Therapeutic bacteriophages, commonly called as phages, are a promising potential alternative to antibiotics in the management of bacterial infections of a wide range of organisms including cultured fish. Their natural immunogenicity often induces the modulation of a variated collection of immune responses within several types of immunocytes while promoting specific mechanisms of bacterial clearance.
- aquaculture
- bacteriophages
- disease management
- fish
- immunology
- lytic enzymes
- pathogens
1. Phage Biology and Spatial Distribution
2. Phage’s Life Cycle
The phages like any other viruses depend on the metabolism of their bacterial host for reproduction. During the reproductive process, most phage types completely consume the resources of their host and kill them when releasing their progeny [6]. Initially, phages must infect their host bacteria through the binding of specific receptors that selectively sense specific components of the target bacterial cell wall such as the lipopolysaccharide in Gram-negative, or peptidoglycan in Gram-positive, capsular polysaccharides, and superficial appendages such as pili and flagella [7,8,9]. Following the classical viral reproductive strategies, once the phage inserts their nucleic acid into the bacterium’s cytoplasm, the host cellular machinery is highjacked to induce extensive replication through the lytic cycle (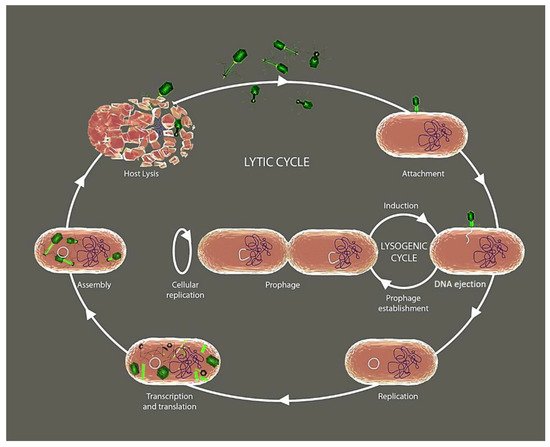
3. Phage Lytic Enzymes and Depolymerases
Lysins derived from phages degrade bacterial peptidoglycans and are classified into five groups, depending on the bonds these enzymatic proteins cleave in the bacterial peptidoglycan [16][11]. Although their function is exclusively to degrade the cell wall of bacteria, the lytic enzymes of phages present a tremendous structural diversity and a significant number of different mechanisms of action [17,18,19,20][12][13][14][15].
In general, lysins are more likely to lyse Gram-positive bacteria because their cell wall peptidoglycan is directly exposed on the cell surface unlike Gram-negative bacteria. However, the study of phages or their lysins has been limited to a few fish pathogens such as Streptococcus agalactiae, Lactococcus garvieae, Renibacterium salmoninarum, Streptococcus iniae, and S. dysgalactiae, which are highly associated with disease outbreaks in fish farms.
4. Interactions between Phage and the Fish Immune System
4.1. Phage-Mediated Activation of Inflammation
4.1. Phage-Mediated Activation of Inflammation
Bacteriophage treatment was associated with opposite shifts in the inflammatory response in several test models, both in vivo and in vitro [16][17][18][19]. However, the results seem to depend not only on the cellular or animal model used but also on the type of phage applied and the panel of cytokines analyzed. Phage therapy in humans can also modify the levels of some cytokines produced by blood cells in treated patients [20]. In fish, some researchers have analyzed the cytokines’ response to the presence of bacteriophages alone or the coinfection of phages with their target bacteria. For example, phage therapy reduced the expression of the proinflammatory cytokines tnfa and il1b in the inflammatory response generated by Pseudomonas aeruginosa infection in zebrafish embryos [21][22]. Besides, using the adult zebrafish (Danio rerio) and the E. tarda model of infection, other authors also showed that although a phage treatment induced the expression of cytokine genes at specific time points, a robust proinflammatory response was undetected in the host [23]. Furthermore, a recent study has shown that a phage lysate of A. hydrophila induced a more robust immune response in Cyprinus carpio when compared to a formalin killed vaccine [24]. As a proof-of-concept, a novel commercial preparation containing three bacterial phages (BAFADOR4.2. Phage-Specific Adaptive Responses
4.2. Phage-Specific Adaptive Responses
Due to the protein structure of the phage envelope, these proteins are the target of the adaptive immune system, which response with the production of neutralizing antibodies against them. Early studies with mice and even amphibians showed that phage exposure of the animals induced primary and secondary antibody responses [26][27][28]. It is expected that some phage epitopes stimulate an antibody response in experimental models. However, antibody production depends on the route of phage administration, the application schedule and dose, and individual features of a phage. Consequently, the results of studies where an antibody response to phages has been verified are very heterogeneous. Phagocytosis by immune patrolling cells seems to be a significant process of bacteriophage neutralization within animal bodies [29]. Moreover, although blood in humans and animals, including fish, is deemed sterile, genomic analysis has shown a rich phage community, which inevitably comes into continuous contact with immune cells in this rich fluid [30]. Despite these mechanisms of phagocytosis, antigen presentation, and antibody production by the immune cells against phages, the number of antibodies produced does not affect phage therapy outcomes. On the other hand, due to the numerous and constant presence of large numbers of phages in our microbiota, it is not surprising that a low but stable background of antibodies against them is produced. Therefore, in some human or animal tests, high antibody levels have not been found against the phages used. Phage-derived RNA and ssDNA could directly contribute to B cell activation and the synthesis of anti-bacteriophage antibodies [31][32]. Despite the production of antibodies by animals against phage core or tail proteins, the induction of antibodies seems irrelevant for treating infections because the antibacterial effects of phages are faster than antibody formation in acute infections [33]. Conversely, the production of antibodies against phages could interfere with the outcome of the infection in chronic infections [34]. However, no robust studies have demonstrated an antibody-mediated immune response after inoculation or experimental infection with phages in fish.5. Potential of Phage Therapy in Aquaculture Settings
