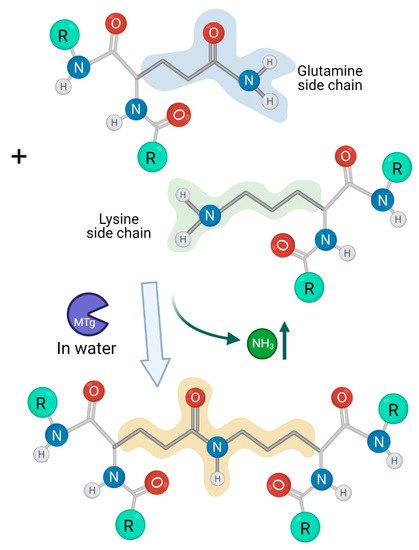
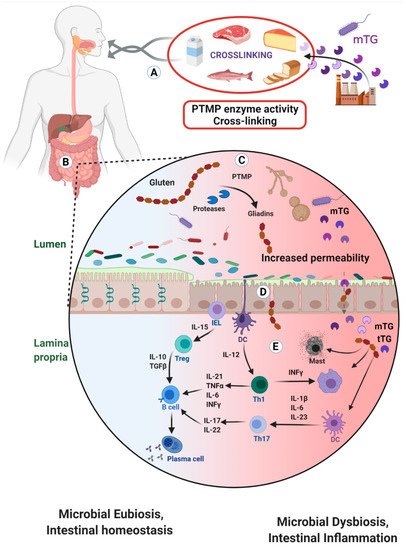
2. Microbial Transglutaminase Cross-Linked Complexes Are Pathogenic
2.1. Trans-Enterocytic Transport of Gliadin and mTG
2.2. Compromised Tight Junction Functional Integrity
-
Pathogenic prokaryotes are powerful disruptors of human intestinal permeability [28][29]. Since mTG present a survival factor for the luminal microbes and since the mTG compromises some basic enteric physical and immune protective mechanisms, it might support luminal and mucosal pathobionts activities;
-
Gliadins and gluten are known to open the tight junction gap by stimulating zonulin release [28]. As an integral part of the mTG-gliadin neo-complex, the gluten/gliadin part of the complex can drive gut permeability. It should be noticed that this mechanism is not only shared between the CD patient, but also by their closed relative and to some degree the broader normal population [30][31];
2.3. Enhances Enteric Epithelial Gliadins Uptake and Transportation
2.4. Suppression of Mechanical and Immunological Enteric Protective Barriers
2.5. Contributes to Luminal Microbiotic, Dysbiotic and Pathobiotic Proliferation
2.6. Potential mTG-Gliadin Complexes Uptake and Presentation by Mucosal Dendritic Cells
3. mTG in the Human Gut Lumen
-
Are the mTG-gliadins cross-linked complexes destroyed in the stomach? As mentioned above, those covalent iso-peptide bonds are extremely resistant to the luminal proteases, reducing agents and detergents;
-
Microbial transglutaminase is temperature dependent and is active up to 60° Celsius. In reality, many food products are not boiled before consumption or during processing, and some populations prefer eating raw meat. Just as a reminder, analyzing supermarket shelves’ meat and meat products, many were found to contain transglutaminase [59]. Intriguingly, mTG gliadin docked complexes turn more immunogenic when heated to 90° Celsius [10][17]. It is logical to speculate that during denaturation, epitopes are exteriorized and are exposed to the immune system. Regarding mTG activity and temperature, the newly identified cold Atlantic cod TG opens a new area of thermostable mTG application for boiled/heated/cooked food product’s manufacturing [60];
-
Microbial transglutaminase is active at pH-4.0 and above. However, gastric physiology and pathophysiology show that upon eating or post-prandially, gastric acidity is neutralized. Large pediatric, adult and elderly people are chronically consuming acid suppressor medications, infants and elderly have higher gastric pH and alkaline reflux is not rare. Notably, the stomach pH is differentially distributed and some areas are less acidic [10]. In summary, it is suggested that active mTG can execute its functions in the duodenum, small and large bowel. The cross-linked complexes are created ex-vivo, while processing the food, they are stomach passage resistant and are immunogenic.
