Ischemic stroke is the leading cause of mortality and long-term disability worldwide. Disruption of the blood–brain barrier (BBB) is a prominent pathophysiological mechanism, responsible for a series of subsequent inflammatory cascades that exacerbate the damage to brain tissue. However, the benefit of recanalization is limited in most patients because of the narrow therapeutic time window. Recently, mesenchymal stem cells (MSCs) have been assessed as excellent candidates for cell-based therapy in cerebral ischemia, including neuroinflammatory alleviation, angiogenesis and neurogenesis promotion through their paracrine actions. In addition, accumulating evidence on how MSC therapy preserves BBB integrity after stroke may open up novel therapeutic targets for treating cerebrovascular diseases.
- blood–brain barrier
- permeability
- cell therapy
- matrix metalloproteinases
- inflammation
- ischemic stroke
- mesenchymal stem cell
- molecular mechanism
Note: The entry will be online only after author check and submit it.
1. Introduction
2. Potential Mechanisms of Blood–Brain Barrier Preservation by MSCs Following Ischemia
2.1. MMP Regulation and Attenuating Leukocytes Infiltrations
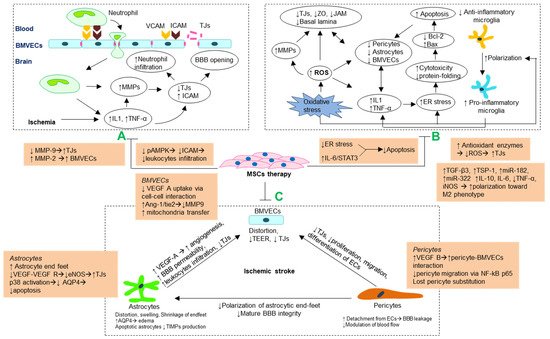
2.2. Antioxidant and Anti-Inflammatory Mechanism
2.3. Stabilizing Morphology and Crosstalk of Cellular Components Blood–Brain Barrier
2.3.1. Brain Microvascular Endothelial Cells
2.3.2. Astrocytes
2.3.3. Pericytes
| Reference | Signaling Pathway |
Component of BBB | Molecular Mechanism | Model | Number of Cells and Sources | Route | Time Treatment/Passage | |||
|---|---|---|---|---|---|---|---|---|---|---|
| [68] | [36] | ICAM/AMPK | MMPs, ICAM-1 | ↓ICAM-1 ↓neutrophil infiltration, ↓MMP-9 |
tMCAO | 2 × 10 | 5 | BMMSC |
ICV | 15 min/3 |
| [146] | [99] | P38 | AQP-4 astrocytes |
↓AQP-4, ↓neuroinflammatory, ↓apoptotic astrocytes | tMCAO | 2 × 10 | 5 | BMMSC | ICV | 20 min/3 |
| [27] | VEGF/eNOS | Astrocytes endfeet | ↑density of astrocytic endfeet, ↑VEGF/eNOS-dependent TJs | LPS | 1 × 10 | 6 | BMMSC | IV | 4 h/6 | |
| [115] | [62] | IL-6/STAT3 | Astrocytes | ↑IL-6 ↑anti-apoptosis of astrocytes |
HIBD | 2 × 10 | 5 | BMMSC |
ICV | 5 days/3–5 |
| [35] | [85] | VEGF/Flk1 Ang1/Tie2 | Astrocytes BMVECs | ↑Ang1/Tie2 → ↑occludins and VEGF/Flk1 expression ↑vascular maturation |
tMCAO | 3 × 10 | 6 | BMMSC | IV | 24 h/- |
| [108] | [55] | PRDX4 | BMVECs | ↑PRDX4-mediated antioxidant ↓ ROS |
tMCAO | 2 × 10 | 6 | BMMSC |
IV | 24 h/5–10 |
| [95] | [40] | - | BMVECs | ↓Neutrophil infiltration ↓Endothelial damage |
GCI | 1 × 10 | 6 | ADMSC |
IV | Immediately/2 |
| [125] | [72] | ANXA1-FPR | BMVECs | ↓endothelial resistance | UCO OGD | BMMSC-EVs 2doses ~2 × 10 | 7 | IV | 1,4 days/- | |
| [134] | [82] | Mitochondrial TNTs |
BMVECs | Transfer mitochondrial to BMVECs via TNTs→↓oxidative stress | tMCAO | 5 × 10 | 5 | BMMSC | IA | 24 h/3–5 |