During the fish and shellfish production cycle, these animals are already in daily contact with billions of bacteriophages, which assures us that they are safe. However, in their use against bacterial infections where massive phage production is required, we must consider several factors. As phage treatments constantly require isolating the bacterium causing the disease, once a helpful phage is characterized against this bacterial strain, a stable batch of technically challenging preparations must be produced for field use. Consequently, one of the most critical challenge for microbiologists working directly or indirectly with aquaculture is the standardization of stocks used to treat infections or combat biofilms in aquaculture facilities. These stocks require strict quality control for purity, viability, and stability, implying that the correct conservation of the stocks is necessary for preparations containing single or mixed phages (phage cocktail). Titer, dosage, and quality of phage preparations are crucial parameters in standardizing experiments in the laboratory and experimental infections in field trials. Since we know that while some phages can grow exponentially inside a bacterial population from a low initial concentration, other phages need to maintain a relationship between the number of bacteria and the number of phage particles to achieve an adequate performance. Therefore, we must empirically verify this critical parameter. Very recently, a phage cocktail containing seven bacteriophages (three against A. hydrophila and four against P. fluorescens) has been tested in the European eel (Anguilla anguilla) and rainbow trout (Oncorhynchus mykiss), reducing the mortality of fish challenged with strains of these two species of bacteria [56,197]. Cocktails have also been used successfully in laboratory tests or small field trials in food protection or veterinary and human medicine [198,199,200,201]. In these and other studies, many phages (cocktail) are used to carry out the experiments, but in most cases, only the phage that has presented better results in vitro is subsequently characterized [117,118,133,202]. Second, it would be desirable to know phage genetics with sufficient precision. After all, we must consider that when we intend to use bacteriophages in aquaculture, they may contain genes for resistance to antibiotics or bacterial virulence genes that can produce noticeable side effects because they replicate exponentially in contact with their target bacteria. We must also remember that many antibiotic residues end up in continental or oceanic waters due to anthropogenic activities. Therefore, we must be aware that even phages isolated from aquatic environments can carry antibiotic resistance genes or virulence factors [203,204]. At present, although each time their number increases, not all phages used in in vitro or in vivo assays against fish or shellfish bacterial pathogens have been entirely genetically analyzed or characterized (| Gram-Negative Targets |
Source | Enrichment ɸ | Characterization Method | Phage Strains Name | Family * | Genome Length | References |
|---|---|---|---|---|---|---|---|
| Aeromonas hydrophila | River water | No | TEM | ɸ2 and ɸ5 | Myoviridae | ~20 kb | [52] |
| Fishponds; Polluted rivers | Single | TEM | N21, W3, G65, Y71 and Y81 | Myoviridae; Podoviridae | n.d. | [53] | |
| Stream water | Single | TEM, dsDNA | pAh-1 | Myoviridae | ~64 kb | [54] | |
| Sea water | Single | TEM, DNA sequencing | Akh-2 | Siphoviridae | 114,901 bp | [55] | |
| Carp tissues | Single | TEM | AHP-1 | Myoviridae | n.d. | [56] | |
| Lake water | Single | TEM, dsDNA, DNA sequencing | AhyVDH1 | Myxoviridae | 39,175 bp | [57] | |
| River water | No | TEM, dsDNA, DNA sequencing | MJG | Podoviridae | 45,057 bp | [58] | |
| Sewage water | Single | TEM | AH1 | n.d. | n.d. | [59] | |
| Striped catfish pond water | Single | TEM, dsDNA, DNA sequencing | PVN02 | Myoviridae | 51,668 bp | [60][61] | |
| River water | TEM, dsDNA | pAh1-C pAh6-C |
Myoviridae | 55 kb 58 kb |
[62] | ||
| Wastewater | |||||||
| CHOED | |||||||
| ALME | |||||||
| CHOD | |||||||
| CHOB | |||||||
| Several shapes | |||||||
| ~47–48 kb | |||||||
| [ | |||||||
| 98 | |||||||
| ] | |||||||
| Sewage water | Double | dsDNA | VP-2 VA-1 |
n.d. | n.d. | [51] | |
| Water samples from fish farms | |||||||
| [ | |||||||
| 130 | |||||||
| ] | |||||||
| Yersinia ruckeri | |||||||
| Wastewater containing suspended trout feces from a settling pond at a trout farm | |||||||
| Single | |||||||
| TEM | |||||||
| NC10 | |||||||
| Podoviridae | |||||||
| n.d. | |||||||
| [ | |||||||
| 49 | |||||||
| ] | |||||||
| Sewage | No | TEM | YerA41 (several phages) | icosahedral head, contractile tail | n.d. | [131] | |
| Sewage | No | TEM, DNA sequencing, dsDNA | R1-37 | Myoviridae | ~270 kb | [132][133] |
| Gram-Positive Targets | Source | Enrichment ɸ | Characterization Method | Phage Strains Name | Family * | Genome Length | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactococcus garvieae | L. garvieae isolated from diseased yellowtail | No | TEM, dsDNA | PLgY(16) | Siphoviridae | n.d. | [134] | |||||
| Yellowtail (Y) Water (W) Sediments (S) |
Single | TEM, dsDNA | PLgW1-6 PLgY16 PLgY30 PLgY886 PLgS1 |
Siphoviridae | >20 kbp | [135][136][137] | ||||||
| Domestic compost | Single | TEM, DNA sequencing | GE1 | Siphoviridae | 24,847 bp | [138] | ||||||
| L. garvieae host | No | TEM, DNA sequencing | PLgT-1 | Siphoviridae | 29,284 bp | [139][140][141] | ||||||
| Rainbow trout farm water | Single | TEM, DNA sequencing | WP-2 | Picovirinae | 18,899 bp | [142] | ||||||
| Streptococcus agalactiae | Tilapia pond | No | TEM | HN48 | Caudoviridae | n.d. | [143] | |||||
| S. iniae | S. iniae host | No | TEM, dsDNA | vB_SinS-44 vB_SinS-45 vB_SinS-46 vB_SinS-48 | Siphoviridae | ~51.7 kb ~28.4 kb ~66.3 kb ~27.5 kb |
[144] | |||||
| Weissella ceti | W. ceti host strain | No | TEM | PWc | Siphoviridae | 38,783 bp | [145]No | TEM, dsDNA, DNA sequencing | Ahp1 | Podoviridae | ~42 kb | [63] |
| Aeromonas punctata | Stream water | Single | TEM, dsDNA | IHQ1 | Myoviridae | 25–28 kb | [64] | |||||
| Aeromonas salmonicida | River waters, two passing through fish farms | Single | TEM, DNA sequencing | SW69-9 L9-6 Riv-10 |
Myoviridae | 173,097 bp, 173,578 bp and 174,311 bp | [65] | |||||
| River water | Single | TEM, DNA sequencing | phiAS5 | Myoviridae | 225,268 bp | [66] | ||||||
| Sediment of a Rainbow trout culture farm | Single | TEM, dsDNA, DNA sequencing | PAS-1 | Myoviridae | ~48 kb | [67] | ||||||
| Wastewater from a seafood market | No | TEM, DNA sequencing | AsXd-1 | Siphoviridae | 39,014 bp | [68] | ||||||
| Sewage network water from a lift station | Single | TEM | AS-A AS-D AS-E |
Myoviridae | n.d. | [40][41] | ||||||
| River water | No | TEM | HER 110 | Myoviridae | n.d. | [69][70] | ||||||
| Aeromonas spp. | Gastrointestinal content of variated fish species | No | TEM, DNA sequencing | phiA8-29 | Myoviridae | 144,974 bp | [71][72] | |||||
| Citrobacter freundii | Sewage water | No | TEM, DNA sequencing | IME-JL8 | Siphoviridae | 49,838 bp | [73] | |||||
| Edwardsiella ictaluri | Water from catfish ponds | Single | TEM, dsDNA, DNA sequencing | eiAU eiDWF eiMSLS |
Siphoviridae | 42.80 kbp 42.12 kbp 42.69 kbp |
[74][75] | |||||
| River water | Multiple | DNA Sequencing | PEi21 | Myoviridae | 43,378 bp | [76][77] | ||||||
| Striped catfish kidney and liver | Single | TEM, dsDNA | MK7 | Myoviridae | ~34 kb | [78] | ||||||
| Edwardsiella tarda | Seawater | Single | TEM, dsDNA | ETP-1 | Podoviridae | ~40 kb | [23] | |||||
| River water | No | TEM, DNA sequencing | pEt-SU | Myoviridae | 276,734 bp | [79] | ||||||
| Wastewater | Single | DNA sequencing | PETp9 | Myoviridae | 89,762 bp | [80] | ||||||
| Fish tissues and rearing seawater | No | TEM, DNA sequencing | GF-2 | Myoviridae | 43,129 bp | [81] | ||||||
