Triple-negative breast cancer is a combative cancer type with a highly inflated histological grade that leads to poor theragnostic value. Gene, protein, and receptor-specific targets have shown effective clinical outcomes in patients with TNBC. Cells are frequently exposed to DNA-damaging agents. DNA damage is repaired by multiple pathways; accumulations of mutations occur due to damage to one or more pathways and lead to alterations in normal cellular mechanisms, which lead to development of tumors. Advances in target-specific cancer therapies have shown significant momentum; most treatment options cause off-target toxicity and side effects on healthy tissues. PARP (poly(ADP-ribose) polymerase) is a major protein and is involved in DNA repair pathways, base excision repair (BER) mechanisms, homologous recombination (HR), and nonhomologous end-joining (NEJ) deficiency-based repair mechanisms. DNA damage repair deficits cause an increased risk of tumor formation. Inhibitors of PARP favorably kill cancer cells in BRCA-mutations. For a few years, PARPi has shown promising activity as a chemotherapeutic agent in BRCA1- or BRCA2-associated breast cancers, and in combination with chemotherapy in triple-negative breast cancer.
- breast cancer
- PARP (poly(ADP-ribose) polymerase)
- TNBC
- therapeutic target
- DNA damage repair
- signaling pathway
1. Introduction
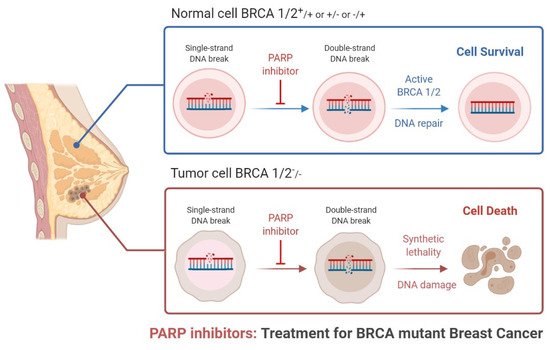
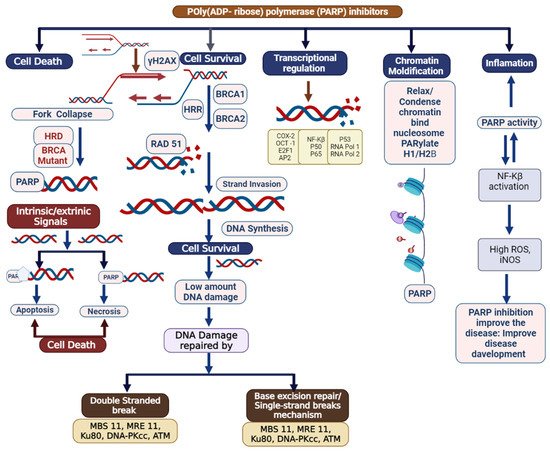
2. Clinical Applications of PARP Inhibitors in TNBC
| Compound Name | Compound Structure | Efficacy | IC50 |
|---|
| Name of the Molecules | Tmax (h) | t (h) | AUC (lgh/ mL) | Cmax (lg/mL) | CL/F (L/h) | Vz/F | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nicotinamide | 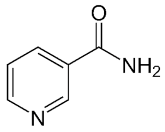 |
PARP inhibitor and by-product of the PARP reaction; many pharmacological actions other than that of inhibiting PARP | 210 μM | ||||||||||||
| 3-aminobenzamide | 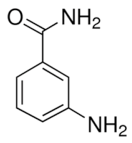 |
Benzamides are free radical scavengers, among other | |||||||||||||
| Olaparib capsule formulation 300 mg | 1.49 (0.57–3.05) |
13.02 (8.23) | 55.20 (67.4) | 8.05 (24.3) | 6.36 (3.47) | 112.1 (59.84) | pharmacological actions | 33 μM | |||||||
| PD128763 | 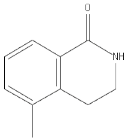 |
Cytoprotective agent, chemosensitizer, and radiosensitizer; adverse effect of compound causes hypothermia | 420 nM | ||||||||||||
| [AUC] | 5.48 (40.5) | 7.55 (3.99) | 127 (107) | [19 | DPQ | 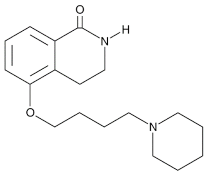 |
A commonly used Warner–Lambert PARP inhibitor compound based on an isoquinoline core |
1 μM | |||||||
| NU1025 | 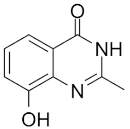 |
Potentiators of anticancer agent cytotoxicity |
400 nM | ||||||||||||
| 4-ANI | 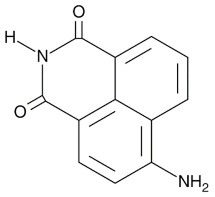 |
PARP in DNA repair and cell death | 180 nM | ||||||||||||
| ] | INO-1001 | 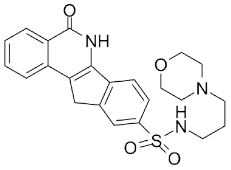 |