4. Should mTG Usage Be Labeled and Declared on Food Products?
For decades, the American regulatory authorities, the FDA, classified mTG in the GRAS category. They followed the manufacturers’ declarations on mTG being non-toxic, safe, non-allergenic, non-immunogenic and non-pathogenic for public health [3][12][18]. The topic of industrial enzyme production, usage and safety of genetically modified micro-organisms is the subject of intense debate, while continental and national discrepancies are wide [60][61][62][63][64][65][66][67][68]. Multiple issues are raised and the antibiotic resistance gene is of concern [52][61][62][63]. In view of continuous efforts to bioengineer more cost-efficient mTG for industrial applications [8][69][48][49][50][51][60] and in view of the all the detrimental effects of mTG and its trans-amidated complexes used for food processing (Figure 2), public health against the side effects of mTG should be a prime priority. The worldwide food and industrial safety regulatory authorities should reassess the updated observations; hence, consider the alleviation of the GRAS status and enforce the labelling of this heavily used processed food additive.
5. Should the Customers Be Warned for a Potential Health Risk of mTG Consumption?
Based on the widely criticized GRAS category, the detrimental effects of the mTG and its cross-linked complexes and the updated scientific literature, the national and international food regulatory authorities should reassess the “processing aid” classification of the enzyme. The mTG should be labeled as a food ingredient and meet standards that require maintaining public health.
6. Warnings for Use of Microbial Transglutaminase
References
- Yu, J.; Pian, Y.; Ge, J.; Guo, J.; Zheng, Y.; Jiang, H.; Hao, H.; Yuan, Y.; Jiang, Y.; Yang, M. Functional and structural characterization of the antiphagocytic properties of a novel transglutaminase from streptococcus suis. J. Biol. Chem. 2015, 290, 19081–19092.
- Xia, X.; Qin, W.; Zhu, H.; Wang, X.; Jiang, J.; Hu, J. How Streptococcus suis serotype 2 attempts to avoid attack by host immune defenses. J. Microbiol. Immunol. Infect. 2019, 52, 516–525.
- Lerner, A.; Matthias, T. Microbial Transglutaminase is Beneficial to Food Industries but a Caveat to Public Health. Med One 2019, 4, e190001.
- Doti, N.; Caporale, A.; Monti, A.; Sandomenico, A.; Selis, F.; Ruvo, M. A recent update on the use of microbial transglutaminase for the generation of biotherapeutics. World J. Microbiol. Biotechnol. 2020, 36, 1–14.
- Miwa, N. Innovation in the food industry using microbial transglutaminase: Keys to success and future prospects. Anal. Biochem. 2020, 597, 113638.
- Deweid, L.; Avrutina, O.; Kolmar, H. Microbial transglutaminase for biotechnological and biomedical engineering. Biol. Chem. 2019, 400, 257–274.
- Steffen, W.; Ko, F.C.; Patel, J.; Lyamichev, V.; Albert, T.J.; Benz, J.; Rudolph, M.G.; Bergmann, F.; Streidl, T.; Kratzsch, P.; et al. Discovery of a microbial transglutaminase enabling highly site-specific labeling of proteins. J. Biol. Chem. 2017, 292, 15622.
- Chan, S.K.; Lim, T.S. Bioengineering of microbial transglutaminase for biomedical applications. Appl. Microbiol. Biotechnol. 2019, 103, 2973–2984.
- Lerner, A.; Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 2015, 14, 479–489.
- Lerner, A.; Matthias, T. Microbial transglutaminase should be considered as an environmental inducer of celiac disease. World J. Clin. Cases 2019, 7, 3912–3914.
- Lerner, A.; Matthias, T. Microbial transglutaminase: A new potential player in celiac disease. Clin. Immunol. 2019, 199, 37–43.
- Lerner, A.; Matthias, T. Processed food additive microbial transglutaminase and its cross-linked gliadin complexes are potential public health concerns in celiac disease. Int. J. Mol. Sci. 2020, 21, 1127.