| [133] | [81] | Mitochondrial TNTs |
hUVECs | Transfer mitochondrial to hUVECs via TNTs→↓oxidative stress | OGD RO |
- | - | 4 h/3–5 | ||
| [142] | [92] | TGF-β Smad2/3 |
BMVECs | ↑VEGF, ↑Ang-1 | pMCAO | 2 × 10 | 6 | BMMSC | IV | 3 h/3 |
| [36] | [76] | VEGF | Gap junctionBMVECs | ↑gap junction-mediated cell-cell interaction ↓glucose, ↓VEGF uptake in ECs |
pMCAO | 5 × 10 | 5 | BMMSC |
IV | 24 h/9 |
| [37] | [100] | NF-kB p65 | Pericytes | ↓NF-kB p65→↓pericyte migration | SCI | BMMSC-EVs 1 dose ~2 × 10 | 6 | IV | 30 min/3–5 | |
| [110] | [57] | ER stress | ER Astrocytes |
↓ER stress-induced apoptosis ↓inflammation |
tMCAO | 2 × 10 | 6 | 3 doses ADMSC |
IV | 0, 12, 24 h/2 |
| [94] | [37] | - | - | ↓pro-inflammatory, ↓polarize towards M1-phenotype | pMCAO | 4 × 10 | 6 | cells/kg hAMSC | IV | 24 h/- |
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596.
- Johnson, C.O.; Nguyen, M.; Roth, G.A.; Nichols, E.; Alam, T.; Abate, D.; Abd-Allah, F.; Abdelalim, A.; Abraha, H.N.; Abu-Rmeileh, N.M.; et al. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458.
- Candelario-Jalil, E. Injury and repair mechanisms in ischemic stroke: Considerations for the development of novel neurotherapeutics. Curr. Opin. Investig. Drugs (Lond. Engl. 2000) 2009, 10, 644–654.
- Neuwelt, E.A. Mechanisms of disease: The blood-brain barrier. Neurosurgery 2004, 54, 131–142.
- Persidsky, Y.; Ramirez, S.; Haorah, J.; Kanmogne, G.D. Blood–brain Barrier: Structural Components and Function Under Physiologic and Pathologic Conditions. J. Neuroimmune Pharmacol. 2006, 1, 223–236.
- Saunders, N.R.; Liddelow, S.; Dziegielewska, K.M. Barrier Mechanisms in the Developing Brain. Front. Pharmacol. 2012, 3, 46.
- Goldstein, G.W.; Betz, A.L. The blood-brain barrier. Sci. Am. 1986, 255, 74–83.
- Schoknecht, K.; Shalev, H. Blood-brain barrier dysfunction in brain diseases: Clinical experience. Epilepsia 2012, 53, 7–13.
- Yang, Y.; Rosenberg, G.A. Blood-Brain Barrier Breakdown in Acute and Chronic Cerebrovascular Disease. Stroke 2011, 42, 3323–3328.
- Qin, W.; Li, J.; Zhu, R.; Gao, S.; Fan, J.; Xia, M.; Zhao, R.C.; Zhang, J. Melatonin protects blood-brain barrier integrity and permeability by inhibiting matrix metalloproteinase-9 via the NOTCH3/NF-κB pathway. Aging 2019, 11, 11391–11415.
- Malemud, C.J. Matrix metalloproteinases (MMPs) in health and disease: An overview. Front. Biosci. 2006, 11, 1696–1701.
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.; Rosenberg, G.A. Matrix Metalloproteinase-Mediated Disruption of Tight Junction Proteins in Cerebral Vessels is Reversed by Synthetic Matrix Metalloproteinase Inhibitor in Focal Ischemia in Rat. Br. J. Pharmacol. 2006, 27, 697–709.
- Rosell, A.; Lo, E.H. Multiphasic roles for matrix metalloproteinases after stroke. Curr. Opin. Pharmacol. 2008, 8, 82–89.
