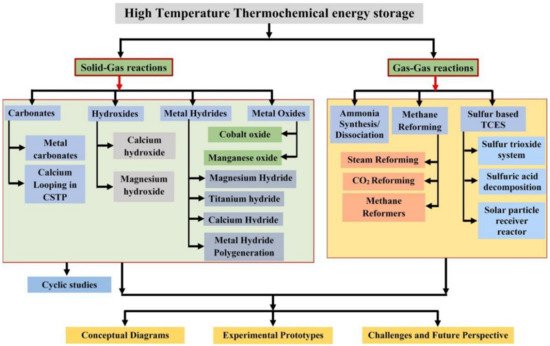
Thermochemical heat storage (TCHS) based on CaO/CaCO3 cycles has broad application prospects due to many advantages, such as high heat storage density, high exothermic temperature, low energy loss, low material price, and good coupling with CSP plants.
- thermochemical heat storage
- CaO/CaCO3 cycles
- solar energy
- CaO-based material
1. Introduction
SHS | LHS | TCHS | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Heat storage density | Low ~0.2 GJ/m3 | Medium ~0.3–0.5 GJ/m3 | High ~0.5–3 GJ/m3 | ||||||||||||||||||
Working temperature | Low | Low or medium | Medium or high | ||||||||||||||||||
Advantages | Mature technology | Low price | Long service life | Small heat storage volume | Simple system | High heat storage density | Small thermal losses | Long-distance transportation | |||||||||||||
Disadvantages | High thermal losses | Low heat storage density | Poor thermal conductivity | Material corrosion | High thermal losses | Complex technology | High cost |
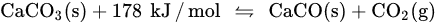
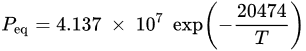
2. CaO/CaCO3 TCHS
System design represents a major contribution to the application of CaO/CaCO3 heat storage in CSP plants. The concept of calcium looping (CaL) TCHS can be traced back to the 1970s [39][42], but most of the subsequent CaL research has focused on CO2 capture [40][43]. Only with the increasing demand for heat storage in recent years has the application of CaL in TCHS been extensively studied. Although the CaO/CaCO3 heat storage technology and the CO2 capture technology have the same chemical reaction principle [41][44], they have remarkable differences in factors, such as reaction conditions and applications [42][45]. When the CaL process is utilized for CO2 capture from the flue gas of coal-fired power plants, the carbonation stage of CaO occurs in a carbonator with the flue gas containing about 15 vol% CO2 to form CaCO3 at the optimal temperature of 600–700 °C [43][44][46,47]. The calcination stage of CaCO3 occurs in a calciner at above 900 °C under a high concentration of CO2 (>90 vol%) for CO2 enrichment, where the required heat is provided by fuel oxygen-enriched combustion [45][48]. When CaL heat storage is implemented in CSP stations, its carbonation and calcination conditions are more flexible. The carbonation reaction is carried out under pure CO2 for high temperature and power generation efficiency in the exothermic stage. Gases with different concentrations of CO2 are fed into the carbonator as required, so the exothermic temperature of 600–900 °C can be reached [37]. Increasing the carbonation pressure can improve the limited temperature of the carbonation reaction [46][49]. In the solar calciner, the reactants generally cannot stay for a long time, so the calcination reaction in the CaO/CaCO3 system needs to be accomplished as soon as possible [32]. Longer reaction time and higher temperature result in more severe sintering of CaO in the calcination stage, which is not beneficial for the carbonation of CaO [47][50]. Thus, a shorter time and lower temperature lead to a higher carbonation of CaO due to the slight sintering [48][49][51,52]. In addition, the solar calciner at low temperature needs fewer solar reflectors, so the cost is also reduced [50][51][53,54]. The calcination kinetics depends not only on the calcination temperature, but also on the calcination atmosphere [52][55]. The different calcination atmospheres for CaO/CaCO3 heat storage were investigated, including CO2 [52][53][55,56], steam [54][57], and inert gases [49][52]. For the calcination under pure CO2 at atmospheric pressure, CaCO3 can only be quickly decomposed at a temperature around 930–950 °C due to the limitation of thermodynamic equilibrium calculated by Equation (2) [49][52][52,55]. If the CO2 partial pressure is higher than the equilibrium pressure, the calcination reaction cannot occur. The utilization of superheated steam (SHS) can reduce the calcination temperature to as low as 680 °C to save energy, and the calcined CaO has strong heat storage activity [54][57]. Nevertheless, the separation of vapor and CO2 needs energy consumption. That is because the heat for cooling vapor is difficult to utilize. CO2 after separation and purification is easier to use and store [55][58]. The calcination temperature of CaCO3 can be noticeably reduced by using inert gases, such as helium (He) or nitrogen (N2). Compared with pure N2, the calcination rate under pure He is faster due to the high diffusivity of CO2 in He and the high thermal conductivity of He, and the calcination temperature is as low as 725 °C [49][52]. However, a further issue that needs to be considered is the separation of CO2 and He. The content related to the reaction conditions will be discussed in detail in the next section. Recently, scholars have conducted lots of research on CaO/CaCO3 heat storage, continuously optimizing the integrated process of CaO/CaCO3 heat storage and CSP power generation to improve efficiency [56][57][59,60].3. Effect of Reaction Conditions on Performance of CaO-Based Materials in CaO/CaCO3 TCHS
For the CaO/CaCO3 TCHS system, whether the CaO-based material can maintain high carbonation performance and cyclic stability are key to heat storage. A number of studies have shown that temperature [58][97], pressure [59][60][98,99], atmosphere [61][100], and particle size [62][101] have crucial effects on the sintering rate of CaO-based materials. Therefore, it is necessary to study the reaction conditions in the stages of calcination and carbonation. The heat storage performances of CaO-based materials are evaluated by the effective conversion [58][97] and heat storage density [59][98], respectively. The effective conversion denotes the ratio of the mass of CaO reacted during each carbonation cycle to the total mass of the sample before the carbonation, which is defined by Equation (3):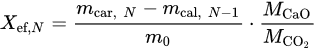 where N denotes the number of TCHS cycles; Xef, N is the effective conversion of CaO-based materials after N TCHS cycles; mcar, N and mcal, N−1 denote the mass of the sample after the Nth carbonation and the N-1th calcination, respectively, g; m0 represents the original mass of the sample, g; MCaO and MCO2 represent the molar masses of CaO and CO2, respectively, g/mol.