| Flavobacterium columnare | River water | Single | TEM, DNA sequencing | FCL-2 | Myoviridae | 47,142 bp | [82][83][84] | |||||
| Fishpond’s water and bottom sediments | No | TEM, dsDNA | FCP1-FCP9 | Podoviridae | n.d. | [42] | ||||||
| Flavobacterium psychrophilum | Rainbow trout farm water | Single/double | TEM, dsDNA | ø (FpV-1 to FpV-22) | Podoviridae Siphoviridae Myoviridae |
(~8 to ~90 kb) | [85][86] | |||||
| Ayu kidneys and pondwater collected from ayu farms | Multiple | TEM, dsDNA | PFpW-3, PFpC-Y PFpW-6, PFpW-7 PFpW-8 |
Myoviridae; Podoviridae; Siphoviridae | n.d. | [87] | ||||||
| Photobacterium damselae subsp. damselae | Raw oysters | Single | TEM, dsDNA | Phda1 | Myoviridae | 35.2–39.5 kb | [88] | |||||
| Gastrointestinal tract of lollipop catshark | Single | TEM, DNA sequencing | vB_Pd_PDCC-1 | Myoviridae | 237,509 bp | [89] | ||||||
| Pseudomonas plecoglossicida | Ayu pond water and diseased fish | No | TEM, DNA sequencing | PPpW-3 PPpW-4 |
Myoviridae Podoviridae | 43,564 bp 41,386 bp |
[90][91] | |||||
| Pseudomonas aeruginosa | Wastewater | No | TEM, DNA sequencing | MBL | n.d. | 42,519 bp | [92] | |||||
| Shewanella spp. | Wastewater from a marketplace |
Single | TEM, DNA sequencing | SppYZU01 to SppYZU10 | Myoviridae; Siphoviridae. | SppYZU01 (43.567 bp) SppYZU5 (54.319 bp) |
[93] | |||||
| Tenacibaculum maritimum | Seawater | Multiple | TEM, DNA sequencing | PTm1 PTm5 |
Myoviridae | 224,680 bp 226,876 bp |
[94] | |||||
| Vibrio alginolyticus | Aquaculture tank water | Single | TEM, DNA sequencing | VEN | Podoviridae | 44,603 bp | [95] | |||||
| Marine sediment | No | TEM, DNA sequencing | ValKK3 | Myoviridae | 248,088 bp | [96] | ||||||
| Marine water | Single | TEM, dsDNA | St2 Grn1 |
Myoviridae | 250,485 bp 248,605 bp | [97] | ||||||
| Vibrio anguillarum | Soft tissues from clams and mussels | No | TEM, dsDNA | 309 ALMED Multiple |
TEM, DNA sequencing | ø H1, H7, S4-7, H4, H5 H8, H20 S4-18, 2E-1, H2 |
Myoviridae Siphoviridae Podoviridae | ~194–195 kb ~50 kb ~45–51 kb |
[99] | |||
| Vibrio campbellii | Host strain (V. campbellii) isolated form a dead shrimp | No | TEM, DNA sequencing | HY01 | Siphoviridae | 41.772 bp | [100] | |||||
| Hepatopancreas of Pacific white shrimp |
Single | dsDNA, DNA sequencing | vB_Vc_SrVc9 | Autographiviridae | ~43.15 kb | [101] | ||||||
| Vibrio harveyi | Shrimp farm, hatcheries and marine water | Multiple | TEM, dsDNA | A | Siphoviridae | n.d. | [102] | |||||
| Vibrio harveyi | No | TEM, dsDNA | VHML | Myovirus-like | n.d. | [103] | ||||||
| Shrimp pond water | Single | TEM, dsDNA | PW2 | Siphoviridae | ~46 kb | [104] | ||||||
| Water and sediment samples | Single | TEM, dsDNA | VHM1, VHM2 VHS1 |
Myoviridae, Siphoviridae |
~55 kb, ~66 kb ~69 kb |
[105] | ||||||
| Hatchery water and oyster tissues | Single | TEM, dsDNA | vB_VhaS-a vB_VhaS-tm |
Siphoviridae | ~82 kb ~59 kb |
[106] | ||||||
| Commercial clam samples | Multiple | Genomic analysis, dsDNA | ø VhCCS-01 VhCCS-02 VhCCS-04 VhCCS-06 VhCCS-17 VhCCS-20 VhCCS-19 VhCCS-21 |
Siphoviridae, Myoviridae |
n.d. | [107] | ||||||
| Oyster, clam, shrimp, and seawater samples | No | TEM, DNA sequencing | VHP6b | Siphoviridae | 78,081 bp | [108] | ||||||
| shrimp hatchery and farm water, oysters from estuaries, coastal sea water |
Multiple | TEM, dsDNA | Viha10 Viha8 Viha9 Viha11 Viha1 to Viha7 |
Siphoviridae - Siphoviridae Myoviridae (Viha4) |
n.d. ~44–94 kb ~85 kb (Viha4) |
[109][110] | ||||||
| Seawater sample | Single | TEM | VhKM4 | Myoviridae | n.d. | [111] | ||||||
| Vibrio ordalii | Macerated specimens of mussels | No | TEM, DNA sequencing | B_VorS-PVo5 | Siphoviridae | 80,578 bp | [112] | |||||
| Vibrio parahaemolyticus | Sewage sample | No | TEM, dsDNA | VPp1 | Tectiviridae | ~15 kb | [113] | |||||
| Polluted seawater | No | TEM, dsDNA | KVP40 KVP41 |
Myoviridae | n.d. | [114][115] | ||||||
| Seawater or mussels | Single | dsDNA | SPA2 SPA3 |
n.d. | ~21 kb | [116] | ||||||
| Coastal water | Single | TEM, DNA sequencing | pVP-1 | Siphoviridae | 111,506 bp | [117][118] | ||||||
| V. parahaemolyticus isolated from sewage samples collected from an aquatic product market | No | TEM, DNA sequencing | vB_VpS_BA3 vB_VpS_CA8 | Siphoviridae | 58,648 bp 58,480 bp |
[119] | ||||||
| Shrimp pond water | Single | TEM, DNA sequencing | VP-1 | Myoviridae | 150,764 bp | [120] | ||||||
| Coastal sand sediment | double | TEM, DNA sequencing | VpKK5 | Siphoviridae | 56,637 bp | [121][122] | ||||||
| Vibrio splendidus | Raw sewage obtained from local hatcheries | Single | TEM | PVS-1, PVS-2 PVS-3 |
Myoviridae; Siphoviridae | n.d. | [123] | |||||
| Seawater near a fish farm cage | Single | TEM, DNA sequencing | vB_VspP_pVa5 | Podoviridae | 78,145 bp | [124] | ||||||
| Vibrio coralliilyticus | sewage in oyster hatchery | Single | TEM | pVco-14 | Siphoviridae | n.d. | [125] | |||||
| Vibrio vulnificus | Seawater sample | Single | TEM, DNA sequencing | SSP002 | Siphoviridae | 76,350 bp | [126][127] | |||||
| Abalone samples | No | TEM, sequencing | VVPoo1 | Siphoviridae | 76,423 bp | [128] | ||||||
| Initial host strain (V. vulnificus) | No | TEM | VV1 VV2 VV3 VV4 |
Tectiviridae | n.d. | [129] | ||||||
| Vibrio sp. | Sewage draining exits | Single | TEM, DNA sequencing | VspDsh-1 VpaJT-1 ValLY-3 ValSw4-1 VspSw-1 |
Siphoviridae | 46,692 bp 60,177 bp 76,310 bp 79,545 bp 113,778 bp |
References
- Ghosh, C.; Sarkar, P.; Issa, R.; Haldar, J. Alternatives to Conventional Antibiotics in the Era of Antimicrobial Resistance. Trends Microbiol. 2019, 27, 323–338.
- Golkar, Z.; Bagasra, O.; Pace, D.G. Bacteriophage therapy: A potential solution for the antibiotic resistance crisis. J. Infect. Dev. Ctries 2014, 8, 129–136.
- Wigington, C.H.; Sonderegger, D.; Brussaard, C.P.; Buchan, A.; Finke, J.F.; Fuhrman, J.A.; Lennon, J.T.; Middelboe, M.; Suttle, C.A.; Stock, C.; et al. Re-examination of the relationship between marine virus and microbial cell abundances. Nat. Microbiol. 2016, 1, 15024.
- Zhang, R.; Li, Y.; Yan, W.; Wang, Y.; Cai, L.; Luo, T.; Li, H.; Weinbauer, M.G.; Jiao, N. Viral control of biomass and diversity of bacterioplankton in the deep sea. Commun. Biol. 2020, 3, 256.
- Brum, J.R.; Schenck, R.O.; Sullivan, M.B. Global morphological analysis of marine viruses shows minimal regional variation and dominance of non-tailed viruses. ISME J. 2013, 7, 1738–1751.
- Dion, M.B.; Oechslin, F.; Moineau, S. Phage diversity, genomics and phylogeny. Nat. Rev. Microbiol. 2020, 18, 125–138.
- Washizaki, A.; Yonesaki, T.; Otsuka, Y. Characterization of the interactions between Escherichia coli receptors, LPS and OmpC, and bacteriophage T4 long tail fibers. Microbiologyopen 2016, 5, 1003–1015.
- Dunne, M.; Hupfeld, M.; Klumpp, J.; Loessner, M.J. Molecular Basis of Bacterial Host Interactions by Gram-Positive Targeting Bacteriophages. Viruses 2018, 10, 397.
- Bertozzi Silva, J.; Storms, Z.; Sauvageau, D. Host receptors for bacteriophage adsorption. FEMS Microbiol. Lett. 2016, 363, fnw002.