- Chelluboina, B.; Nalamolu, K.R.; Mendez, G.G.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Veeravalli, K.K. Mesenchymal Stem Cell Treatment Prevents Post-Stroke Dysregulation of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases. Cell. Physiol. Biochem. 2017, 44, 1360–1369.
- Paolinelli, R.; Corada, M.; Orsenigo, F.; Dejana, E. The molecular basis of the blood brain barrier differentiation and maintenance. Is it still a mystery? Pharmacol. Res. 2011, 63, 165–171.
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418.
- Larrue, V.; von Kummer, R.; Müller, A.; Bluhmki, E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: A secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 2001, 32, 438–441.
- Warach, S.; Latour, L.L. Evidence of Reperfusion Injury, Exacerbated by Thrombolytic Therapy, in Human Focal Brain Ischemia Using a Novel Imaging Marker of Early Blood–Brain Barrier Disruption. Stroke 2004, 35, 2659–2661.
- Nagpal, A.; Choy, F.C.; Howell, S.; Hillier, S.; Chan, F.; Hamilton-Bruce, M.A.; Koblar, S.A. Safety and effectiveness of stem cell therapies in early-phase clinical trials in stroke: A systematic review and meta-analysis. Stem Cell Res. Ther. 2017, 8, 1–13.
- Lees, J.S.; Sena, E.S.; Egan, K.; Antonic-Baker, A.; Koblar, S.; Howells, D.; MacLeod, M.R. Stem Cell-Based Therapy for Experimental Stroke: A Systematic Review and Meta-Analysis. Int. J. Stroke 2012, 7, 582–588.
- Wagenaar, N.; Nijboer, C.H.; Van Bel, F. Repair of neonatal brain injury: Bringing stem cell-based therapy into clinical practice. Dev. Med. Child Neurol. 2017, 59, 997–1003.
- Yong, K.W.; Choi, J.R.; Mohammadi, M.; Mitha, A.P.; Sanati-Nezhad, A.; Sen, A. Mesenchymal Stem Cell Therapy for Ischemic Tissues. Stem Cells Int. 2018, 2018, 1–11.
- Zhang, P.; Li, J.; Liu, Y.; Chen, X.; Kang, Q. Transplanted human embryonic neural stem cells survive, migrate, differentiate and increase endogenous nestin expression in adult rat cortical peri-infarction zone. Neuropathology 2009, 29, 410–421.
- Tae-Hoon, L.; Yoon-Seok, L. Transplantation of mouse embryonic stem cell after middle cerebral artery occlusion. Acta Cir. Bras. 2012, 27, 333–339.
- Huang, L.; Wong, S.; Snyder, E.Y.; Hamblin, M.H.; Lee, J.-P. Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury. Stem Cell Res. Ther. 2014, 5, 1–16.
- Leu, S.; Lin, Y.-C.; Yuen, C.-M.; Yen, C.-H.; Kao, Y.-H.; Sun, C.-K.; Yip, H.-K. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J. Transl. Med. 2010, 8, 63.
- Park, H.J.; Shin, J.Y.; Na Kim, H.; Oh, S.H.; Song, S.K.; Lee, P.H. Mesenchymal stem cells stabilize the blood–brain barrier through regulation of astrocytes. Stem Cell Res. Ther. 2015, 6, 187.
- Fan, Y.; Shen, F.; Frenzel, T.; Zhu, W.; Ye, J.; Liu, J.; Chen, Y.; Su, H.; Young, W.L.; Yang, G.-Y. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann. Neurol. 2009, 67, 488–497.
- Chau, M.J.; Deveau, T.C.; Song, M.; Gu, X.; Chen, N.; Wei, L. iPSC Transplantation Increases Regeneration and Functional Recovery After Ischemic Stroke in Neonatal Rats. Stem Cells 2014, 32, 3075–3087.
- Honmou, O.; Onodera, R.; Sasaki, M.; Waxman, S.G.; Kocsis, J.D. Mesenchymal stem cells: Therapeutic outlook for stroke. Trends Mol. Med. 2012, 18, 292–297.
- Klingemann, H.; Matzilevich, D.; Marchand, J. Mesenchymal Stem Cells—Sources and Clinical Applications. Transfus. Med. Hemotherapy 2008, 35, 2.