- Matthias, T.; Lerner, A. Microbial Transglutaminase Is Immunogenic and Potentially Pathogenic in Pediatric Celiac Disease. Front. Pediatr. 2018, 6, 389.
- Lerner, A.; Matthias, T. Don’t forget the exogenous microbial transglutaminases: It is immunogenic and potentially pathogenic. AIMS Biophys. 2016, 3, 546–552.
- Lerner, A.; Benzvi, C. “Let Food Be Thy Medicine”: Gluten and Potential Role in Neurodegeneration. Cells 2021, 10, 756.
- Martin, A.; de Vivo, G.D.; Ricotta, M.; Iannuzzi, M.; Gentile, V. Transglutaminases as possible therapeutic targets in neurodegenerative diseases. Recent Pat. CNS Drug Discov. 2010, 3, 195–202.
- Stricker, S.; De Laffolie, J.; Rudloff, S.; Komorowski, L.; Zimmer, K.P. Intracellular localization of microbial transglutaminase and its influence on the transport of gliadin in enterocytes. J. Pediatr. Gastroenterol. Nutr. 2019, 68, e43–e50.
- Lerner, A.; Matthias, T. Possible association between celiac disease and bacterial transglutaminase in food processing: A hypothesis. Nutr. Rev. 2015, 73, 544–552.
- Lerner, A.; Aminov, R.; Matthias, T. Transglutaminases in Dysbiosis as Potential Environmental Drivers of Autoimmunity. Front. Microbiol. 2017, 8, 66.
- Chen, L.; Ullah, N.; Li, C.; Hackman, R.M.; Li, Z.; Xu, X.; Guanghong Zhou, G.; Feng, X. Incorporated glucosamine adversely affects the emulsifying properties of whey protein isolate polymerized by transglutaminase. J. Dairy Sci. 2017, 100, 3413–3423.
- Hu, X.; Ren, J.; Zhao, M.; Cui, C.; He, P. Emulsifying properties of the transglutaminase-treated crosslinked product between peanut protein and fish (Decapterus maruadsi) protein hydrolysates. J. Sci. Food Agric. 2010, 91, 578–585.
- Li, F.; Lu, J.; Kong, X.; Hyeon, T.; Ling, D. Dynamic Nanoparticle Assemblies for Biomedical Applications. Adv. Mater. 2017, 29, 1605897.
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K. Application of nanotechnology in food science: Perception and overview. Front. Microbiol. 2017, 8, 1501.
- Faust, J.J.; Masserano, B.M.; Mielke, A.H.; Abraham, A.; Capco, D.G. Engineered nanoparticles induced brush border disruption in a human model of the intestinal epithelium. Adv. Exp. Med. Biol. 2014, 811, 55–72.
- Lama, S.; Merlin-Zhang, O.; Yang, C. In vitro and in vivo models for evaluating the oral toxicity of nanomedicines. Nanomaterials 2020, 10, 2177.
- Wang, J.H.; Tang, M.Z.; Yu, X.T.; Xu, C.M.; Yang, H.M.; Tang, J.B. Site-specific, covalent immobilization of an engineered enterokinase onto magnetic nanoparticles through transglutaminase-catalyzed bioconjugation. Colloids Surf. B Biointerfaces 2019, 177, 506–511.
- Ma, T.; Lu, J.; Zhu, J.; Li, X.; Gu, H.; Montalbán-López, M.; Wu, X.; Luo, S.; Zhao, Y.; Jiang, S.; et al. The Secretion of Streptomyces monbaraensis Transglutaminase from Lactococcus lactis and Immobilization on Porous Magnetic Nanoparticles. Front Microbiol. 2019, 10, 1675.
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175.
- Sanders, D.J.; Inniss, S.; Sebepos-Rogers, G.; Rahman, F.Z.; Smith, A.M. The role of the microbiome in gastrointestinal inflammation. Biosci. Rep. 2021, 41, BSR20203850.