- Los, M.; Wegrzyn, G. Pseudolysogeny. Adv. Virus Res. 2012, 82, 339–349.
- Vázquez, R.; García, E.; García, P. Phage Lysins for Fighting Bacterial Respiratory Infections: A New Generation of Antimicrobials. Front. Immunol. 2018, 9, 2252.
- Broendum, S.S.; Buckle, A.M.; McGowan, S. Catalytic diversity and cell wall binding repeats in the phage-encoded endolysins. Mol. Microbiol. 2018, 110, 879–896.
- Cahill, J.; Young, R. Phage Lysis: Multiple Genes for Multiple Barriers. Adv. Virus Res. 2019, 103, 33–70.
- Rodriguez-Rubio, L.; Martínez, B.; Donovan, D.M.; Rodriguez, A.; García, P. Bacteriophage virion-associated peptidoglycan hydrolases: Potential new enzybiotics. Crit. Rev. Microbiol. 2013, 39, 427–434.
- Ghose, C.; Euler, C.W. Gram-Negative Bacterial Lysins. Antibiotics 2020, 9, 74.
- Dufour, N.; Delattre, R.; Chevallereau, A.; Ricard, J.-D.; Debarbieux, L. Phage Therapy of Pneumonia Is Not Associated with an Overstimulation of the Inflammatory Response Compared to Antibiotic Treatment in Mice. Antimicrob. Agents Chemother. 2019, 63, e00379-19.
- Khan Mirzaei, M.; Haileselassie, Y.; Navis, M.; Cooper, C.; Sverremark-Ekström, E.; Nilsson, A.S. Morphologically Distinct Escherichia coli Bacteriophages Differ in Their Efficacy and Ability to Stimulate Cytokine Release in vitro. Front. Microbiol. 2016, 7, 437.
- Secor, P.R.; Michaels, L.A.; Smigiel, K.S.; Rohani, M.G.; Jennings, L.K.; Hisert, K.B.; Arrigoni, A.; Braun, K.R.; Birkland, T.P.; Lai, Y. Filamentous Bacteriophage Produced by Pseudomonas aeruginosa Alters the Inflammatory Response and Promotes Noninvasive Infection in vivo. Infect. Immun. 2017, 85, e00648-16.
- Trend, S.; Chang, B.J.; O’Dea, M.; Stick, S.M.; Kicic, A.; WAERP; AusREC; AREST CF. Use of a Primary Epithelial Cell Screening Tool to Investigate Phage Therapy in Cystic Fibrosis. Front. Pharmacol. 2018, 9, 1330.
- Fattal, B.; Dotan, A.; Tchorsh, Y.; Parpari, L.; Shuval, H. Penetration of E. coli and F2 Bacteriophage into Fish Tissues. Schr. Ver. Wasser- Boden-und Lufthyg. 1988, 78, 27–38.
- Cafora, M.; Deflorian, G.; Forti, F.; Ferrari, L.; Binelli, G.; Briani, F.; Ghisotti, D.; Pistocchi, A. Phage Therapy against Pseudomonas aeruginosa Infections in a Cystic Fibrosis Zebrafish Model. Sci. Rep. 2019, 9, 1–10.
- Cafora, M.; Forti, F.; Briani, F.; Ghisotti, D.; Pistocchi, A. Phage Therapy Application to Counteract Pseudomonas aeruginosa Infection in Cystic Fibrosis Zebrafish Embryos. JoVE (J. Vis. Exp.) 2020, e61275.
- Nikapitiya, C.; Chandrarathna, H.; Dananjaya, S.; De Zoysa, M.; Lee, J. Isolation and Characterization of Phage (ETP-1) Specific to Multidrug Resistant Pathogenic Edwardsiella tarda and Its in vivo Biocontrol Efficacy in Zebrafish (Danio rerio). Biologicals 2020, 63, 14–23.
- Yun, S.; Jun, J.W.; Giri, S.S.; Kim, H.J.; Chi, C.; Kim, S.G.; Kim, S.W.; Kang, J.W.; Han, S.J.; Kwon, J. Immunostimulation of Cyprinus carpio Using Phage Lysate of Aeromonas hydrophila. Fish Shellfish. Immunol. 2019, 86, 680–687.
- Schulz, P.; Robak, S.; Dastych, J.; Siwicki, A.K. Influence of Bacteriophages Cocktail on European Eel (Anguilla anguilla) Immunity and Survival after Experimental Challenge. Fish Shellfish. Immunol. 2019, 84, 28–37.
- Lin, H.; Caywood, B.E.; Rowlands, D., Jr. Primary and Secondary Immune Responses of the Marine Toad (Bufo marinus) to Bacterophage F2. Immunology 1971, 20, 373.
- Bradley, S.; Kim, Y.; Watson, D. Immune Response by the Mouse to Orally Administered Actinophage. Proc. Soc. Exp. Biol. Med. 1963, 113, 686–688.
- Young, R.; Ruddle, F.H. Inactivation of T-2 Bacteriophage by Sensitized Leucocytes in vitro. Nature 1965, 208, 1105–1106.
- Van Belleghem, J.D.; Dąbrowska, K.; Vaneechoutte, M.; Barr, J.J.; Bollyky, P.L. Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses 2019, 11, 10.
- Van Belleghem, J.D.; Clement, F.; Merabishvili, M.; Lavigne, R.; Vaneechoutte, M. Pro-and Anti-Inflammatory Responses of Peripheral Blood Mononuclear Cells Induced by Staphylococcus aureus and Pseudomonas aeruginosa Phages. Sci. Rep. 2017, 7, 1–13.
- Bekeredjian-Ding, I.B.; Wagner, M.; Hornung, V.; Giese, T.; Schnurr, M.; Endres, S.; Hartmann, G. Plasmacytoid Dendritic Cells Control TLR7 Sensitivity of Naive B Cells via Type I IFN. J. Immunol. 2005, 174, 4043–4050.
- Hashiguchi, S.; Yamaguchi, Y.; Takeuchi, O.; Akira, S.; Sugimura, K. Immunological Basis of M13 Phage Vaccine: Regulation under MyD88 and TLR9 Signaling. Biochem. Biophys. Res. Commun. 2010, 402, 19–22.
- Hodyra-Stefaniak, K.; Miernikiewicz, P.; Drapała, J.; Drab, M.; Jończyk-Matysiak, E.; Lecion, D.; Kaźmierczak, Z.; Beta, W.; Majewska, J.; Harhala, M. Mammalian Host-Versus-Phage Immune Response Determines Phage Fate in vivo. Sci. Rep. 2015, 5, 1–13.
- Krut, O.; Bekeredjian-Ding, I. Contribution of the Immune Response to Phage Therapy. J. Immunol. 2018, 200, 3037–3044.
- Schulz, P.; Pajdak-Czaus, J.; Robak, S.; Dastych, J.; Siwicki, A.K. Bacteriophage-based Cocktail Modulates Selected Immunological Parameters and Post-challenge Survival of Rainbow Trout (Oncorhynchus mykiss). J. Fish Dis. 2019, 42, 1151–1160.
- Moye, Z.D.; Woolston, J.; Abbeele, P.v.d.; Duysburgh, C.; Verstrepen, L.; Das, C.R.; Marzorati, M.; Sulakvelidze, A. A Bacteriophage Cocktail Eliminates Salmonella Typhimurium from the Human Colonic Microbiome While Preserving Cytokine Signaling and Preventing Attachment to and Invasion of Human Cells by Salmonella in vitro. J. Food Prot. 2019, 82, 1336–1349.
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage Applications for Food Production and Processing. Viruses 2018, 10, 205.
- Fischer, S.; Kittler, S.; Klein, G.; Glünder, G. Impact of a Single Phage and a Phage Cocktail Application in Broilers on Reduction of Campylobacter Jejuni and Development of Resistance. PLoS ONE 2013, 8, e78543.
- Sulakvelidze, A. Using Lytic Bacteriophages to Eliminate or Significantly Reduce Contamination of Food by Foodborne Bacterial Pathogens. J. Sci. Food Agric. 2013, 93, 3137–3146.