- Lalu, M.M.; Montroy, J.; Dowlatshahi, D.; Hutton, B.; Juneau, P.; Wesch, N.; Zhang, S.Y.; McGinn, R.; Corbett, D.; Stewart, D.J.; et al. From the Lab to Patients: A Systematic Review and Meta-Analysis of Mesenchymal Stem Cell Therapy for Stroke. Transl. Stroke Res. 2019, 11, 345–364.
- Satani, N.; Cai, C.; Giridhar, K.; McGhiey, D.; George, S.; Parsha, K.; Nghiem, D.M.; Valenzuela, K.S.; Riecke, J.; Vahidy, F.; et al. World-Wide Efficacy of Bone Marrow Derived Mesenchymal Stromal Cells in Preclinical Ischemic Stroke Models: Systematic Review and Meta-Analysis. Front. Neurol. 2019, 10, 405.
- Li, Z.; Dong, X.; Tian, M.; Liu, C.; Wang, K.; Li, L.; Liu, Z.; Liu, J. Stem cell-based therapies for ischemic stroke: A systematic review and meta-analysis of clinical trials. Stem Cell Res. Ther. 2020, 11, 1–13.
- Liu, L.; Eckert, M.A.; Riazifar, H.; Kang, D.-K.; Agalliu, D.; Zhao, W. From Blood to the Brain: Can Systemically Transplanted Mesenchymal Stem Cells Cross the Blood-Brain Barrier? Stem Cells Int. 2013, 2013, 1–7.
- Cheng, Z.; Wang, L.; Qu, M.; Liang, H.; Li, W.; Li, Y.; Deng, L.; Zhang, Z.; Yang, G.-Y. Mesenchymal stem cells attenuate blood-brain barrier leakage after cerebral ischemia in mice. J. Neuroinflamm. 2018, 15, 1–11.
- Yoshida, Y.; Takagi, T.; Kuramoto, Y.; Tatebayashi, K.; Shirakawa, M.; Yamahara, K.; Doe, N.; Yoshimura, S. Intravenous Administration of Human Amniotic Mesenchymal Stem Cells in the Subacute Phase of Cerebral Infarction in a Mouse Model Ameliorates Neurological Disturbance by Suppressing Blood Brain Barrier Disruption and Apoptosis via Immunomodulation. Cell Transplant. 2021, 30, 09636897211024183.
- Yang, Y.; Rosenberg, G.A. Matrix metalloproteinases as therapeutic targets for stroke. Brain Res. 2015, 1623, 30–38.
- Nam, H.S.; Kwon, I.; Lee, B.H.; Kim, H.; Kim, J.; An, S.; Lee, O.-H.; Lee, P.H.; Kim, H.O.; Namgoong, H.; et al. Effects of Mesenchymal Stem Cell Treatment on the Expression of Matrix Metalloproteinases and Angiogenesis during Ischemic Stroke Recovery. PLoS ONE 2015, 10, e0144218.
- Chung, T.N.; Kim, J.H.; Choi, B.Y.; Chung, S.P.; Kwon, S.W.; Suh, S.W. Adipose-derived mesenchymal stem cells reduce neuronal death after transient global cerebral ischemia through prevention of blood-brain barrier disruption and endothelial damage. Stem Cells Transl. Med. 2014, 4, 178–185.
- Wang, C.; Börger, V.; Sardari, M.; Murke, F.; Skuljec, J.; Pul, R.; Hagemann, N.; Dzyubenko, E.; Dittrich, R.; Gregorius, J.; et al. Mesenchymal Stromal Cell–Derived Small Extracellular Vesicles Induce Ischemic Neuroprotection by Modulating Leukocytes and Specifically Neutrophils. Stroke 2020, 51, 1825–1834.
- Yang, L.; Froio, R.M.; Sciuto, T.E.; Dvorak, A.M.; Alon, R.; Luscinskas, F.W. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-α-activated vascular endothelium under flow. Blood 2005, 106, 584–592.
- Hardie, D.G.; Carling, D.; Carlson, M. The amp-activated/snf1 protein kinase subfamily: Metabolic Sensors of the Eukaryotic Cell? Annu. Rev. Biochem. 1998, 67, 821–855.
