Essential oil (EO)-based mouthwashes have been used for oral health maintenance due to their antimicrobial and anti-inflammatory properties. The aim was to review clinical trials that assessed the role of EO-based mouthwashes in controlling gingivitis in patients undergoing fixed orthodontic treatment (OT). The Patients, Interventions, Control and Outcome (PICO) format was based on the following: (a) P: Patients undergoing fixed OT (b) Intervention: EO-based mouth-wash; Control: Mouthwashes that did not contain EOs or no mouthwash (d) Outcome: Control of gingivitis measured by clinical indices.
- essential oil
- mouthwash
- gingivitis
1. Introduction
Essential oils (EOs) are organic compounds that are extracted from plants with various distillation methods [1]. Historically, they have been utilized in process of manufacturing perfumes due to their strong and characteristic scent, as well as in food and beverage industries [2]. The EO-derivatives possess anti-inflammatory and antimicrobial properties and have been used in the field of clinical dentistry and related research [3]. Lavender, peppermint, cinnamon and clove oils have been shown to have an inhibitory effect on different bacteria and fungi [3]. Recently, EO-derivatives were found to be efficient in the management of orofacial pain due to their analgesic properties [4,5]. Furthermore, studies indicated that EOs are capable of the management of dental anxiety before certain surgical procedures [6,7].
Patients undergoing fixed orthodontic treatment (OT) are more prone to gingival inflammation because fixed orthodontic appliances are bulky and create a favorable environment for plaque accumulation [8,9]. Mechanical plaque removal poses a challenge for orthodontic patients and different strategies have been implemented in order to control plaque formation, prevent the development of gingivitis and maintain oral health [9]. More specifically, chemotherapeutic agents with antimicrobial properties, such as 0.12% chlorhexidine (CHX), have been proposed as an adjunct to the standard oral hygiene protocol [10]. However, prolonged use of these agents has been associated with side effects, such as hypersensitivity reactions, burning sensation and changes in taste and tooth color [11,12]. Another potential approach for the management of oral health in orthodontic patients is the use of EO-containing mouthwashes due to their antimicrobial and anti-inflammatory properties [13]. A recent study [13] investigated the effectiveness of mouthwash with 1% Matricaria chamomilla L. (MTC) in the management of gingivitis during OT, by comparing it to CHX and a placebo mouthwash. The authors concluded that the use of both CHX and MTC mouthwash significantly reduced gingival inflammation compared to the use of the placebo mouthwash [13]. Listerine ® is another EO-containing mouthwash that has been studied in the literature [14].
2. Research
A meticulous search of indexed literature revealed 63 studies. After title and abstract screening and removal of the duplicate studies, 17 studies were retrieved and evaluated in more detail. Eleven studies were excluded after full-text evaluation because they did not meet the eligibility criteria. In total, six studies (three RCTs, three non-RCTs) were included and processed for data extraction ( Figure 1 ).
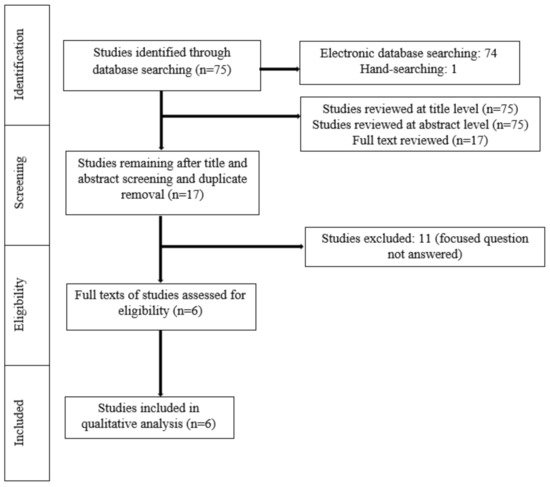