- Hollon, J.; Puppa, E.L.; Greenwald, B.; Goldberg, E.; Guerrerio, A.; Fasano, A. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with Non-Celiac gluten sensitivity. Nutrients 2015, 7, 1565–1576.
- Cardoso-Silva, D.; Delbue, D.; Itzlinger, A.; Moerkens, R.; Withoff, S.; Branchi, F.; Schumann, M. Intestinal barrier function in gluten-related disorders. Nutrients 2019, 11, 2325.
- Kumar, M.D.; Singh, A.K.; Shama, H.; Deshwal, G.K. Histone cross-linking by transglutaminase. Biochem. Biophys. Res. Commun. 2002, 293, 1453–1457.
- Dieli-Crimi, R.; Cénit, M.C.; Núñez, C. The genetics of celiac disease: A comprehensive review of clinical implications. J. Autoimmun. 2015, 64, 26–41.
- Perry, A.S.; Baird, A.M.; Gray, S.G. Epigenetic methodologies for the study of celiac disease. Methods Mol. Biol. 2015, 1326, 131–158.
- Miyoshi, Y.; Tanabe, S.; Suzuki, T. Cellular zinc is required for intestinal epithelial barrier maintenance via the regulation of claudin-3 and occludin expression. Am. J. Physiol. Liver Physiol. 2016, 311, G105–G116.
- Lerner, A.; Neidhöfer, S.; Matthias, T. The Gut Microbiome Feelings of the Brain: A Perspective for Non-Microbiologists. Microorganisms 2017, 5, 66.
- Boukhettala, N.; Claeyssens, S.; Bensifi, M.; Maurer, B.; Abed, J.; Lavoinne, A.; Déchelotte, P.; Coëffier, M. Effects of essential amino acids or glutamine deprivation on intestinal permeability and protein synthesis in HCT-8 cells: Involvement of GCN2 and mTOR pathways. Amino Acids 2012, 42, 375–383.
- Obrenovich, M. Leaky Gut, Leaky Brain? Microorganisms 2018, 6, 107.
- Lerner, A.; Makhoul, B.F.; Eliakim, R. Neurological Manifestations of Celiac Disease in Children and Adults Affiliations Celiac disease and environment View project Neurological Manifestations of Celiac Disease in Children and Adults. Eur. Neurol. J. 2012, 4, 15–20.
- Zelnik, N.; Pacht, A.; Obeid, R.; Lerner, A. Range of neurologic disorders in patients with celiac disease. Pediatrics 2004, 113, 1672–1676.
- Vojdani, A.; Lerner, A.; Vojdani, E. Cross-Reactivity and Sequence Homology between Al-Pha-Synuclein and Food Products: A Step Further for Parkinson’s Disease Synucleinopathy. Cells 2021, 10, 1111.
- Lerner, A.; Sobolevskaia, P.; Churilov, L.; Shoenfeld, Y. Alpha-enolase involvement in intestinal and extraintestinal manifestations of celiac disease. J. Transl. Autoimmun. 2021, 4, 100109.
- Gatta, N.G.; Cammarota, G.; Iannaccone, M.; Gentile, V. Transglutaminase Activity as a Possible Molecular Mechanism in the Etiopathogenesis of Neurodegenerative Diseases. J. Biochem. Mol. Biol. Res. 2016, 2, 157–165.
- Lebreton, C.; Ménard, S.; Abed, J.; Moura, I.C.; Coppo, R.; Dugave, C.; Monteiro, R.C.; Fricot, A.; Traore, M.G.; Martin Griffin, M. Interactions among secretory immunoglobulin A, CD71, and transglutaminase-2 affect permeability of intestinal epithelial cells to gliadin peptides. Gastroenterology 2012, 143, 698–707.e4.
- Recktenwald, C.V.; Hansson, G.C. The reduction-insensitive bonds of the MUC2 mucin are isopeptide bonds. J. Biol. Chem. 2016, 291, 13580–13590.
- Pian, Y.; Wang, P.; Liu, P.; Zheng, Y.; Zhu, L.; Wang, H.; Xu, B.; Yuan, Y.; Jiang, Y. Proteomics identification of novel fibrinogen-binding proteins of Streptococcus suis contributing to antiphagocytosis. Front. Cell. Infect. Microbiol. 2015, 5, 19.