- Ren, G.; Zhao, X.; Zhang, L.; Zhang, J.; L’Huillier, A.; Ling, W.; Roberts, A.I.; Le, A.D.; Shi, S.; Shao, C.; et al. Inflammatory Cytokine-Induced Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1 in Mesenchymal Stem Cells Are Critical for Immunosuppression. J. Immunol. 2010, 184, 2321–2328.
- Wu, T.-Y.; Liang, Y.-H.; Wu, J.-C.; Wang, H.-S. Interleukin-1β Enhances Umbilical Cord Mesenchymal Stem Cell Adhesion Ability on Human Umbilical Vein Endothelial Cells via LFA-1/ICAM-1 Interaction. Stem Cells Int. 2019, 2019, 1–13.
- Rosenberg, G.A.; Estrada, E.Y.; Dencoff, J.E. Matrix Metalloproteinases and TIMPs Are Associated With Blood-Brain Barrier Opening After Reperfusion in Rat Brain. Stroke 1998, 29, 2189–2195.
- Fujimoto, M.; Takagi, Y.; Aoki, T.; Hayase, M.; Marumo, T.; Gomi, M.; Nishimura, M.; Kataoka, H.; Hashimoto, N.; Nozaki, K. Tissue Inhibitor of Metalloproteinases Protect Blood—Brain Barrier Disruption in Focal Cerebral Ischemia. Br. J. Pharmacol. 2008, 28, 1674–1685.
- Tejima, E.; Guo, S.; Murata, Y.; Arai, K.; Lok, J.; van Leyen, K.; Rosell, A.; Wang, X.; Lo, E.H. Neuroprotective effects of overexpressing tissue inhibitor of metalloproteinase TIMP-1. J. Neurotrauma 2009, 26, 1935–1941.
- Gardner, J.; Ghorpade, A. Tissue inhibitor of metalloproteinase (TIMP)-1: The TIMPed balance of matrix metalloproteinases in the central nervous system. J. Neurosci. Res. 2003, 74, 801–806.
- Menge, T.; Zhao, Y.; Zhao, J.; Wataha, K.; Gerber, M.; Zhang, J.; Letourneau, P.; Redell, J.; Shen, L.; Wang, J.; et al. Mesenchymal Stem Cells Regulate Blood-Brain Barrier Integrity Through TIMP3 Release After Traumatic Brain Injury. Sci. Transl. Med. 2012, 4, 161ra150.
- Lakhan, S.E.; Kirchgessner, A.; Tepper, D.; Leonard, A. Matrix Metalloproteinases and Blood-Brain Barrier Disruption in Acute Ischemic Stroke. Front. Neurol. 2013, 4, 32.
- Muir, E.; Adcock, K.; Morgenstern, D.; Clayton, R.; von Stillfried, N.; Rhodes, K.; Ellis, C.; Fawcett, J.; Rogers, J. Matrix metalloproteases and their inhibitors are produced by overlapping populations of activated astrocytes. Mol. Brain Res. 2002, 100, 103–117.
- Huang, W.; Wang, T.; Zhang, D.; Zhao, T.; Dai, B.; Ashraf, A.; Wang, X.; Xu, M.; Millard, R.W.; Fan, G.-C.; et al. Mesenchymal Stem Cells Overexpressing CXCR4 Attenuate Remodeling of Postmyocardial Infarction by Releasing Matrix Metalloproteinase-9. Stem Cells Dev. 2012, 21, 778–789.
- Song, K.; Li, Y.; Zhang, H.; An, N.; Wei, Y.; Wang, L.; Tian, C.; Yuan, M.; Sun, Y.; Xing, Y.; et al. Oxidative Stress-Mediated Blood-Brain Barrier (BBB) Disruption in Neurological Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 1–27.
- Huang, Y.; Wang, J.; Cai, J.; Qiu, Y.; Zheng, H.; Lai, X.; Sui, X.; Wang, Y.; Lu, Q.; Zhang, Y.; et al. Targeted homing of CCR2-overexpressing mesenchymal stromal cells to ischemic brain enhances post-stroke recovery partially through PRDX4-mediated blood-brain barrier preservation. Theranostics 2018, 8, 5929–5944.