Random allocation of the participants to the groups was performed in three studies [11,13,15]. In the study by Bauer Faria et al. [17], 31 patients undergoing fixed OT used 3 different mouthwashes in a random order. Initially, all the participants were instructed to use a mouthwash containing 0.5% Zingiber officinale (ZO), then 0.12% CHX and finally a placebo mouthwash [17]. Tufekci et al. (10), reported that the age and gender of the participants could play a significant role in their compliance and could therefore influence the findings of the study. As a result, patients were matched for both these variables [10]. In the study by Akbulut [16], no randomization of the participants was mentioned. In five studies [10,11,13,15,17], the number of participants ranged between 30 and 79. In the study by Akbulut [16], the groups were formed according to the number of mini screws. More specifically, 38 patients were divided into 4 groups, with each group consisting of 15 mini screws [16]. The number of males and females ranged between 4 and 20 and 17 and 27, respectively [10,13,15,16,17]. One study [11] did not report the number of male and female participants. In five studies [10,11,13,15,17], the age of the patients ranged between 10 and 64 years. In the study by Akbulut [16], the age of the participants was not mentioned. In all studies [10,11,13,15,16,17], a variation was identified at the clinical indices used to assess the gingival status of the participants (Plaque Index, Visible Plaque Index, Modified Plaque Index, Bleeding Index, Gingival Bleeding Index, Gingival Index, and Modified Gingival Index). The duration of follow-up also varied among studies [10,11,13,15,16,17] from baseline (0 days of treatment) to 180 days of intervention ( Table 1 ). All studies [10,11,13,15,16,17] included participants who used a mouthwash that contained EOs (test group) and those who either used mouthwashes that did not contain EOs or did not use any mouthwash (control group) during OT with fixed appliances. A power analysis was performed in three studies [10,11,17] ( Table 2 ).
| Authors | Type of Study | Participants | Gender | Age in Years (Range) |
Clinical Indices | Duration of Follow-Up | ||
|---|---|---|---|---|---|---|---|---|
| Tufekci et al. (2008) [ | 10 | ] | Non-RCT | 47 | 20 M 27 F |
16.6 years * (10–64) |
PI, BI and MGI | 90 and 180 days |
| Chen et al. (2013) [ | 11 | ] | RCT | 79 | NR | 17.7 ± 3.9 years | PI, BI and MGI | 90 and 180 days |
| Goes et al. (2016) [ | 13 | ] | RCT | 30 | 4 M 26 F |
28.8 ± 3.28 years (10–40) |
VPI and GBI | 15 days |
| Alves et al. (2010) [ | 15 | ] | RCT | 30 | 10 M 20 F |
12–21 years | VPI and GI | 60 days |
| Akbulut ** (2020) [ | 16 | ] | Non-RCT | 38 (60 mini screws) | 18 M 20 F |
NR | MPI and MGI | 21 days |
| Bauer Faria et al. (2021) [ | 17 | ] | Non-RCT | 31 | 14 M 17 F |
19.96 years (12–35) |
GBI | 7 days |
| Authors (Year) | Test Group ( | n | = Number of Patients) | Control Group ( | n | = Number of Patients) | Power Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tufekci et al. (2008) [ | 10 | ] | EO-based MW ( | n | = 24) | No MW ( | n | = 23) | Yes | ||||
| Chen et al. (2013) [ | 11 | ] | EO-based MW 1 ( | n | = 28) EO-based MW 2 ( | n | = 25) | No MW ( | n | = 26) | Yes | ||
| Goes et al. (2016) [ | 13 | ] | EO-based MW ( | n | = 10) | CHX ( | n | = 10) Placebo MW ( | n | = 10) | No | ||
| Alves et al. (2010) [ | 15 | ] | EO-based MW ( | n | = 10) | Placebo MW ( | n | = 10) No MW ( | n | = 10) | No | ||
| Akbulut (2020) [ | 16 | ] | EO-based MW ( | n | = NR) | CHX ( | n | = NR) Povidone-iodine MW ( | n | = NR) No MW ( | n | = NR) | No |
| Bauer Faria et al. (2021) [ | 17 | ] | EO-based MW ( | n | = 31) | CHX ( | n | = 31) Placebo MW ( | n | = 31) | Yes |
The participants in the test group used a variation of mouthwashes that contained EOs, including Listerine ® [10,11,15,16] , 1% MTC [13], 0.5% ZO [17] and 2.5% Fructus mume (FM) [11]. Moreover, different mouthwashes were used by subjects in the control group, including 0.12% CHX [13,16,17], 7.5% povidone-iodine [16] and placebo [13,15,17]. In four studies [10,11,15,16], the participants in the control group were instructed to brush and floss, but not use any mouthwash. In four studies [10,11,13,15] the mouthwashes were used twice daily. However, two studies did not report a specific protocol regarding the daily frequency of usage of the mouthwashes [16,17] ( Table 3 ).