- Silva, Y.J.; Moreirinha, C.; Pereira, C.; Costa, L.; Rocha, R.J.; Cunha, Â.; Gomes, N.C.; Calado, R.; Almeida, A. Biological Control of Aeromonas salmonicida Infection in Juvenile Senegalese Sole (Solea senegalensis) with Phage AS-A. Aquaculture 2016, 450, 225–233.
- Duarte, J.; Pereira, C.; Costa, P.; Almeida, A. Bacteriophages with Potential to Inactivate Aeromonas hydrophila in Cockles: In vitro and in vivo Preliminary Studies. Antibiotics 2021, 10, 710.
- Prasad, Y.; Kumar, D.; Sharma, A. Lytic Bacteriophages Specific to Flavobacterium columnare Rescue Catfish, Clarias batrachus (Linn.) from Columnaris Disease. J. Environ. Biol. 2011, 32, 161–168.
- Hsu, C.; Lo, C.; Liu, J.; Lin, C. Control of the Eel (Anguilla Japonica) Pathogens, Aeromonas Hydrophila and Edwardsiella tarda, by Bacteriophages. J. Fish. Soc. Taiwan 2000, 27, 21–31.
- Blanco-Picazo, P.; Roscales, G.; Toribio-Avedillo, D.; Gómez-Gómez, C.; Avila, C.; Ballesté, E.; Muniesa, M.; Rodríguez-Rubio, L. Antibiotic Resistance Genes in Phage Particles from Antarctic and Mediterranean Seawater Ecosystems. Microorganisms 2020, 8, 1293.
- Castillo, D.; Kauffman, K.; Hussain, F.; Kalatzis, P.; Rørbo, N.; Polz, M.F.; Middelboe, M. Widespread Distribution of Prophage-Encoded Virulence Factors in Marine Vibrio Communities. Sci. Rep. 2018, 8, 1–9.
- Christiansen, R.H.; Madsen, L.; Dalsgaard, I.; Castillo, D.; Kalatzis, P.G.; Middelboe, M. Effect of Bacteriophages on the Growth of Flavobacterium psychrophilum and Development of Phage-Resistant Strains. Microb. Ecol. 2016, 71, 845–859.
- Castillo, D.; Christiansen, R.H.; Dalsgaard, I.; Madsen, L.; Middelboe, M. Bacteriophage Resistance Mechanisms in the Fish Pathogen Flavobacterium psychrophilum: Linking Genomic Mutations to Changes in Bacterial Virulence Factors. Appl. Environ. Microbiol. 2015, 81, 1157–1167.
- Middelboe, M.; Holmfeldt, K.; Riemann, L.; Nybroe, O.; Haaber, J. Bacteriophages Drive Strain Diversification in a Marine Flavobacterium: Implications for Phage Resistance and Physiological Properties. Environ. Microbiol. 2009, 11, 1971–1982.
- Welch, T.J. Characterization of a Novel Yersinia ruckeri Serotype O1-specific Bacteriophage with Virulence-neutralizing Activity. J. Fish Dis. 2020, 43, 285–293.
- Verner–Jeffreys, D.W.; Algoet, M.; Pond, M.J.; Virdee, H.K.; Bagwell, N.J.; Roberts, E.G. Furunculosis in Atlantic Salmon (Salmo salar L.) Is Not Readily Controllable by Bacteriophage Therapy. Aquaculture 2007, 270, 475–484.
- Silva, Y.J.; Costa, L.; Pereira, C.; Mateus, C.; Cunha, A.; Calado, R.; Gomes, N.C.; Pardo, M.A.; Hernandez, I.; Almeida, A. Phage Therapy as an Approach to Prevent Vibrio anguillarum Infections in Fish Larvae Production. PLoS ONE 2014, 9, e114197.
- Le, T.S.; Nguyen, T.H.; Vo, H.P.; Doan, V.C.; Nguyen, H.L.; Tran, M.T.; Tran, T.T.; Southgate, P.C.; Kurtböke, D.İ. Protective Effects of Bacteriophages against Aeromonas hydrophila Causing Motile Aeromonas septicemia (MAS) in Striped Catfish. Antibiotics 2018, 7, 16.
- Liu, J.; Gao, S.; Dong, Y.; Lu, C.; Liu, Y. Isolation and Characterization of Bacteriophages against Virulent Aeromonas hydrophila. BMC Microbiol. 2020, 20, 1–13.
- Easwaran, M.; Dananjaya, S.; Park, S.C.; Lee, J.; Shin, H.; De Zoysa, M. Characterization of Bacteriophage PAh-1 and Its Protective Effects on Experimental Infection of Aeromonas hydrophila in Zebrafish (Danio Rerio). J. Fish Dis. 2017, 40, 841–846.
- Akmal, M.; Rahimi-Midani, A.; Hafeez-ur-Rehman, M.; Hussain, A.; Choi, T.-J. Isolation, Characterization, and Application of a Bacteriophage Infecting the Fish Pathogen Aeromonas hydrophila. Pathogens 2020, 9, 215.
- Chandrarathna, H.; Nikapitiya, C.; Dananjaya, S.; De Silva, B.; Heo, G.-J.; De Zoysa, M.; Lee, J. Isolation and Characterization of Phage AHP-1 and Its Combined Effect with Chloramphenicol to Control Aeromonas hydrophila. Braz. J. Microbiol. 2020, 51, 409–416.
- Cheng, Y.; Gao, D.; Xia, Y.; Wang, Z.; Bai, M.; Luo, K.; Cui, X.; Wang, Y.; Zhang, S.; Xiao, W. Characterization of Novel Bacteriophage AhyVDH1 and Its Lytic Activity Against Aeromonas hydrophila. Curr. Microbiol. 2021, 78, 329–337.
- Cao, Y.; Li, S.; Wang, D.; Zhao, J.; Xu, L.; Liu, H.; Lu, T.; Mou, Z. Genomic Characterization of a Novel Virulent Phage Infecting the Aeromonas hydrophila Isolated from Rainbow Trout (Oncorhynchus mykiss). Virus Res. 2019, 273, 197764.
- Wu, J.-L.; Lin, H.-M.; Jan, L.; Hsu, Y.-L.; CHANG, L.-H. Biological Control of Fish Bacterial Pathogen, Aeromonas hydrophila, by Bacteriophage AH 1. Fish Pathol. 1981, 15, 271–276.
- Tu, V.Q.; Nguyen, T.-T.; Tran, X.T.; Millard, A.D.; Phan, H.T.; Le, N.P.; Dang, O.T.; Hoang, H.A. Complete Genome Sequence of a Novel Lytic Phage Infecting Aeromonas hydrophila, an Infectious Agent in Striped Catfish (Pangasianodon hypophthalmus). Arch. Virol. 2020, 165, 2973–2977.
- Hoang Hoang, A.; Xuan Tran, T.T.; Nga, L.E.P.; Oanh Dang, T.H. Selection of Phages to Control Aeromonas hydrophila–an Infectious Agent in Striped Catfish. Biocontrol Sci. 2019, 24, 23–28.
- Jun, J.W.; Kim, J.H.; Shin, S.P.; Han, J.E.; Chai, J.Y.; Park, S.C. Protective Effects of the Aeromonas Phages PAh1-C and PAh6-C against Mass Mortality of the Cyprinid Loach (Misgurnus anguillicaudatus) Caused by Aeromonas hydrophila. Aquaculture 2013, 416, 289–295.
- Wang, J.-B.; Lin, N.-T.; Tseng, Y.-H.; Weng, S.-F. Genomic Characterization of the Novel Aeromonas hydrophila Phage Ahp1 Suggests the Derivation of a New Subgroup from PhiKMV-like Family. PLoS ONE 2016, 11, e0162060.
- Haq, I.U.; Chaudhry, W.N.; Andleeb, S.; Qadri, I. Isolation and Partial Characterization of a Virulent Bacteriophage IHQ1 Specific for Aeromonas punctata from Stream Water. Microb. Ecol. 2012, 63, 954–963.
- Vincent, A.T.; Paquet, V.E.; Bernatchez, A.; Tremblay, D.M.; Moineau, S.; Charette, S.J. Characterization and Diversity of Phages Infecting Aeromonas salmonicida subsp. salmonicida. Sci. Rep. 2017, 7, 1–10.