Heat storage density represents the maximum heat that can be released per unit mass of CaO-based materials during each carbonation reaction, which is defined by Equation (4):
where N denotes the number of TCHS cycles; Xef, N is the effective conversion of CaO-based materials after N TCHS cycles; mcar, N and mcal, N−1 denote the mass of the sample after the Nth carbonation and the N-1th calcination, respectively, g; m0 represents the original mass of the sample, g; MCaO and MCO2 represent the molar masses of CaO and CO2, respectively, g/mol.
Heat storage density represents the maximum heat that can be released per unit mass of CaO-based materials during each carbonation reaction, which is defined by Equation (4):
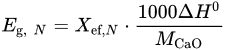 where Eg, N is the heat storage density of CaO-based materials, kJ/kg; ΔH0 denotes the standard reaction heat (178 kJ/mol for 0 °C; 165.5 kJ/mol for 900 °C).
where Eg, N is the heat storage density of CaO-based materials, kJ/kg; ΔH0 denotes the standard reaction heat (178 kJ/mol for 0 °C; 165.5 kJ/mol for 900 °C).
4. Performance of CaO-Based Materials in CaO/CaCO3 TCHS
It has been a consensus that the effective conversion of CaO plays a decisive role in the CaO/CaCO3 cycles heat storage. Prieto et al. [63][112] pointed out that the inactivation of CaO was a major defect for the CSP-CaL system. As the number of CaO/CaCO3 heat storage cycles increases, the activity of CaO decreases rapidly, and usually reaches a lower conversion over 20 cycles [64][113]. On the one hand, the carbonation occurs rapidly under high CO2 pressure at high temperature, so the generated CaCO3 layer blocks pores of the unreacted CaO [65][61]. On the other hand, due to the low Tammann temperature of calcium-based materials, CaO grains are sintered under harsh calcination conditions during multiple CaO/CaCO3 heat storage cycles [47][50]. The deactivation characteristics of CaO in the heat storage cycles are mainly related to the CaO precursor and the calcination/carbonation conditions. Calcium-based materials include a variety of natural ores, such as limestone, dolomite, and calcium-rich industrial waste such as carbide slag, steel slag, and fly ash [66][114].5. Improvement on Cyclic Thermal Storage Stability of CaO-Based Materials in CaO/CaCO3 TCHS
The heat storage performance of natural calcium-based materials, such as limestone and dolomite, declines rapidly with the number of heat storage cycles, which has an adverse effect on the CaO/CaCO3 TCHS. The lower the performance of CaO, the higher the inert solid content of the heat storage system for transportation, preheating, and cooling, resulting in a large amount of energy loss [67]. Studies have shown that the overall efficiency of CSP-CaL power plants increased by more than 10%, as the effective conversion of calcium-based materials increased from 0.07 to 0.5 [68][63]. Thus, it is beneficial to improve the cyclic heat storage performance of calcium-based materials and prepare calcium-based heat storage materials with high efficiency and stable performance, which have become the focus of attention of researchers. Adding a dopant with a high Tammann temperature to calcium-based materials is one of the most common methods to slow down the sintering of CaO-based materials. The supporter dispersed between the CaO grains plays a supporting role, which prevents the agglomeration of the CaO grains at high temperatures to a certain extent and enhances the sintering resistance of the CaO-based material [69][123].6. Improvement on Optical and Thermal Properties of CaO-Based Materials in CaO/CaCO3 TCHS
