You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Abdullah Hoter and Version 2 by Catherine Yang.
Inflammatory bowel disease (IBD) is a multifactorial human intestinal disease that arises from numerous, yet incompletely defined, factors. Two main forms, Crohn’s disease (CD) and ulcerative colitis (UC), lead to a chronic pathological form. Heat shock proteins (HSPs) are stress-responsive molecules involved in various pathophysiological processes.
- heat shock proteins
- inflammatory bowel disease
- HSPs targeting
1. Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract that implies dysregulated intestinal homeostasis. Clinically, IBD falls into two major types: Crohn’s disease (CD), which can impact any part of the gastrointestinal tract, and ulcerative colitis (UC), which displays restricted pathology to the colon [1] (see Figure 1). In fact, a complete knowledge of the exact mechanism that leads to the disease is still obscure; however, several factors contributing to the pathogenesis of IBD have been identified [1][2][1,2].
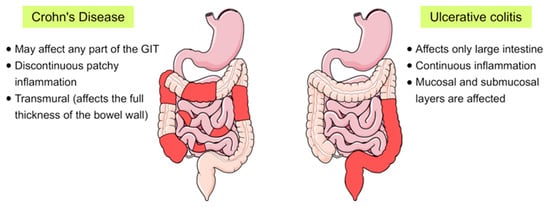
Figure 1. Schematic representation showing the main differences between the two main forms of inflammatory bowel disease (IBD), Crohn’s disease and ulcerative colitis, in terms of their location and the pattern of the affected areas (red-coloured) in the gastrointestinal tract (GIT).
IBD results from multifaceted factors which include genetic predisposition, environmental factors, smoking, dietary factors, intestinal microbiota and alterations in the function of the immune system [2][3][2,3]. It is believed that a complex interplay between these factors and host innate immunity can initiate the disease [1][3][1,3]. In accordance with these observations, our group has recently revealed that pro-inflammatory mediators including TNFα, MCP1 and IL-1β, whose levels are frequently elevated in IBD, can induce endoplasmic reticulum (ER) stress and alter the function and trafficking of key proteins of the intestinal brush border membranes [4]. Owing to the aforementioned complex factors and overlapping mechanisms along with the heterogenic nature of IBD, the disease has been recently identified as an ailment of “complex and variable” nature [5].
Heat shock proteins (HSPs) are highly conserved, stress-induced molecules that are ubiquitously expressed in all eukaryotic cells. These proteins are classically categorized according to their molecular mass into six major families whose members range from 10 to 170 kDa [6][7][6,7]. In addition, Kampinga and his colleagues provided an alternative classification that names HSP members in the form of a letter/number combination [8] (Table 1).
Table 1.
Summary of heat shock proteins (HSP) families and their common members.
| HSP Family | Alternative Family Name | Number of Members | Common Selected Members |
|---|
| HSP110 | HSPH | 4 | HSPH1 (HSP105), HSPH2 (HSP110, HSPA4) |
| HSP90 | HSPC | 5 | HSPC2 (HSP90α), HSPC3 (HSP90β), HSPC4 (GRP94, HSP90B1, GP96, endoplasmin), HSPC5 (TRAP1, HSP75, HSP90L) |
| HSP70 | HSPA | 13 | HSPA1A (HSP70-1), HSPA1B (HSP70-2) HSPA5 (BIP, GRP78), HSPA6 (HSP70B′), HSPA8 (HSC70), HSPA9 (GRP75) |
| HSP60 and HSP10 (Chaperonins) | HSPD and HSPE | 14 | HSPD1 (HSP60), HSPE1 (HSP10) |
| HSP40 | DNAJ | 50 | DNAJA1, DNAJB1 (HSPF1 and HSP40), DNAJC1 |
| Small HSPs | HSPB | 11 | HSPB1 (HSP27), HSPB4 (CRYAA) and HSPB5 (CRYAB) |
2. Heat Shock Proteins in IBD
2.1. HSP90
There is accumulating evidence linking HSP90 expression to the pathogenesis of IBD. Expression of HSP90 was found to be elevated in the intestinal mucosa of patients with UC at the time of diagnosis and to be reduced after therapy [9][30]. It has been therefore postulated that HSP90, together with other chaperones like HSP70 and HSP60, contributes to the development and maintenance of IBD [9][30]. Previous contradicting results were reported by Stahl and his colleagues who demonstrated the absence of significant differences between HSP90 levels in healthy and IBD patients [10][31]. However, fluctuations in the aforementioned levels of HSPs before and after IBD treatments supported the suggestion of utilizing HSPs as useful biomarkers in this disease [9][30]. A pioneering work by de Zoeten et al. has revealed a key role for Foxp3+ T-regulatory cells (Tregs) in intestinal homeostasis. A reduced Foxp3+ Tregs population has been linked to autoimmune diseases and allograft rejection. These Tregs highly express various histone/protein deacetylases (HDACs) that influence many cellular activities including gene expression, protein function and chromatin remodeling [11][32]. Interestingly, pan-HDAC inhibition, that has been used for cancer treatment, could enrich Treg production and impact the acetylation status of other non-histone proteins. Of particular importance, targeted inhibition of the HDAC6 isoform (HDAC6i) has been shown to enhance the suppressive functions of Tregs, regulate the HSR and affect HSP90 acetylation [11][32].