- Kim, J.H.; Son, J.S.; Choi, Y.J.; Choresca, C.H., Jr.; Shin, S.P.; Han, J.E.; Jun, J.W.; Park, S.C. Complete Genome Sequence and Characterization of a Broad-Host Range T4-like Bacteriophage PhiAS5 Infecting Aeromonas salmonicida subsp. salmonicida. Vet. Microbiol. 2012, 157, 164–171.
- Kim, J.; Son, J.; Choi, Y.; Choresca, C.; Shin, S.; Han, J.; Jun, J.; Kang, D.; Oh, C.; Heo, S. Isolation and Characterization of a Lytic Myoviridae Bacteriophage PAS-1 with Broad Infectivity in Aeromonas salmonicida. Curr. Microbiol. 2012, 64, 418–426.
- Yang, Z.; Yuan, S.; Chen, L.; Liu, Q.; Zhang, H.; Ma, Y.; Wei, T.; Huang, S. Complete Genome Analysis of Bacteriophage AsXd-1, a New Member of the Genus Hk97virus, Family Siphoviridae. Arch. Virol. 2018, 163, 3195–3197.
- Imbeault, S.; Parent, S.; Lagacé, M.; Uhland, C.F.; Blais, J.-F. Using Bacteriophages to Prevent Furunculosis Caused by Aeromonas salmonicida in Farmed Brook Trout. J. Aquat. Anim. Health 2006, 18, 203–214.
- Petrov, V.; Karam, J. Diversity of Structure and Function of DNA Polymerase (Gp43) of T4-Related Bacteriophages. Biochemistry (Moscow) 2004, 69, 1213–1218.
- He, Y.; Yang, H. The Gastrointestinal Phage Communities of the Cultivated Freshwater Fishes. FEMS Microbiol. Lett. 2015, 362, fnu027.
- He, Y.; Huang, Z.; Zhang, X.; Zhang, Z.; Gong, M.; Pan, X.; Wei, D.; Yang, H. Characterization of a Novel Lytic Myophage, PhiA8-29, Infecting Aeromonas Strains. Arch. Virol. 2019, 164, 893–896.
- Jia, K.; Yang, N.; Zhang, X.; Cai, R.; Zhang, Y.; Tian, J.; Raza, S.H.A.; Kang, Y.; Qian, A.; Li, Y. Genomic, Morphological and Functional Characterization of Virulent Bacteriophage IME-JL8 Targeting Citrobacter freundii. Front. Microbiol. 2020, 11, 2967.
- Walakira, J.; Carrias, A.; Hossain, M.; Jones, E.; Terhune, J.; Liles, M. Identification and Characterization of Bacteriophages Specific to the Catfish Pathogen, Edwardsiella ictaluri. J. Appl. Microbiol. 2008, 105, 2133–2142.
- Carrias, A.; Welch, T.J.; Waldbieser, G.C.; Mead, D.A.; Terhune, J.S.; Liles, M.R. Comparative Genomic Analysis of Bacteriophages Specific to the Channel Catfish Pathogen Edwardsiella ictaluri. Virol. J. 2011, 8, 1–12.
- Yasuike, M.; Kai, W.; Nakamura, Y.; Fujiwara, A.; Kawato, Y.; Hassan, E.S.; Mahmoud, M.M.; Nagai, S.; Kobayashi, T.; Ototake, M. Complete Genome Sequence of the Edwardsiella ictaluri-Specific Bacteriophage PEi21, Isolated from River Water in Japan. Genome Announc. 2014, 2, e00228-14.
- Hassan, E.S.; Mahmoud, M.M.; Kawato, Y.; Nagai, T.; Kawaguchi, O.; Iida, Y.; Yuasa, K.; Nakai, T. Subclinical Edwardsiella ictaluri Infection of Wild Ayu Plecoglossus Altivelis. Fish Pathol. 2012, 47, 64–73.
- Hoang, H.A.; Yen, M.H.; Ngoan, V.T.; Nga, L.P.; Oanh, D.T. Virulent Bacteriophage of Edwardsiella ictaluri Isolated from Kidney and Liver of Striped Catfish Pangasianodon Hypophthalmus in Vietnam. Dis. Aquat. Org. 2018, 132, 49–56.
- Kim, S.G.; Giri, S.S.; Yun, S.; Kim, H.J.; Kim, S.W.; Kang, J.W.; Han, S.J.; Kwon, J.; Jun, J.W.; Oh, W.T. Genomic Characterization of Bacteriophage PEt-SU, a Novel PhiKZ-Related Virus Infecting Edwardsiella tarda. Arch. Virol. 2020, 165, 219–222.
- Cui, H.; Zhang, J.; Cong, C.; Wang, L.; Li, X.; Murtaza, B.; Xu, Y. Complete Genome Analysis of the Novel Edwardsiella tarda Phage VB_EtaM_ET-ABTNL-9. Arch. Virol. 2020, 165, 1241–1244.
- Yasuike, M.; Nishiki, I.; Iwasaki, Y.; Nakamura, Y.; Fujiwara, A.; Sugaya, E.; Kawato, Y.; Nagai, S.; Kobayashi, T.; Ototake, M. Full-Genome Sequence of a Novel Myovirus, GF-2, Infecting Edwardsiella tarda: Comparison with Other Edwardsiella myoviral Genomes. Arch. Virol. 2015, 160, 2129–2133.
- Almeida, G.M.; Laanto, E.; Ashrafi, R.; Sundberg, L.-R. Bacteriophage Adherence to Mucus Mediates Preventive Protection against Pathogenic Bacteria. MBio 2019, 10, e01984-19.
- Laanto, E.; Bamford, J.K.; Ravantti, J.J.; Sundberg, L.-R. The Use of Phage FCL-2 as an Alternative to Chemotherapy against Columnaris Disease in Aquaculture. Front. Microbiol. 2015, 6, 829.
- Laanto, E.; Sundberg, L.-R.; Bamford, J.K. Phage Specificity of the Freshwater Fish Pathogen Flavobacterium columnare. Appl. Environ. Microbiol. 2011, 77, 7868–7872.
- Christiansen, R.H.; Dalsgaard, I.; Middelboe, M.; Lauritsen, A.H.; Madsen, L. Detection and Quantification of Flavobacterium psychrophilum-Specific Bacteriophages in vivo in Rainbow Trout upon Oral Administration: Implications for Disease Control in Aquaculture. Appl. Environ. Microbiol. 2014, 80, 7683–7693.
- Stenholm, A.R.; Dalsgaard, I.; Middelboe, M. Isolation and Characterization of Bacteriophages Infecting the Fish Pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 2008, 74, 4070–4078.
- Kim, J.H.; Gomez, D.K.; Nakai, T.; Park, S.C. Isolation and Identification of Bacteriophages Infecting Ayu Plecoglossus altivelis Altivelis Specific Flavobacterium psychrophilum. Vet. Microbiol. 2010, 140, 109–115.
- Yamaki, S.; Kawai, Y.; Yamazaki, K. Characterization of a Novel Bacteriophage, Phda1, Infecting the Histamine-producing Photobacterium damselae subsp. damselae. J. Appl. Microbiol. 2015, 118, 1541–1550.
- Veyrand-Quirós, B.; Gómez-Gil, B.; Lomeli-Ortega, C.O.; Escobedo-Fregoso, C.; Millard, A.D.; Tovar-Ramírez, D.; Balcázar, J.L.; Quiroz-Guzmán, E. Use of Bacteriophage VB_Pd_PDCC-1 as Biological Control Agent of Photobacterium Damselae Subsp. Damselae during Hatching of Longfin Yellowtail (Seriola rivoliana) Eggs. J. Appl. Microbiol. 2020, 129, 1497–1510.
- Kawato, Y.; Yasuike, M.; Nakamura, Y.; Shigenobu, Y.; Fujiwara, A.; Sano, M.; Nakai, T. Complete Genome Sequence Analysis of Two Pseudomonas plecoglossicida Phages, Potential Therapeutic Agents. Appl. Environ. Microbiol. 2015, 81, 874–881.