- Xu, B.; Zhang, P.; Li, W.; Liu, R.; Tang, J.; Fan, H. hsdS, belonging to the type i restriction-modification system, contributes to the streptococcus suis serotype 2 survival ability in phagocytes. Front. Microbiol. 2017, 8, 1524.
- Fu, R.Y.; Chen, J.; Li, Y. Heterologous leaky production of transglutaminase in Lactococcus lactis significantly enhances the growth performance of the host. Appl. Environ. Microbiol. 2005, 71, 8481–8490.
- Fu, R.Y.; Chen, J.; Li, Y. Influence of expression of transglutaminase on the growth of Lactococcus lactis. Wei Sheng Wu Xue Bao 2005, 45, 510–515.
- Zhang, N.; Zhang, S.; He, Y.; Chen, X.; Zhang, Y.; Dong, Z. Intein-mediated intracellular production of active microbial transglutaminase in Corynebacterium glutamicum. Enzym. Microb. Technol. 2020, 142, 109680.
- Rickert, M.; Strop, P.; Lui, V.; Melton-Witt, J.; Farias, S.E.; Foletti, D.; Shelton, D.; Jaume Pons, J.; Rajpal, A. Production of soluble and active microbial transglutaminase in Escherichia coli for site-specific antibody drug conjugation. Protein Sci. 2016, 25, 442–455.
- Lerner, A.; Matthias, T.; Aminov, R. Potential effects of horizontal gene exchange in the human gut. Front. Immunol. 2017, 8, 1630.
- Mann, E.R.; Li, X. Intestinal antigen-presenting cells in mucosal immune homeostasis: Crosstalk between dendritic cells, macrophages and B-cells. World J Gastroenterol. 2014, 20, 9653–9664.
- Stagg, A.J. Intestinal Dendritic Cells in Health and Gut Inflammation. Front. Immunol. 2018, 9, 2883.
- Bekiaris, V.; Persson, E.K.; Agace, W.W. Intestinal dendritic cells in the regulation of mucosal immunity. Immunol. Rev. 2014, 260, 86–101.
- Chrobok, N.L.; Sestito, C.; Wilhelmus, M.M.M.; Drukarch, B.; van Dam, A.M. Is monocyte- and macrophage-derived tissue transglutaminase involved in inflammatory processes? Amino Acids 2017, 49, 441–452.
- Ráki, M.; Schjetne, K.W.; Stamnaes, J.; Molberg Jahnsen, F.L.; Issekutz, T.B.; Bogen, B.; Sollid, L.M. Surface expression of transglutaminase 2 by dendritic cells and its potential role for uptake and presentation of gluten peptides to T cells. Scand. J. Immunol. 2007, 65, 213–220.
- Hodrea, J.; Demény, M.Á.; Majai, G.; Sarang, Z.; Korponay-Szabó, I.R.; Fésüs, L. Transglutaminase 2 is expressed and active on the surface of human monocyte-derived dendritic cells and macrophages. Immunol. Lett. 2010, 130, 74–81.
- Kaufmann, A.; Köppel, R.; Widmer, M. Determination of microbial transglutaminase in meat and meat products. Food Addit. Contam. Part. A 2012, 29, 1364–1373.
- Lerner, A.; Ramesh, A.; Matthias, T. The temperature and pH repertoire of the transglutaminase family is expanding. FEBS Open Bio 2020, 10, 492–494.
- Deckers, M.; Vanneste, K.; Winand, R.; Keersmaecker, S.C.J.D.; Denayer, S.; Heyndrickx, M.; Deforce, D.; Fraiture, M.A.; Roosens, N.H.C. Strategy for the identification of micro-organisms producing food and feed products: Bacteria producing food enzymes as study case. Food Chem. 2020, 305, 125431.
- Fraiture, M.A.; Deckers, M.; Papazova, N.; Roosens, N.H.C. Are antimicrobial resistance genes key targets to detect genetically modified microorganisms in fermentation products? Int. J. Food Microbiol. 2020, 331, 108749.