The Tammann temperature of calcium-based materials is relatively low, so CaO grains agglomerate and grow up during cyclic heat storage process at high temperature, leading to the blockage of the pore structure, which is manifested as a gradual decline in heat storage performance [40][69][43,123]. A large number of researches have focused on slowing down the sintering speed of calcium-based heat storage materials to improve cyclic stability. The volumetric heat collection is more suitable for CaO/CaCO3 heat storage system, which requires calcium-based materials with great optical and thermal properties. However, natural calcium-based materials usually have poor optical absorption capacities and thermal conductivity. In recent years, the optical absorption capacity and thermal conductivity of natural calcium-based materials have been given more attention and represent a valuable research direction. Han et al. [70][142] prepared composite materials by impregnating in H3BO3 solution with CaCO3 and adding expanded graphite with high thermal conductivity for heat storage. They found that the thermal conductivity of the composite materials increased by 60% when 3 wt% expanded graphite was added. The expanded graphite exhibited strong sintering resistance. The heat storage density of the composite material was 1313 kJ/kg after 50 cycles, while that of limestone was only 452 kJ/kg. On this basis, Han et al. [71][143] also studied an effective compression method to make graphite nanosheets better support the pore structure of CaO-based materials, so the obtained composites possessed a higher volumetric energy density. However, it is worth noting that CO2 attaches to graphite at high temperatures to form CO gas. More consideration should be given when choosing graphite as an additive. Da et al. [72][79] put forward a new idea to increase the blackness of calcium-based materials to achieve the direct absorption of solar energy in a CaO/CaCO3 heat storage system. Their experiments showed that adding black FeMnO3 and Fe2O3 to CaO by the sol-gel method improved the optical absorption properties of the materials. When the molar ratio of Ca/Mn/Fe was 100:4:8, the solar absorption of the composite reached 89.81% as exhibited in Figure 2. In addition, the existence of FeMnO3 and Fe2O3 also enhanced the sintering resistance of CaO. The effective conversion of Ca/Fe/Mn composites remained as high as 0.8 after 20 cycles.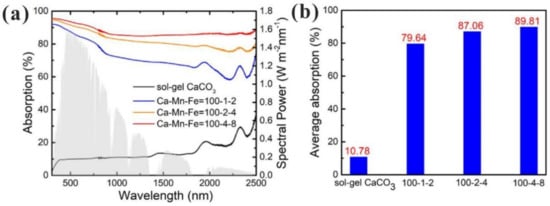
Additives | Doping Ratio (wt%) | Carbonation Pressure (bar) | Cycles | Effective Conversion | Reference | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
SiO2 | 10% | 1 | 20 | 0.30 |
[78] |
[126] |
|||||||||||
SiO2 | 30% | 1 | 20 | 0.34 |
[78] |
[126] |
|||||||||||
SiO2 | 5% | 1 | 20 | 0.20 |
[79] |
[127] |
|||||||||||
SiO2 | 37.5% | 1 | 45 | 0.20 |
[80] |
[129] |
|||||||||||
SiO2 | 20% | 5 | 50 | 0.29 |
[81] |
[130] |
|||||||||||
Al2O3 | 20% | 5 | 50 | 0.62 |
[81] |
[130] |
|||||||||||
Al2O3 | 5% | 1 | 20 | 0.55 |
[82] |
[131] |
|||||||||||
ZrO2 | 5% | 1 | 10 | 0.22 |
[83] |
[109] |
|||||||||||
ZrO2 | 20% | 5 | 50 | 0.67 |
[81] |
[130] |
|||||||||||
ZrO2 | 40% | 5 | 50 | 0.45 |
[81] |
[130] |
|||||||||||
ZnO | 20% | 5 | 50 | 0.07 |
[81] |
[130] |
|||||||||||
Fe2O3 | 20% | 5 | 50 | 0.08 |
[81] |
[130] |
|||||||||||
Ni | 20% | 5 | 50 | 0.14 |
[81] |
[130] |
|||||||||||
BaCO3 | 9.5% | 5 | 50 | 0.09 |
[81] |
[130] |
|||||||||||
Li2SO4 | 5% | 1 | 11 | 0.48 |
[84] |
[137] |
|||||||||||
Al2O3/CeO2 | 5%/5% | 13 | 30 | 0.79 |
[85] |
[136] |
|||||||||||
Graphite | 20% | 5 | 50 | 0.25 |
[81] |
[130] |
|||||||||||
H3BO3/Graphite | 3% | 1 | 50 | 0.41 |
[70] |
[142] |
|||||||||||
Mn/Fe | - | 1 | 20 | 0.80 |
[72] |
[79] |
|||||||||||
Al/Citric acid | - | 1 | 20 | 0.7 |
[86] |
[133] |
|||||||||||
Acetic acid(Ac) | - | 1 | 30 | 0.56 |
[87] |
[140] |
|||||||||||
Mg/Ac | - | 1 | 30 | 0.70 |
[87] |
[140] |
|||||||||||
NaY | 20% | 5 | 50 | 0.23 |
[81] |
[130] |
|||||||||||
HY | 20% | 5 | 50 | 0.16 |
[81] |
[130] |
|||||||||||
Mor | 20% | 5 | 50 | 0.15 |
[81] |
[130] |