Potent enhancer of radiation sensitivity and enhances radiation-induced cell killing by interfering with DNA repair mechanisms, resulting in necrotic cell death | 105 nM | |||||||||||
| Veliparib monotherapy 40 mg (10 mg, fasting) | E7449 | 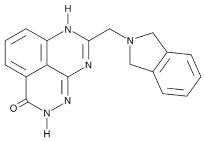 |
Antitumor activity of E7449; a novel PARP 1/2 and tankyrase 1/2 inhibitor | 1 nM | |||||||||||
| CEP-8983 | 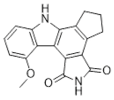 |
Increases the sensitivity of chemoresistant tumor cells to temozolomide | 20 nM | ||||||||||||
| [ | 18 | ] | |||||||||||||
| Olaparib tablet formulation 300 mg single dose (fasted) | 1.50 (0.50–5.85) |
12.2 (5.31) |
43.6 (54.3) [AUCt] 43.0 (55.2) [AUC] |
7.00 (35.0) | 7.95 (4.23) | 146 (142) | [19] | ||||||||
| Olaparib tablet formulation 300 mg single dose (fed) | 4.00 (1.00–12.0) |
12.2 (5.31) |
46.0 (56.6) [AUCt | Pamiparib (BGB-290) |
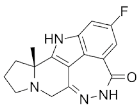 |
Pamiparib has potent PARP trapping, the capability to penetrate the brain, and can be used for the research of various cancers including solid tumors | 0.9 nM | ||||||||
| ] | 45.4 (57.1)1.2 ± 0.8 | 5.9 ± 1.3 | 2.23 ± 0.82 [AUCt] 2.43 ± 1.07 [AUC] |
0.36 ± 0.13 | 19.0 ± 7.36 | NA | [20][21] | ||||||||
| Veliparib monotherapy 40 mg (10 mg, fed) | 1.2 ± 0.7 | 5.8 ± 1.2 | 2.45 ± 0.93 [AUCt] 2.65 ± 1.17 [AUCt] |
0.37 ± 0.12 | 17.3 ± 6.41 | NA | |||||||||
| Veliparib monotherapy 40 mg (40 mg, fasting) | 1.3 ± 0.9 | 5.8 ± 1.3 | 2.24 ± 0.98 [AUCt] 2.45 ± 1.24 [AUCt] |
0.34 ± 0.12 | 19.5 ± 7.66 | NA | ISO | 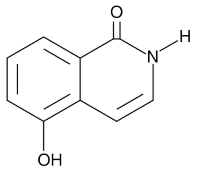 |
|||||||
| Veliparib monotherapy 40 mg (40 mg, fed) | PARP in DNA repair and cell death | 390 nM | |||||||||||||
| 2.5 ± 1.1 | 5.8 ± 1.4 | 2.14 ± 0.80 [AUCt] 2.35 ± 1.06 [AUCt] |
0.28 ± 0.09 | 19.7 ± 7.51 | NA | Olaparib (Lynparza) |
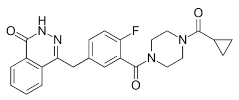 |
Use in a BRCA1 | |||||||
| Veliparib metabolite M8 | 2.4 (3.5–9.8)-positive patient with metastatic triple-negative breast cancer, without the initial use of platinum-based chemotherapy, showed significant rapid near-resolution of large liver metastasis while patient experienced gout-like symptoms | 1 nM | |||||||||||||
| – | 0.3–1.9 [AUCint] |
0.011 (0.007–0.014) |
NA | NA | [20][21] | Niraparib (Zejula) | 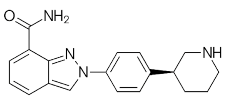 |
Niraparib in combination with pembrolizumab in patients with triple-negative breast cancer | 4 nM | ||||||
| Niraparib 300 mg/day | 3.1 (2.0–6.1) | a | 14.117 (AUC24)b |
1.921 | NA | NA[12] | Talazoparib (Talzenna) |
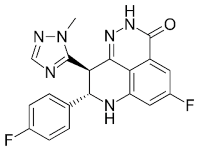 |
Ferm line BRCA-mutant, HER2-negative locally advanced or metastatic breast cancer | 0.6 nM | |||||
| Niraparib metabolite: unlabeled M1 plasma | 9.02 | 78.4 | 41.2 (AUCt) | 476 | NA | NA | [15] | Veliparib (ABT-888) | 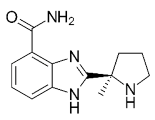 |
Received orphan drug status for lung cancer |
2 nM | Fluzoparib (SHR-3162) |
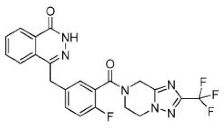 |