| Authors (Year) | Type of MW | Concentration of MW | Daily Frequency of Usage | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test-Group | Control-Group | Test-Group | Control-Group | Test-Group | Control-Group | |||||
| Tufekci et al. (2008) [ | 10 | ] | Listerine | ® | No MW | NR | NA | 2× daily | NA | |
| Chen et al. (2013) [ | 11 | ] | Listerine | ® | Fructus mume |
No MW | NR 2.5% |
NA | 2× daily 2× daily |
NA |
| Goes et al. (2016) [ | 13 | ] | Matricaria chamomilla L. | CHX Placebo |
1% | 0.12% NR |
2× daily | 2× daily 2× daily |
||
| Alves et al. (2010) [ | 15 | ] | Listerine | ® | Placebo No MW |
NR | NR NA |
2× daily | 2× daily NA |
|
| Akbulut (2020) [ | 16 | ] | Listerine | ® | CHX Povidone iodine No MW |
NR | 0.12% 7.5% NA |
NR | NR NR NA |
|
| Bauer Faria et al. (2021) [ | 17 | ] | Zingiber officinale | CHX Distilled water |
0.5% | 0.12% NA |
NR | NR NA |
||
In the study by Tufekci et al. [10], all clinical indices were significantly higher in the group that did not use a mouthwash compared with the Listerine ® group after 90 and 180 days. It was concluded that Listerine ® is efficient in controlling plaque accumulation and gingivitis in patients undergoing fixed OT [10]. In the study by Alves et al. [15], Listerine ® was also found to be more successful in controlling gingivitis compared with a placebo mouthwash. Chen et al. [11] reported that the use of both Listerine ® and FM mouthwashes resulted in a significant reduction in bleeding of gingival tissue compared with the use of no mouthwash. Furthermore, Listerine ® and FM mouthwashes were found to be equally efficient in promoting oral health [11]. In another study [16], the use of both Listerine ® and CHX significantly improved the oral hygiene status of patients with orthodontic mini screws compared with the use of povidone iodine or no mouthwash. Goes et al. [13] reported that both CHX and MTC mouthwashes were more effective in controlling plaque accumulation and gingival bleeding compared with a placebo mouthwash. Moreover, there were no differences when CHX was compared with the MTC mouthwash [13]. In the study by Bauer Faria et al. [17], ZO mouthwash had higher efficiency in controlling gingival inflammation compared with CHX and a placebo mouthwash ( Table 4 ).
| Authors (Year) | Main Outcomes | Side-Effects/Complications | Conclusion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tufekci et al. (2008) [ | 10 | ] | BI, MGI and PI were significantly higher in the group that did not use a mouthwash compared with the Listerine | ® | group after 90 and 180 days. | N/R | Listerine | ® | is effective in decreasing plaque accumulation and gingival bleeding in orthodontic patients. | ||
| Chen et al. (2013) [ | 11 | ] |
| No complications were mentioned by the patients. | Listerine | ® | and FM mouthwashes lead to decreased bleeding of gingival tissue in patients undergoing fixed orthodontic treatment. | ||||
| Goes et al. (2016) [ | 13 | ] |
|
|
| ||||||
| Alves et al. (2010) [ | 15 | ] |
| N/R | Listerine | ® | is an effective adjunct to oral hygiene in patients undergoing orthodontic treatment. | ||||
| Akbulut (2020) [ | 16 | ] |
| N/R | CHX, Listerine | ® | and povidone-iodine are effective in promoting oral health in patients with orthodontic mini screws. | ||||
| Bauer Faria et al. (2021) [ | 17 | ] | GBI was significantly higher in the CHX group compared with the ZO group after 7 days. | ZO and CHX have a low taste tolerance. | ZO reduces gingival bleeding and oral biofilm accumulation. |
3. Conclusions
Based upon the limited evidence available, EO-based mouthwashes seem to be effective for the management of gingivitis among patients undergoing fixed OT. Further well-designed and power-adjusted clinical trials are needed.