- Park, S.C.; Shimamura, I.; Fukunaga, M.; Mori, K.-I.; Nakai, T. Isolation of Bacteriophages Specific to a Fish Pathogen, Pseudomonas plecoglossicida, as a Candidate for Disease Control. Appl. Environ. Microbiol. 2000, 66, 1416–1422.
- Khairnar, K.; Raut, M.P.; Chandekar, R.H.; Sanmukh, S.G.; Paunikar, W.N. Novel Bacteriophage Therapy for Controlling Metallo-Beta-Lactamase Producing Pseudomonas aeruginosa Infection in Catfish. BMC Vet. Res. 2013, 9, 1–9.
- Yang, Z.; Tao, X.; Zhang, H.; Rao, S.; Gao, L.; Pan, Z.; Jiao, X. Isolation and Characterization of Virulent Phages Infecting Shewanella baltica and Shewanella putrefaciens, and Their Application for Biopreservation of Chilled Channel Catfish (Ictalurus punctatus). Int. J. Food Microbiol. 2019, 292, 107–117.
- Kawato, Y.; Istiqomah, I.; Gaafar, A.Y.; Hanaoka, M.; Ishimaru, K.; Yasuike, M.; Nishiki, I.; Nakamura, Y.; Fujiwara, A.; Nakai, T. A Novel Jumbo Tenacibaculum maritimum Lytic Phage with Head-Fiber-like Appendages. Arch. Virol. 2020, 165, 303–311.
- Kokkari, C.; Sarropoulou, E.; Bastias, R.; Mandalakis, M.; Katharios, P. Isolation and Characterization of a Novel Bacteriophage Infecting Vibrio alginolyticus. Arch. Microbiol. 2018, 200, 707–718.
- Lal, T.M.; Sano, M.; Hatai, K.; Ransangan, J. Complete Genome Sequence of a Giant Vibrio Phage ValKK3 Infecting Vibrio alginolyticus. Genom. Data 2016, 8, 37–38.
- Kalatzis, P.G.; Bastías, R.; Kokkari, C.; Katharios, P. Isolation and Characterization of Two Lytic Bacteriophages, ΦSt2 and ΦGrn1; Phage Therapy Application for Biological Control of Vibrio alginolyticus in Aquaculture Live Feeds. PLoS ONE 2016, 11, e0151101.
- Higuera, G.; Bastías, R.; Tsertsvadze, G.; Romero, J.; Espejo, R.T. Recently Discovered Vibrio anguillarum Phages Can Protect against Experimentally Induced Vibriosis in Atlantic Salmon, Salmo salar. Aquaculture 2013, 392, 128–133.
- Tan, D.; Gram, L.; Middelboe, M. Vibriophages and Their Interactions with the Fish Pathogen Vibrio anguillarum. Appl. Environ. Microbiol. 2014, 80, 3128–3140.
- Nuidate, T.; Kuaphiriyakul, A.; Surachat, K.; Mittraparp-Arthorn, P. Induction and Genome Analysis of HY01, a Newly Reported Prophage from an Emerging Shrimp Pathogen Vibrio campbellii. Microorganisms 2021, 9, 400.
- Lomelí-Ortega, C.O.; Martínez-Sández, A.; Barajas-Sandoval, D.R.; Reyes, A.G.; Magallón-Barajas, F.; Veyrand-Quíros, B.; Gannon, L.; Harrison, C.; Michniewski, S.; Millard, A. Isolation and Characterization of Vibriophage VB_Vc_SrVc9: An Effective Agent in Preventing Vibrio campbellii Infections in Brine Shrimp Nauplii (Artemia franciscana). J. Appl. Microbiol. 2021, 131, 36–49.
- Vinod, M.; Shivu, M.; Umesha, K.; Rajeeva, B.; Krohne, G.; Karunasagar, I.; Karunasagar, I. Isolation of Vibrio harveyi Bacteriophage with a Potential for Biocontrol of Luminous Vibriosis in Hatchery Environments. Aquaculture 2006, 255, 117–124.
- Oakey, H.; Owens, L. A New Bacteriophage, VHML, Isolated from a Toxin-producing Strain of Vibrio harveyi in Tropical Australia. J. Appl. Microbiol. 2000, 89, 702–709.
- Phumkhachorn, P.; Rattanachaikunsopon, P. Isolation and Partial Characterization of a Bacteriophage Infecting the Shrimp Pathogen Vibrio harveyi. Afr. J. Microbiol. Res 2010, 4, 1794–1800.
- Stalin, N.; Srinivasan, P. Efficacy of Potential Phage Cocktails against Vibrio harveyi and Closely Related Vibrio Species Isolated from Shrimp Aquaculture Environment in the South East Coast of India. Vet. Microbiol. 2017, 207, 83–96.
- Wang, Y.; Barton, M.; Elliott, L.; Li, X.; Abraham, S.; O’Dea, M.; Munro, J. Bacteriophage Therapy for the Control of Vibrio harveyi in Greenlip Abalone (Haliotis laevigata). Aquaculture 2017, 473, 251–258.
- Crothers-Stomps, C.; Høj, L.; Bourne, D.; Hall, M.; Owens, L. Isolation of Lytic Bacteriophage against Vibrio harveyi. J. Appl. Microbiol. 2010, 108, 1744–1750.
- Patil, J.R.; Desai, S.N.; Roy, P.; Durgaiah, M.; Saravanan, R.S.; Vipra, A. Simulated Hatchery System to Assess Bacteriophage Efficacy against Vibrio harveyi. Dis. Aquat. Org. 2014, 112, 113–119.
- Karunasagar, I.; Shivu, M.; Girisha, S.; Krohne, G.; Karunasagar, I. Biocontrol of Pathogens in Shrimp Hatcheries Using Bacteriophages. Aquaculture 2007, 268, 288–292.
- Shivu, M.M.; Rajeeva, B.C.; Girisha, S.K.; Karunasagar, I.; Krohne, G.; Karunasagar, I. Molecular Characterization of Vibrio harveyi Bacteriophages Isolated from Aquaculture Environments along the Coast of India. Environ. Microbiol. 2007, 9, 322–331.
- Lal, T.M.; Sano, M.; Ransangan, J. Isolation and Characterization of Large Marine Bacteriophage (Myoviridae), VhKM4 Infecting Vibrio harveyi. J. Aquat. Anim. Health 2017, 29, 26–30.
- Echeverría-Vega, A.; Morales-Vicencio, P.; Saez-Saavedra, C.; Ceh, J.; Araya, R. The Complete Genome Sequence and Analysis of VB_VorS-PVo5, a Vibrio Phage Infectious to the Pathogenic Bacterium Vibrio Ordalii ATCC-33509. Stand. Genom. Sci. 2016, 11, 1–8.
- Peng, Y.; Ding, Y.; Lin, H.; Wang, J. Isolation, Identification and Lysis Properties Analysis of a Vibrio parahaemolyticus Phage VPp1. Mar. Sci. 2013, 37, 96–101.
- Matsuzaki, S.; Inoue, T.; Tanaka, S.; Koga, T.; Kuroda, M.; Kimura, S.; Imai, S. Characterization of a Novel Vibrio parahaemolyticus Phage, KVP241, and Its Relatives Frequently Isolated from Seawater. Microbiol. Immunol. 2000, 44, 953–956.
- Matsuzaki, S.; Tanaka, S.; Koga, T.; Kawata, T. A Broad-host-range Vibriophage, KVP40, Isolated from Sea Water. Microbiol. Immunol. 1992, 36, 93–97.
- Onarinde, B.A.; Dixon, R.A. Prospects for Biocontrol of Vibrio parahaemolyticus Contamination in Blue Mussels (Mytilus edulus)—A Year-Long Study. Front. Microbiol. 2018, 9, 1043.
- Kim, J.H.; Jun, J.W.; Choresca, C.H.; Shin, S.P.; Han, J.E.; Park, S.C. Complete Genome Sequence of a Novel Marine Siphovirus, PVp-1, Infecting Vibrio parahaemolyticus. J. Virol. 2012, 86, 7013–7014.
- Jun, J.W.; Kim, H.J.; Yun, S.K.; Chai, J.Y.; Park, S.C. Eating Oysters without Risk of Vibriosis: Application of a Bacteriophage against Vibrio parahaemolyticus in Oysters. Int. J. Food Microbiol. 2014, 188, 31–35.