Inhibitor of poly-adenosine diphosphate(ADP)ribose polymerase (PARP) 1/2 being developed for the treatment of BRCA1/2-mutant solid tumors. | 1.5 nM |
| Name of Drug | Types of Inhibitors | Prior Treatment | Type of Population | Status | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|
| AZD1775 in patent with TNBC LYNPARZATM |
PARP Inhibitor, patent with TNBC |
Olaparib in combination with AZD6738 mutated (ATM) | Inhibitor of Ataxia-Telangiectasia and WEE1 inhibitor |
Phase II | NCT03330847 |
| AZD1775 in patent with TNBC LYNPARZATM |
PARP Inhibitor, patent with TNBC |
Olaparib with radiation therapy, after chemotherapy |
Inhibitor of ataxia-telangiectasia |
Phase I | NCT03109080 |
| AZD1775, LYNPARZATM |
Patent with TNBC | Olaparib with atezolizumab | Inhibitor of PD-L1 |
Phase II | NCT02849496 |
| AZD1775, LYNPARZATM |
Patent with TNBC | Oolaparib with paclitaxel and carboplatin |
Inhibitor of germline BRCA mutated |
Phase II/III | NCT03150576, NCT02789332 |
| AZD1775, LYNPARZATM |
Patent with TNBC | Olaparib with AZD2171 orally |
Inhibitor of VEGFR tyrosine kinase | Phase I/II | NCT01116648 |
| AZD1775, LYNPARZATM |
Patent with TNBC | Olaparib with PI3K inhibitor, BKM120 | Inhibitor of BKM120 | Phase I | NCT01623349 |
| AZD1775, LYNPARZATM |
Patent with TNBC | Olaparib with onalespib | Inhibitor of heat shock protein 90 | Phase I | NCT02898207 |
| AZD1775, LYNPARZATM |
Patent with TNBC | Olaparib with AZD2014 |
mTORC1/2 or Oral AKT inhibitor |
Phase I/II | NCT02208375 |
| PARP1/2 inhibitor Veliparib |
Patent with TNBC | Veliparib in combination with cyclophosphamide |
Inhibitor of EGFR, HER2, BRCA, and tyrosine kinase |
Phase II and failed in phase III trials |
NCT01306032 |
| PARP1/2 inhibitor Veliparib |
Inhibitor of tyrosine kinase, HER2, and BRCA |
Veliparib in combination with carboplatin |
Patients with TNBC | Completed phase I study | NCT01251874 |
| PARP1/2 inhibitor Veliparib |
Inhibitor of EGFR, BRCA, and tyrosine kinase |
Veliparib with vinorelbine |
Patients with TNBC | Completed phase I | NCT01281150 |
| PARP1/2 inhibitor Veliparib |
Inhibitor of EGFR, HER2, BRCA, and tyrosine kinase |
Veliparib with cisplatin | Patients with TNBC | Completed phase I | NCT01104259 |
| PARP1/2 inhibitor Veliparib |
Inhibitor of EGFR, HER2, BRCA, and tyrosine kinase |
Veliparib with pegylation | Patients with TNBC | Completed phase I | NCT01145430 |
| PARP1/2 inhibitor Veliparib |
Inhibitor of EGFR, HER2, BRCA, and tyrosine kinase |
Veliparib with pegylation | Patients with TNBC | Completed phase I | NCT01145430 |
| PARP1/2 inhibitor Veliparib |
Inhibitor of EGFR, HER2, BRCA, and tyrosine kinase |
Veliparib with lapatinib | Patients with TNBC | Phase I | NCT02158507 |
| PARP1/2 inhibitor Veliparib |
Inhibitor of EGFR, HER2, BRCA, and tyrosine kinase |
Veliparib in combination with irinotecan HCl | Patients with TNBC | Phase I I | NCT00576654 |
| PARP1/2 inhibitor Veliparib |