2.2. HSP70
Generally, elevated expression of HSP70 has been reported in patients with intestinal inflammatory diseases compared with healthy individuals [12][21]. Studies with experimentally induced colitis in mice have shown that overexpression of HSPs was not a universal response for all HSPs. In colorectal mucosa, the expression levels of HSP70 and HSP40 were increased, whereas the levels of HSP25, HSP32 and HSP90 remained unaltered [13][43]. Similar findings, reflecting comparatively high HSP70 mucosal expression, have been reported in UC and CD human patients compared with healthy controls [14][44]. Notably, HSP70 expression can change in response to treatment type and duration. For instance, a six-month treatment of UC patients with 5-aminosalicylic acid (5-ASA) preparations and probiotics resulted in modulation of the HSP70 expression pattern from high to normal in healthy individuals [9][30]. These observations support the notion that IBD treatments including chemotherapeutics and antibiotics act to eliminate intestinal commensal bacteria which harbour epitopes necessary for appropriate HSP70 induction [15][45]. Moreover, the previous results suggest that measurement of HSP levels in the intestinal mucosa imply a strong potential for monitoring the response to treatment in IBD [9][30].2.3. HSP60
Current reports strongly consider the mitochondrial chaperonin HSP60 as a major player in IBD pathogenesis and treatment [16][78]. To this end, Cappello and his colleagues enumerated several reasons supporting this hypothesis. Among these are: (a) The ability of HSP60 to stimulate pro-inflammatory cytokines [17][18][19][20][79,80,81,82]; (b) The variability of HSP60 levels in UC mucosa in response to the disease status [21][22][73,83]; (c) The implication of HSP60 in other inflammatory conditions such as atherosclerosis [23][24][23,84]; and finally (d) The “molecular mimicry” resulting from shared epitopes among human HSP60 and HSP60 from variant intestinal pathogenic microorganisms that ultimately leads to cross-reactivity and development of autoimmunity [25][26][27][28][25,85,86,87]. In addition, previous studies have revealed that HSP60 is upregulated in the lamina propria of the intestine in IBD patients compared to healthy individuals [22][83]. These findings suggest a potential role for HSP60 in inflammatory response activation [16][78]. In accordance with this conclusion, single or co-administration of 5-aminosalicylic acid (5-ASA) with probiotics to IBD patients resulted in mitigation of inflammation and simultaneous reduction of HSP60 expression levels [21][16][73,78]. An imbalance in gut microbiota has also been reported to impact HSP60 function in IBD [29][88]. Dietary probiotics led to lowered levels of HSP60 together with alteration in its post-translational modifications in mice models [30][89]. These reduced HSP60 concentrations have been attributed to HSP60 secretion into the extracellular milieu either in its soluble free form or via exosomes [31][90].2.4. Small HSPs
The fact that sHSPs are broadly associated with several intestinal pathologies makes them interesting targets to investigate in IBD. Alpha B-crystallin (CRYAB or HSPB5), a significant member of sHSPs, has been recently shown to regulate the intestinal inflammatory response in the intestinal mucosa. In vivo and in vitro studies have implicated CRYAB in the suppression of proinflammatory cytokines (e.g., TNF-α, IL-6, IL-1β and IL-8) through inhibiting the formation of the IKK complex [32][106].
HSP27 is another inducible sHSP whose expression is relatively higher in colonic epithelium as compared to the small intestine. Similar to HSP70, the abundant HSP27 colonic expression has been attributed to stimulation by commensal microbes that exist in large amounts in the colon [33][34][48,52]. In addition, many bacteria and bacterial products can trigger HSP27 induction [35][69]. These include lipopolysaccharide (LPS), short-chain fatty acids (SCFAs), soluble factors from probiotic bacteria including Lactobacillus GG and Bifdobacterium breve and sporulating factor from Bacillus subtilis [36][37][38][39][40][76,107,108,109,110]. In line with this information, SCFAs such as butyrate, have been demonstrated to play beneficial roles in the treatment of IBD when supplemented with classical therapeutic agents like mesalazine and corticosteroids [41][36][42][75,76,77].