- Yang, M.; Liang, Y.; Huang, S.; Zhang, J.; Wang, J.; Chen, H.; Ye, Y.; Gao, X.; Wu, Q.; Tan, Z. Isolation and Characterization of the Novel Phages VB_VpS_BA3 and VB_VpS_CA8 for Lysing Vibrio parahaemolyticus. Front. Microbiol. 2020, 11, 259.
- Matamp, N.; Bhat, S.G. Genome Characterization of Novel Lytic Myoviridae Bacteriophage ΦVP-1 Enhances Its Applicability against MDR-Biofilm-Forming Vibrio parahaemolyticus. Arch. Virol. 2020, 165, 387–396.
- Lal, T.M.; Sano, M.; Ransangan, J. Genome Characterization of a Novel Vibriophage VpKK5 (Siphoviridae) Specific to Fish Pathogenic Strain of Vibrio parahaemolyticus. J. Basic Microbiol. 2016, 56, 872–888.
- Lal, T.M.; Ransangan, J. Complete Genome Sequence of VpKK5, a Novel Vibrio parahaemolyticus Lytic Siphophage. Genome Announc. 2015, 3, e01381-14.
- Li, Z.; Li, X.; Zhang, J.; Wang, X.; Wang, L.; Cao, Z.; Xu, Y. Use of Phages to Control Vibrio splendidus Infection in the Juvenile Sea Cucumber Apostichopus japonicus. Fish Shellfish. Immunol. 2016, 54, 302–311.
- Katharios, P.; Kalatzis, P.G.; Kokkari, C.; Sarropoulou, E.; Middelboe, M. Isolation and Characterization of a N4-like Lytic Bacteriophage Infecting Vibrio splendidus, a Pathogen of Fish and Bivalves. PLoS ONE 2017, 12, e0190083.
- Kim, H.J.; Jun, J.W.; Giri, S.S.; Chi, C.; Yun, S.; Kim, S.G.; Kim, S.W.; Kang, J.W.; Han, S.J.; Kwon, J. Application of the Bacteriophage PVco-14 to Prevent Vibrio coralliilyticus Infection in Pacific Oyster (Crassostrea gigas) Larvae. J. Invertebr. Pathol. 2019, 167, 107244.
- Lee, H.S.; Choi, S.; Choi, S.H. Complete Genome Sequence of Vibrio vulnificus Bacteriophage SSP002. J. Virol. 2012, 86, 7711.
- Lee, H.S.; Choi, S.; Shin, H.; Lee, J.-H.; Choi, S.H. Vibrio vulnificus Bacteriophage SSP002 as a Possible Biocontrol Agent. Appl. Environ. Microbiol. 2014, 80, 515–524.
- Kim, H.-J.; Kim, Y.-T.; Kim, H.B.; Choi, S.H.; Lee, J.-H. Characterization of Bacteriophage VVP001 and Its Application for the Inhibition of Vibrio Vulnificus Causing Seafood-Borne Diseases. Food Microbiol. 2021, 94, 103630.
- Srinivasan, P.; Ramasamy, P. Morphological Characterization and Biocontrol Effects of Vibrio vulnificus Phages against Vibriosis in the Shrimp Aquaculture Environment. Microb. Pathog. 2017, 111, 472–480.
- Chen, L.; Fan, J.; Yan, T.; Liu, Q.; Yuan, S.; Zhang, H.; Yang, J.; Deng, D.; Huang, S.; Ma, Y. Isolation and Characterization of Specific Phages to Prepare a Cocktail Preventing Vibrio Sp. Va-F3 Infections in Shrimp (Litopenaeus vannamei). Front. Microbiol. 2019, 10, 2337.
- Stevenson, R.; Airdrie, D. Isolation of Yersinia ruckeri Bacteriophages. Appl. Environ. Microbiol. 1984, 47, 1201–1205.
- Kiljunen, S.; Hakala, K.; Pinta, E.; Huttunen, S.; Pluta, P.; Gador, A.; Lönnberg, H.; Skurnik, M. Yersiniophage ΦR1-37 Is a Tailed Bacteriophage Having a 270 Kb DNA Genome with Thymidine Replaced by Deoxyuridine. Microbiology 2005, 151, 4093–4102.
- Leskinen, K.; Pajunen, M.I.; Vilanova, M.V.G.-R.; Kiljunen, S.; Nelson, A.; Smith, D.; Skurnik, M. YerA41, a Yersinia ruckeri Bacteriophage: Determination of a Non-Sequencable DNA Bacteriophage Genome via RNA-Sequencing. Viruses 2020, 12, 620.
- Park, K.; Matsuoka, S.; Nakai, T.; Muroga, K. A Virulent Bacteriophage of Lactococcus garvieae (Formerly Enterococcus Seriolicida) Isolated from Yellowtail Seriola quinqueradiata. Dis. Aquat. Org. 1997, 29, 145–149.
- Park, K.H.; Kato, H.; Nakai, T.; Muroga, K. Phage Typing of Lactococcus garvieae (Formerly Enterococcus Seriolicida) a Pathogen of Cultured Yellowtail. Fish. Sci. 1998, 64, 62–64.
- Nakai, T.; Sugimoto, R.; Park, K.-H.; Matsuoka, S.; Mori, K.; Nishioka, T.; Maruyama, K. Protective Effects of Bacteriophage on Experimental Lactococcus garvieae Infection in Yellowtail. Dis. Aquat. Org. 1999, 37, 33–41.
- Ooyama, T.; Hirokawa, Y.; Minami, T.; Yasuda, H.; Nakai, T.; Endo, M.; Ruangpan, L.; Yoshida, T. Cell-Surface Properties of Lactococcus Garvieae Strains and Their Immunogenicity in the Yellowtail Seriola quinqueradiata. Dis. Aquat. Org. 2002, 51, 169–177.
- Eraclio, G.; Tremblay, D.M.; Lacelle-Côté, A.; Labrie, S.J.; Fortina, M.G.; Moineau, S. A Virulent Phage Infecting Lactococcus garvieae, with Homology to Lactococcus lactis Phages. Appl. Environ. Microbiol. 2015, 81, 8358–8365.
- Hoai, T.D.; Nishiki, I.; Yoshida, T. Properties and Genomic Analysis of Lactococcus garvieae Lysogenic Bacteriophage PLgT-1, a New Member of Siphoviridae, with Homology to Lactococcus lactis Phages. Virus Res. 2016, 222, 13–23.
- Hoai, T.; Yoshida, T. Induction and Characterization of a Lysogenic Bacteriophage of Lactococcus garvieae Isolated from Marine Fish Species. J. Fish Dis. 2016, 39, 799–808.
- Hoai, T.D.; Nishiki, I.; Fujiwara, A.; Yoshida, T.; Nakai, T. Comparative Genomic Analysis of Three Lytic Lactococcus garvieae Phages, Novel Phages with Genome Architecture Linking the 936 Phage Species of Lactococcus lactis. Mar. Genom. 2019, 48, 100696.
- Ghasemi, S.M.; Bouzari, M.; Yoon, B.H.; Chang, H.-I. Comparative Genomic Analysis of Lactococcus garvieae Phage WP-2, a New Member of Picovirinae Subfamily of Podoviridae. Gene 2014, 551, 222–229.
- Luo, X.; Liao, G.; Liu, C.; Jiang, X.; Lin, M.; Zhao, C.; Tao, J.; Huang, Z. Characterization of Bacteriophage HN 48 and Its Protective Effects in Nile Tilapia Oreochromis niloticus against Streptococcus agalactiae Infections. J. Fish Dis. 2018, 41, 1477–1484.
- Wright, E.; Elliman, J.; Owens, L. Induction and Characterization of Lysogenic Bacteriophages from Streptococcus iniae. J. Appl. Microbiol. 2013, 114, 1616–1624.
- Hoai, T.D.; Mitomi, K.; Nishiki, I.; Yoshida, T. A Lytic Bacteriophage of the Newly Emerging Rainbow Trout Pathogen Weissella Ceti. Virus Res. 2018, 247, 34–39.