Inhibitor of EGFR, HER2, BRCA, and tyrosine kinase |
Veliparib with cisplatin |
Patients with TNBC | Phase II | NCT02595905 |
| AZD2281 and Ku-0059436 PARP1/2 inhibitor (Selective) |
PARP inhibitor; BRCA Mutated |
Olaparib alone, or in combination with durvalumab MEDI4736 against PD-L1 |
HER2-negative treated mTNBC |
Phase-II | NCT00679783 NCT03544125 NCT02484404 NCT03167619 NCT02681562 NCT02484404 |
| PARP1/2 inhibitor Veliparib |
Inhibitor of EGFR, HER2, BRCA, and tyrosine kinase |
Veliparib plus carboplatin | Patients with TNBC | Phase III | NCT02032277 |
| Iniparib BSI-201 and SAR240550 | Competitive PARP inhibitor; ability to form adducts with many cysteine-containing proteins | Combination with gemcitabine and carboplatin |
Patients with TNBC | Phase II | NCT00813956 NCT01045304 NCT01130259 |
| Iniparib BSI-201 and SAR240550 | Competitive PARP inhibitor; ability to form adducts with many cysteine-containing proteins | Combination of iniparib with paclitaxel for TNBC compared to paclitaxel alone |
Patients with TNBC | Competed phase II | NCT01204125 |
| Iniparib BSI-201 and SAR240550 | Competitive PARP inhibitor; ability to form adducts with many cysteine-containing proteins | Iniparib with irinotecan | Patients with TNBC | Phase II trial | NCT01173497 |
| Niraparib | ≥1 anti-HER2 treatment; PARP inhibitor |
Niraparib plus trastuzumab IV |
Metastatic HER2+ breast cancer |
Phase Ib/II (recruiting) | NCT03368729 |
| Niraparib | PARP inhibitor | One anthracycline and/or taxane in the (neo-) adjuvant or Niraparib |
Advanced/metastatic BRCA1- like |
Phase-II, Active, not recruiting | NCT02826512 |
| Niraparib | PARP inhibitor | ≥1 line of therapy Niraparib plus everolimus |
Patients with TNBC | Phase I Recruiting | NCT03154281 |
| Niraparib | Germline BRCA mutation-positive (PARP inhibitors) |
≤2 prior cytotoxic regimens and Niraparib versus physician‘s choice |
Advanced or metastatic breast cancer |
Phase III Active, not yet recruiting |
NCT01905592 (BRAVO) |
| Niraparib | Metastatic TNBC inhibitors (PARP inhibitors) |
≤2 lines of cytotoxic therapy, Niraparib plus pembrolizumab |
Advanced or metastatic TNBC |
Phase I/II Active, not yet recruiting |
NCT02657889 (KEYNOTE-162) |
| veliparib | Metastatic TNBC inhibitors (PARP inhibitors) |
≤2 lines of cytotoxic Chemotherapy, Carboplatin, and paclitaxel with or without veliparib |
Locally advanced unresectable BRCA associated |
Phase III Recruiting | NCT02163694 |
| veliparib | Metastatic TNBC inhibitors (PARP inhibitors) |
Veliparib with temozolomide versus veliparib with carboplatin and paclitaxel versus placebo with carboplatin and paclitaxel ≤2 lines of cytotoxic chemotherapy |
Metastatic TNBC |
Randomized phase II, Ongoing |
NCT01506609 |
| veliparib | Metastatic TNBC inhibitors (PARP inhibitors) |
Veliparib versus atezolizumab versus veliparib plus atezolizumab |
Stage III–IV TNBC | Randomized phase II Ongoing |
NCT02849496 |
