The blood and tissues of vertebrate animals and mammals contain small endogenous metal nanoparticles. These nanoparticles were observed to be composed of individual atoms of iron, copper, zinc, silver, gold, platinum, and other metals. Metal nanoparticles can bind proteins and produce proteinaceous particles called proteons. A small fraction of the entire pool of nanoparticles is usually linked with proteins to form proteons. These endogenous metal nanoparticles, along with engineered zinc and copper nanoparticles at subnanomolar levels, were shown to be lethal to cultured cancer cells. These nanoparticles appear to be elemental crystalline metal nanoparticles. It was discovered that zinc nanoparticles produce no odor response but increase the odor reaction if mixed with an odorant. Some other metal nanoparticles, including copper, silver, gold, and platinum nanoparticles, do not affect the responses to odorants. The sources of metal nanoparticles in animal blood and tissues may include dietary plants and gut microorganisms. The solid physiological and biochemical properties of metal nanoparticles reflect their importance in cell homeostasis and disease.
- proteons
- blood
- olfaction
- cancer
- prions
1. Introduction
2. Metal Nanoparticles in Blood and Tissues of Vertebrates and Mammals
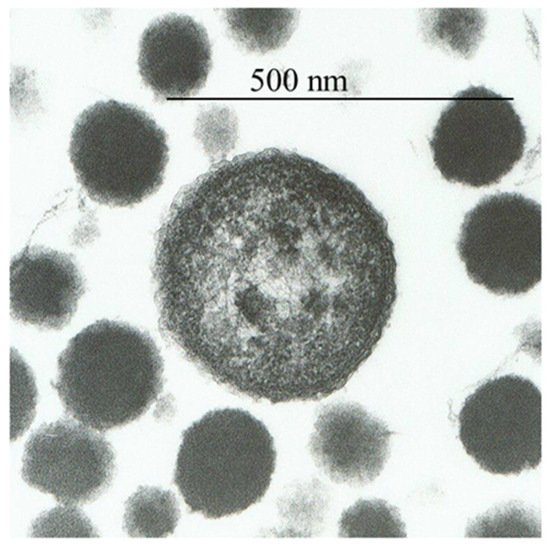
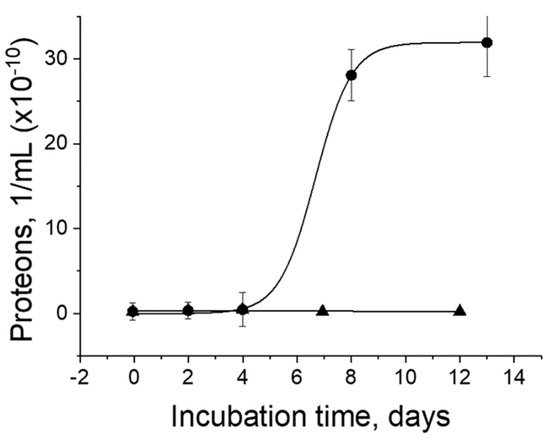
3. Metal Nanoparticles and Prions
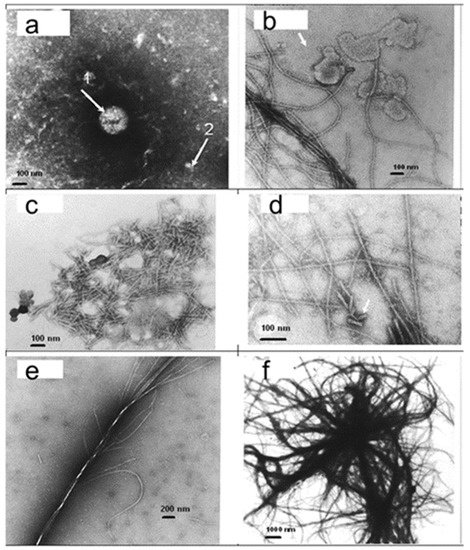
4. Metal Nanoparticles Are Lethal to Cancer Cells
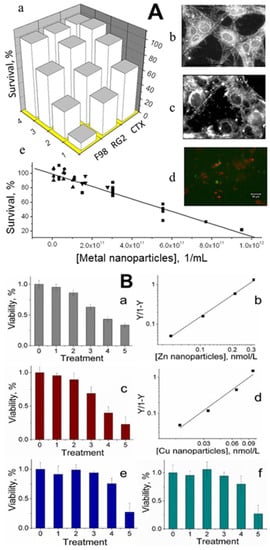
Cancer is a disease that should not affect us if our defense systems are intact. All healthy animals and humans have small metal nanoparticles in their blood that may be dominant components of the body’s defense mechanisms against cancer. Small concentrations of metal nanoparticles of size 1–2 nm isolated from animal blood were observed to be toxic to cultured cancer cells. [3][4][3,4]. After incubation with these nanoparticles, the viability of two rat glioma cell lines (F98 and RG2) decreased by 90% and 75%, respectively, while that of normal rat astrocytes (CTX) was reduced by only 25%. Total suppression of growth in-vitro required ≈ 1 × 1012 metal nanoparticles/mL (i.e., a few nmol/L), a concentration smaller than what is typically found in a healthy animal [3] (Figure 4A). Engineered zinc and copper metal nanoparticles of size 1 nm–2 nm were lethal to cultured RG2 glioma cancer cells. Cell death was confirmed by a colorimetric assay to assess cell metabolic activity, showing that the relative viability of RG2 glioma cells was reduced in a dose-dependent manner at subnanomolar concentrations of the nanoparticles. Noncancerous astrocytes were not affected under the same conditions [4] (Figure 4B). Synthetic copper nanoparticles have been found to be toxic to cultured cancer cells, including U937 (human histiocytic lymphoma) and HeLa cells (human cervical cancer origin), at concentrations of 1–500 μmol/L, [31][32][33][34][41,42,43,44], which are a few orders of magnitude higher than those of engineered zinc and copper nanoparticles [4].