- Chen, X.; Liang, H.; Xi, Z.; Yang, Y.; Shan, H.; Wang, B.; Zhong, Z.; Xu, C.; Yang, G.-Y.; Sun, Q.; et al. BM-MSC Transplantation Alleviates Intracerebral Hemorrhage-Induced Brain Injury, Promotes Astrocytes Vimentin Expression, and Enhances Astrocytes Antioxidation via the Cx43/Nrf2/HO-1 Axis. Front. Cell Dev. Biol. 2020, 8, 302.
- Chi, L.; Huang, Y.; Mao, Y.; Wu, K.; Zhang, L.; Nan, G. Tail Vein Infusion of Adipose-Derived Mesenchymal Stem Cell Alleviated Inflammatory Response and Improved Blood Brain Barrier Condition by Suppressing Endoplasmic Reticulum Stress in a Middle Cerebral Artery Occlusion Rat Model. Med. Sci. Monit. 2018, 24, 3946–3957.
- Evans, M.A.; Broughton, B.R.S.; Drummond, G.R.; Ma, H.; Phan, T.G.; Wallace, E.M.; Lim, R.; Sobey, C.G. Amnion epithelial cells–a novel therapy for ischemic stroke? Neural Regen. Res. 2018, 13, 1346.
- Chen, X.; Yang, B.; Tian, J.; Hong, H.; Du, Y.; Li, K.; Li, X.; Wang, N.; Yu, X.; Wei, X. Dental Follicle Stem Cells Ameliorate Lipopolysaccharide-Induced Inflammation by Secreting TGF-β3 and TSP-1 to Elicit Macrophage M2 Polarization. Cell. Physiol. Biochem. 2018, 51, 2290–2308.
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc. Res. 2019, 115, 1205–1216.
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019, 2019, 7132708.
- Gu, Y.; He, M.; Zhou, X.; Liu, J.; Hou, N.; Bin, T.; Zhang, Y.; Li, T.; Chen, J. Endogenous IL-6 of mesenchymal stem cell improves behavioral outcome of hypoxic-ischemic brain damage neonatal rats by supressing apoptosis in astrocyte. Sci. Rep. 2016, 6, srep18587.
- Borlongan, C.V.; Zhang, Y.; Yu, S.; Tuazon, J.P.; Lee, J.-Y.; Corey, S.; Kvederis, L.; Kingsbury, C.; Kaneko, Y. Neuroprotective effects of human bone marrow mesenchymal stem cells against cerebral ischemia are mediated in part by an anti-apoptotic mechanism. Neural Regen. Res. 2019, 14, 597–604.
- Kaufman, R.J. Orchestrating the unfolded protein response in health and disease. J. Clin. Investig. 2002, 110, 1389–1398.
- Szegezdi, E.; Logue, S.; Gorman, A.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885.
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a Major Cytokine in the Central Nervous System. Int. J. Biol. Sci. 2012, 8, 1254–1266.
- McArthur, S.; Loiola, R.A.; Maggioli, E.; Errede, M.; Virgintino, D.; Solito, E. The restorative role of annexin A1 at the blood–brain barrier. Fluids Barriers CNS 2016, 13, 17.
- Luo, Z.Z.; Gao, Y.; Sun, N.; Zhao, Y.; Wang, J.; Tian, B.; Shi, J. Enhancing the interaction between annexin-1 and formyl peptide receptors regulates microglial activation to protect neurons from ischemia-like injury. J. Neuroimmunol. 2014, 276, 24–36.
- Chen, K.; Bao, Z.; Gong, W.; Tang, P.; Yoshimura, T.; Wang, J.M. Regulation of inflammation by members of the formyl-peptide receptor family. J. Autoimmun. 2017, 85, 64–77.
- He, H.-Q.; Ye, R.D. The Formyl Peptide Receptors: Diversity of Ligands and Mechanism for Recognition. Molecules 2017, 22, 455.
- Maia, L.; De Moraes, C.N.; Dias, M.C.; Martinez, J.B.; Caballol, A.O.; Testoni, G.; De Queiroz, C.M.; Peña, R.D.; Landim-Alvarenga, F.; De Oliveira, E. A proteomic study of mesenchymal stem cells from equine umbilical cord. Theriogenology 2017, 100, 8–15.