| veliparib | Metastatic TNBC inhibitors PARP inhibitors) |
Cisplatin and placebo versus cisplatin and veliparib ≤1 line of cytotoxic chemotherapy for metastatic disease |
Metastatic TNBC and/or BRCA mutation-associated breast cancer |
Phase II Active, not recruiting | NCT02595905 |
| veliparib | Metastatic TNBC inhibitors PARP inhibitors) |
Temozolomide and veliparib ≥1 chemotherapy regimen |
Metastatic TNBC and/or BRCA mutation-associated breast cancer |
Phase II, Active, not recruiting | NCT01009788 |
| Talazoparib | Neoadjuvant therapy | None | Primary breast cancer ≥1 cm with a deleterious BRCA mutation |
Phase II, Active, not recruiting | NCT02282345 |
| Talazoparib | Advanced TNBC and HR deficiency and advanced HER2-negative breast cancer or other solid tumors with a mutation in HR pathway genes |
≥1 line of therapy | Talazoparib | Phase II, Recruiting | NCT02401347 |
| Talazoparib | Metastatic TNBC inhibitors PARP inhibitors |
Platinum-containing regimen with disease progression > 8 weeks |
Metastatic breast cancer with BRCA mutation |
Phase II Terminated (Primary Analysis and study completed Not stopped | NCT02034916 (ABRAZO) |
| Talazoparib | Metastatic TNBC inhibitors PARP inhibitors |
≤3 chemotherapy-inclusive regimens Talazoparib versus physician‘s choice |
Locally advanced and/or metastatic breast cancer with germline BRCA mutations |
Phase III Completed | NCT01945775 (EMBRACA) |
| Rucaparib | Metastatic TNBC inhibitors PARP inhibitors |
≤5 prior chemotherapy Rucaparib regimens in the last 5 years |
Patients presenting with metastatic breast cancer (MBC) | Phase II, Completed | NCT00664781 |
| Rucaparib | Metastatic TNBC inhibitors PARP inhibitors |
≥1 line of chemotherapy, Rucaparib | Patients with a BRCAness genomic signature |
Phase II Completed | NCT02505048 (RUBY) |
| Rucaparib | Stage I–III patients with TNBC or inhibitors PARP inhibitors |
Neoadjuvant chemotherapy Cisplatin with rucaparib | ER/PR+, HER2- negative breast cancer with known BRCA1/2 mutations |
Phase II Completed | NCT01074970 |
| Rucaparib | TNBC inhibitors | ≥3 prior chemotherapy regimens, Rucaparib |
Patients with advanced solid tumors with evidence of germline |
Phase I/II Active, not recruiting |
NCT01482715 |
| Rucaparib | TNBC inhibitors | ≤5 prior chemotherapy regimens in the last 5 years, Rucaparib |
Patients with MBC carriers of a BRCA1/2 | Phase II Completed | NCT00664781 |
| Rucaparib | TNBC inhibitors | ≥1 line of chemotherapy Rucaparib | Patients with a BRCAness genomic signature |
Phase II Completed | NCT02505048 (RUBY) |
| Rucaparib | TNBC inhibitors | Neoadjuvant chemotherapy Cisplatin with rucaparib |
Advanced solid tumors with evidence of germline or somatic BRCA mutation |
Completed | NCT01074970 |
| Rucaparib | TNBC inhibitors | ≥3 prior chemotherapy regimens |
Advanced solid tumors with evidence of germline or somatic BRCA mutation |
Phase I/II Active, not recruiting |
NCT01482715 |
