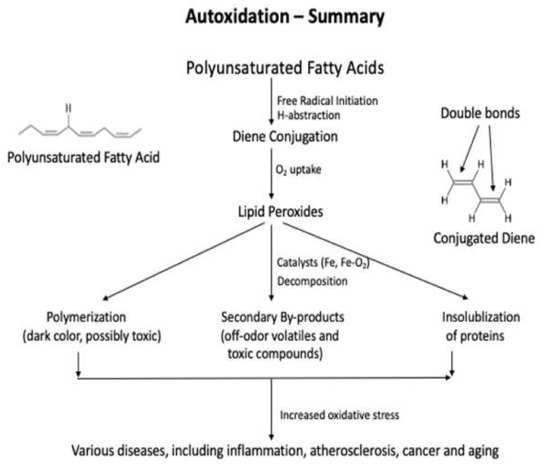
Lipids are significant nutrients for humans and help many functional and regulatory activities in the human body, such as signal transduction, myelination, and synaptic plasticity. Lipids are also involved in the structural developments of the human body . In food, lipid content and fatty acid composition are the two critical congenital parameters to the susceptibility of food to oxidative changes. Lipid content and the fatty acid composition of fat of farm animals varies significantly depending on animal species and the diet. Lipid oxidation causes quality deterioration in food. Depending upon the reaction mechanisms and factors involved, lipid oxidation can be divided into autoxidation, photo-oxidation, and enzyme-catalyzed oxidation. Autoxidation is the most common process of lipid oxidation in foods and is divided into initiation, propagation, and termination stages.
- lipid oxidation
- primary oxidation products
- secondary oxidation products
- antioxidant capacity
1. Introduction
2. Methods Used to Detect the Primary Oxidation Products
The three most used methods for the primary oxidation products in foods are iodometric, ferric thiocyanate, and diene conjugation methods, and they are the direct methods that determine the amount of hydroperoxides formed by oxidation in foods [5][6][40,41]. The peroxide value (PV) analysis is performed using titration or spectrophotometer, and the diene conjugation method determines all the conjugated fatty acids, including lipid peroxides, in the food products after extracting lipids from them. All three methods are used to determine the early stage of lipid oxidation, and their sensitivities are lower than those of the measurements of the secondary products. The values of the primary products can indicate the potentials of lipid oxidation at an early stage when the products are exposed to catalysts or further processed and, thus, may disagree with the sensory quality of the food.2.1. Methods Used to Detect the Primary Oxidation Products
2.1. Peroxide Values: Iodometric and Ferric Thiocyanate Assays
The iodometric assay uses the 2H+ that proceeds, according to the following equations:2.1. Methods Used to Detect the Primary Oxidation Products
The three most used methods for the primary oxidation products in foods are iodometric, ferric thiocyanate, and diene conjugation methods, and they are the direct methods that determine the amount of hydroperoxides formed by oxidation in foods [5][6][40,41]. The peroxide value (PV) analysis is performed using titration or spectrophotometer, and the diene conjugation method determines all the conjugated fatty acids, including lipid peroxides, in the food products after extracting lipids from them. All three methods are used to determine the early stage of lipid oxidation, and their sensitivities are lower than those of the measurements of the secondary products. The values of the primary products can indicate the potentials of lipid oxidation at an early stage when the products are exposed to catalysts or further processed and, thus, may disagree with the sensory quality of the food.2.1.1. Peroxide Values: Iodometric and Ferric Thiocyanate Assays
The iodometric assay uses the 2H+ that proceeds, according to the following equations:The ferric irons (Fe3+) formed Equations (3) and (4) make complexes with thiocyanate, and the absorbance at 500 nm determines the amount of hydroperoxide present in the sample [9][44]. The final amount of Fe2+ oxidized depends on the nature of the solvent and the amount of LOOH present. However, the amount of ferrous iron oxidized depends on the amount of LOOH present in the sample when the solvent conditions are the same. The ferric thiocyanate method is simpler than the iodometric method, and the ferrous iron has lower sensitivity to oxygen than the iodide [10][45]. This method also has been successfully used to determine lipid oxidation levels in insect-based foods [11][12][46,47]. The method is easy, rapid, and sensitive and is responsive to mono- and polyunsaturated fatty acids (PUFA) hydroperoxides.