- Cheng, G.; Ning, J.; Ahmed, S.; Huang, J.; Ullah, R.; An, B.; Hao, H.; Dai, M.; Huang, L.; Wang, X.; et al. Selection and dissemination of antimicrobial resistance in Agri-food production. Antimicrob. Resist. Infect. Control. 2019, 8, 1–13.
- Paul, R.H.; Frestedt, J.; Magurany, K. GRAS from the ground up: Review of the Interim Pilot Program for GRAS notification. Food Chem Toxicol. 2017, 105, 140–150.
- Burdock, G.A.; Carabin, I.G. Generally recognized as safe (GRAS): History and description. Toxicol. Lett. 2004, 150, 3–18.
- Hallagan, J.B.; Hall, R.L.; Drake, J. The GRAS provision—The FEMA GRAS program and the safety and regulation of flavors in the United States. Food Chem. Toxicol. 2020, 138, 111236.
- Faustman, C.; Aaron, D.; Negowetti, N.; Leib, E.B. Ten years post-GAO assessment, FDA remains uninformed of potentially harmful GRAS substances in foods. Crit. Rev. Food Sci. Nutr. 2021, 61, 1260–1268.
- Sewalt, V.; LaMarta, J.; Shanahan, D.; Gregg, L.; Carrillo, R. Letter to the editor regarding “GRAS from the ground up: Review of the Interim Pilot Program for GRAS notification” by Hanlon et al., 2017. Food Chem. Toxicol. 2017, 107, 520–521.
- Duarte, L.; Matte, C.R.; Bizarro, C.V.; Ayub, M.A.Z. Review transglutaminases: Part II—Industrial applications in food, biotechnology, textiles and leather products. World J. Microbiol. Biotechnol. 2020, 36, 11.
- Dekking, E.H.A.; Van Veelen, P.A.; de Ru, A.; Kooy-Winkelaar, E.M.C.; Gröneveld, T.; Nieuwenhuizen, W.F.; Koning, F. Microbial transglutaminases generate T cell stimulatory epitopes involved in celiac disease. J. Cereal Sci. 2008, 47, 339–346.
- Santos, M.; Torne, J. Recent Patents on Transglutaminase Production and Applications: A Brief Review. Recent Pat. Biotechnol. 2009, 3, 166–174.
- Cabrera-Chávez, F.; Rouzaud-Sández, O.; Sotelo-Cruz, N.; Calderón De La Barca, A.M. Transglutaminase treatment of wheat and maize prolamins of bread increases the serum IgA reactivity of celiac disease patients. J. Agric. Food Chem. 2008, 56, 1387–1391.
- Malandain, H. Transglutaminases: A meeting point for wheat allergy, celiac disease, and food safety. Allerg. Immunol. 2005, 37, 397–403.
- Skovbjerg, H.; Norén, O.; Anthonsen, D.; Moller, J.; Sjöström, H. Gliadin is a good substrate of several transglutaminases: Possible implication in the pathogenesis of coeliac disease. Scand. J. Gastroenterol. 2002, 37, 812–817.
- Gerrard, J.A.; Sutton, K.H. Addition of transglutaminase to cereal products may generate the epitope responsible for coeliac disease. Trends Food Sci. Technol. 2005, 16, 510–512.
- Gerrard, J.A.; Cottam, J.R. Protein Cross-linking in Food—Structure, Applications, Implications for Health and Food Safety. In Food Biochemistry and Food Processing; Wiley: Hoboken, NJ, USA, 2012; pp. 207–222.
- Kumar, M.D.; Singh, A.K.; Sharma, H.; Deshwal, G.K. Promising Scope of Transglutaminase as Processing Aid in Food Industries. Food Sci. Rep. 2020, 1, 53–55.
- SR 817.022.108—Verordnung des EDI vom 23. November 2005 Über Lebensmittel Tierischer Herkunft . Available online: https://www.fedlex.admin.ch/eli/cc/2005/801/de (accessed on 23 August 2021).