- Gussenhoven, R.; Klein, L.; Ophelders, D.R.M.G.; Habets, D.H.J.; Giebel, B.; Kramer, B.W.; Schurgers, L.J.; Reutelingsperger, C.P.M.; Wolfs, T.G.A.M. Annexin A1 as Neuroprotective Determinant for Blood-Brain Barrier Integrity in Neonatal Hypoxic-Ischemic Encephalopathy. J. Clin. Med. 2019, 8, 137.
- Cristante, E.; McArthur, S.; Mauro, C.; Maggioli, E.; Romero, I.A.; Wylezinska-Arridge, M.; Couraud, P.O.; Lopez-Tremoleda, J.; Christian, H.C.; Weksler, B.B.; et al. Identification of an essential endogenous regulator of blood-brain barrier integrity, and its pathological and therapeutic implications. Proc. Natl. Acad. Sci. USA 2013, 110, 832–841.
- Sheikh, M.H.; Solito, E. Annexin A1: Uncovering the Many Talents of an Old Protein. Int. J. Mol. Sci. 2018, 19, 1045.
- McArthur, S.; Cristante, E.; Paterno, M.; Christian, H.; Roncaroli, F.; Gillies, G.E.; Solito, E. Annexin A1: A Central Player in the Anti-Inflammatory and Neuroprotective Role of Microglia. J. Immunol. 2010, 185, 6317–6328.
- Kikuchi-Taura, A.; Okinaka, Y.; Saino, O.; Takeuchi, Y.; Ogawa, Y.; Kimura, T.; Gul, S.; Claussen, C.; Boltze, J.; Taguchi, A. Gap junction-mediated cell-cell interaction between transplanted mesenchymal stem cells and vascular endothelium in stroke. Stem Cells 2021, 39, 904–912.
- Weis, S.M.; Cheresh, D.A. Pathophysiological consequences of VEGF-induced vascular permeability. Nat. Cell Biol. 2005, 437, 497–504.
- Zhang, H.-T.; Zhang, P.; Gao, Y.; Li, C.-L.; Wang, H.-J.; Chen, L.-C.; Feng, Y.; Li, R.-Y.; Li, Y.-L.; Jiang, C.-L. Early VEGF inhibition attenuates blood-brain barrier disruption in ischemic rat brains by regulating the expression of MMPs. Mol. Med. Rep. 2017, 15, 57–64.
- Kluge, M.A.; Fetterman, J.L.; Vita, J.A. Mitochondria and Endothelial Function. Circ. Res. 2013, 112, 1171–1188.
- Nawaz, M.; Fatima, F. Extracellular Vesicles, Tunneling Nanotubes, and Cellular Interplay: Synergies and Missing Links. Front. Mol. Biosci. 2017, 4, 50.
- Liu, K.; Ji, K.; Guo, L.; Wu, W.; Lu, H.; Shan, P.; Yan, C. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia–reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc. Res. 2014, 92, 10–18.
- Liu, K.; Guo, L.; Zhou, Z.; Pan, M.; Yan, C. Mesenchymal stem cells transfer mitochondria into cerebral microvasculature and promote recovery from ischemic stroke. Microvasc. Res. 2019, 123, 74–80.
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial transfer from bone-marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012, 18, 759–765.
- Rodriguez, A.-M.; Nakhle, J.; Griessinger, E.; Vignais, M.L. Intercellular mitochondria trafficking highlighting the dual role of mesenchymal stem cells as both sensors and rescuers of tissue injury. Cell Cycle 2018, 17, 712–721.
- Zacharek, A.; Chen, J.; Cui, X.; Li, A.; Li, Y.; Roberts, C.; Feng, Y.; Gao, Q.; Chopp, M. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J. Cereb. Blood Flow Metab. 2007, 27, 1684–1691.
- Valable, S.; Montaner, J.; Bellail, A.; Berezowski, V.; Brillault, J.; Cecchelli, R.; Divoux, D.; MacKenzie, E.T.; Bernaudin, M.; Roussel, S.; et al. VEGF-Induced BBB Permeability is Associated with an MMP-9 Activity Increase in Cerebral ischemia: Both Effects Decreased by ANG-1. Br. J. Pharmacol. 2005, 25, 1491–1504.
- Li, W.-L.; Fraser, J.L.; Yu, S.P.; Zhu, J.; Jiang, Y.-J.; Wei, L. The role of VEGF/VEGFR2 signaling in peripheral stimulation-induced cerebral neurovascular regeneration after ischemic stroke in mice. Exp. Brain Res. 2011, 214, 503–513.