2.1.2. Conjugated Diene Analysis
2.2. Direct Methods to Determine the Secondary Oxidation Products
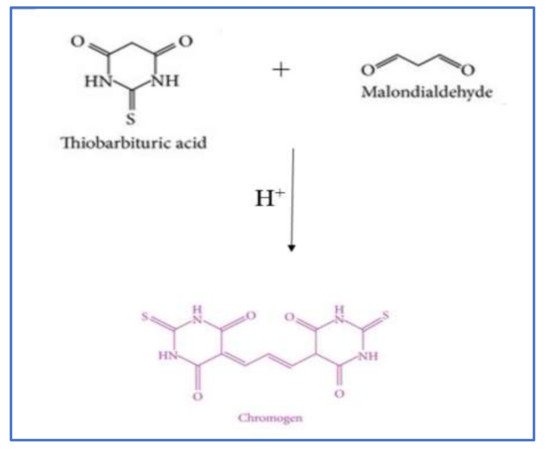
2.2.1. Thiobarbituric Acid Reactive Substances (TBARS) Method
Absorption Spectrometry
Fluorometric Spectrometry
2.2.2. Chromatographic Methods
2.3. Indirect Methods Used to Detect the Secondary Oxidation Products
2.3.1. Fluorometric Method
2.3.2. Sensory Analysis
2.4. Methods Used to Detect the Primary and Secondary Oxidation Products
| Lipid Oxidation Analysis Method | Principle of the Method | Possible Applications | Advantages of the Method | Disadvantages of the Method | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peroxide value (PV): Iodometric and ferric thiocyanate | Oxidation of iodide by hydroperoxides or by oxidation of Fe | 2+ | to Fe | 3+ | . Use a spectrophotometer to obtain the final reading. |
Plant oils and liquid food products, edible insects. | Simple and cheap. Direct readings. Under anaerobic conditions, the sensitivity is high. |
Depend on the titration skills of the person. Only applicable to liquid-based products. |
[7][8][9][10][11][12] | [42,43,44,45,46,47] |
| Conjugated diene analysis | Isomeric hydroperoxides will make conjugated dienes with the removal of oxygen and determine 1,4-dienes produced. Measured at 233 nm. |
Suitable for PUFA-containing foods. | Gives actual values of LDL oxidation during the early stages of oxidation. Simple and cheap. |
Depend on the composition and size of the lipoproteins. Small, conjugated dienes are difficult to detect. |
[4][6][13][14] | [18,41,48,49] | ||||
| TBARS assay | Detect the production of chromogen due to the reaction of MA and TBA. Read absorption at 532 nm. HPLC, GC-MS, and fluorometer are also used. |
Meat and meat-based products. Fish and fish-based products. Can be used in cured meat products, edible insects. |
Simple and fast detection. Easy to detect and low cost. Reproducible and correlate well with sensory attributes. |
MA and TBA can react with other organic compounds present in food. Absorption spectrophotometer is not suitable for detection at low levels. | [19][22][15][16][17][18][20][21][23][24][25][26] | [32,37,39,50,51,52,53,54,55,56,57,58] | ||||
| Chromatography methods | By using the HPLC or GC. Determine the specific compounds produced. | All types of raw and processed foods. Oxidative stress-related diseases. | Sensitive and accurate. Identification and quantification can be made. | The cost of the equipment is high. The complexity of the method and the fact that it is time-consuming. | [19][16][23][24][32][33][34][35] | [32,50,55,56,64,65,66,67] | ||||
| Fluorometric method | Use different fluorescent porphyrins to interact with MDA produced during oxidation. | Animal-based products. Can be used to determine the changes in human serum/plasma. Low moisture foods. | Fast and accurate. Non-destructive Sensitive. Image produced can be further used. Can be used to detect unstable oxidized compounds. |
High cost of the equipment used. Complexity of the method. |
[36][26][37][38][39][40][41] | [38,58,68,69,70,71,72] | ||||
| Sensory analysis | Use trained or untrained human panelist to determine the level of oxidation through sensory attributes, such as odor, taste, and acceptability. | All animals and plant-based foods, cereals. | Gives the overall quality of the food. Direct interpretation. Can be used for liquid, semi-solid, and solid foods. |
Depends on the individual participants and time variations. Depends on the region. Reproducibility difficult. Ethical clearance is needed. |
[42][43][44][45][46] | [73,74,75,76,77] | ||||
| p | -Anisidine test | Determine the level of anisidine produced from the secondary aldehydes produced. | Oil and oil-based products. | Simple, less technical knowledge needed. | Problems in omega-3-rich oils that contain intense colors or containing specific flavorings. | [47][48][49] | [78,79,80] | |||
| Total oxidation index (TOTOX) | Determine the total oxidizied products. | Oil and oil-based products. | Simple calculation. | Similar problem associated with | p | -anisidine test. | [47][48][49] | [78,79,80] |