- Zhao, J.; Chen, L.; Shu, B.; Tang, J.; Zhang, L.; Xie, J.; Qi, S.; Xu, Y. Granulocyte/Macrophage Colony-Stimulating Factor Influences Angiogenesis by Regulating the Coordinated Expression of VEGF and the Ang/Tie System. PLoS ONE 2014, 9, e92691.
- Thurston, G.; Rudge, J.S.; Ioffe, E.; Zhou, H.; Ross, L.; Croll, S.; Glazer, N.; Holash, J.; McDonald, D.M.; Yancopoulos, G.D. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat. Med. 2000, 6, 460–463.
- Zhang, Z.G.; Zhang, L.; Croll, S.D.; Chopp, M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience 2002, 113, 683–687.
- Xiang, J.; Hu, J.; Shen, T.; Liu, B.; Hua, F.; Zan, K.; Zu, J.; Cui, G.; Ye, X. Bone marrow mesenchymal stem cells-conditioned medium enhances vascular remodeling after stroke in type 2 diabetic rats. Neurosci. Lett. 2017, 644, 62–66.
- Zhang, Q.; Zhou, M.; Wu, X.; Li, Z.; Liu, B.; Gao, W.; Yue, J.; Liu, T. Promoting therapeutic angiogenesis of focal cerebral ischemia using thrombospondin-4 (TSP4) gene-modified bone marrow stromal cells (BMSCs) in a rat model. J. Transl. Med. 2019, 17, 1–13.
- Argaw, A.T.; Asp, L.; Zhang, J.; Navrazhina, K.; Pham, T.; Mariani, J.N.; Mahase, S.; Dutta, D.; Seto, J.; Kramer, E.G.; et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Investig. 2012, 122, 2454–2468.
- Argaw, A.T.; Gurfein, B.T.; Zhang, Y.; Zameer, A.; John, G.R. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. USA 2009, 106, 1977–1982.
- Herx, L.M.; Yong, V.W. Interleukin-1β is Required for the Early Evolution of Reactive Astrogliosis Following CNS Lesion. J. Neuropathol. Exp. Neurol. 2001, 60, 961–971.
- Kim, Y.-J.; Park, H.-J.; Lee, G.; Bang, O.Y.; Ahn, Y.H.; Joe, E.; Kim, H.O.; Lee, P.H. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia 2009, 57, 13–23.
- Manley, G.T.; Fujimura, M.; Ma, T.; Noshita, N.; Filiz, F.; Bollen, A.W.; Chan, P.; Verkman, A. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000, 6, 159–163.
- Fu, X.; Li, Q.; Feng, Z.; Mu, D. The roles of aquaporin-4 in brain edema following neonatal hypoxia ischemia and reoxygenation in a cultured rat astrocyte model. Glia 2007, 55, 935–941.
- Tang, G.; Liu, Y.; Zhang, Z.; Lu, Y.; Wang, Y.; Huang, J.; Li, Y.; Chen, X.; Gu, X.; Wang, Y.; et al. Mesenchymal Stem Cells Maintain Blood-Brain Barrier Integrity by Inhibiting Aquaporin-4 Upregulation After Cerebral Ischemia. Stem Cells 2014, 32, 3150–3162.
- Lu, Y.; Zhou, Y.; Zhang, R.; Wen, L.; Wu, K.; Li, Y.; Yao, Y.; Duan, R.; Jia, Y. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Recovery Following Spinal Cord Injury via Improvement of the Integrity of the Blood-Spinal Cord Barrier. Front. Neurosci. 2019, 13, 209.
- Leblanc, N.J.; Guruswamy, R.; ElAli, A. Vascular Endothelial Growth Factor Isoform-B Stimulates Neurovascular Repair After Ischemic Stroke by Promoting the Function of Pericytes via Vascular Endothelial Growth Factor Receptor-1. Mol. Neurobiol. 2018, 55, 3611–3626.
- Tian, X.; Brookes, O.; Battaglia, G. Pericytes from Mesenchymal Stem Cells as a model for the blood-brain barrier. Sci. Rep. 2017, 7, 39676.
- da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213.
