In this Review Article, we discuss the molecular landscape of thyroid cancer and the published and ongoing clinical studies focused on targeted therapies for advanced thyroid cancer. This article serves as a concise resource with up to date literature about this rapidly evolving field.
- thyroid cancer
- targeted therapy
- tyrosine kinase inhibitors
- immunotherapy
The knowledge on thyroid cancer biology has grown over the past decade. Thus, diagnostic and therapeutic strategies to manage thyroid cancer are rapidly evolving. With new insights into tumor biology and cancer genetics, several novel therapies have been approved for the treatment of thyroid cancer. Tyrosine kinase inhibitors (TKIs), such as lenvatinib and sorafenib, have been successfully utilized for the treatment of radioactive iodine (RAI)-refractory metastatic differentiated thyroid cancer (DTC). In addition, pretreatment with mitogen-activated protein kinase (MAPK) inhibitors (trametinib and selumetinib) has been shown to restore RAI avidity in previously RAI-refractory DTCs. Local therapies, such as external beam radiation and radiofrequency/ethanol ablation, have also been employed for treatment of DTC. Vandetanib and cabozantinib are the two TKIs currently approved by the Food and Drug Administration (FDA) for the treatment of medullary thyroid cancer (MTC). Other novel therapies, such as peptide receptor radionuclide therapy and carcinoembryonic antigen (CEA) vaccine, have also been utilized in treating MTC. Ongoing trials on selective rearranged-during-transfection (RET) protooncogene inhibitors, such as LOXO-292 and BLU-667, have demonstrated promising results in the treatment of metastatic MTC resistant to non-selective TKIs. The FDA-approved BRAF/MEK inhibitor combination of dabrafenib and trametinib has revolutionized treatment of BRAFV600E mutation positive anaplastic thyroid cancer. Several other emerging classes of medications, such as gene fusion inhibitors and immune checkpoint inhibitors, are being actively investigated in several clinical trials.
1. Introduction
- Introduction
Thyroid cancer is predicted to affect 52,890 new patients in the USA in 2020, with the incidence being three times higher in women as compared with men [1]. Until recently, thyroid cancer was the most rapidly increasing cancer in the USA, largely, but not solely, due to increasing use of sensitive diagnostic procedures. However, the increase of about 7% per year during the 2000s has slowed to 2% per year in men, and rates have stabilized in women from the period of 2012 to 2016, as a result of the more conservative diagnostic approach that has been recently implemented [1]. Differentiated thyroid cancer (DTC) that arises from follicular cells is further subclassified into papillary thyroid cancer (PTC), which is the most common histological type, follicular thyroid cancer (FTC), and Hürthle cell cancer (HTC). De-differentiated thyroid cancer is classified as poorly differentiated thyroid cancer (PDTC) and anaplastic thyroid cancer (ATC). Medullary thyroid cancer (MTC) arises from neuroendocrine C cells that are derived from the neural crest [2].
The standard of care for DTC consists of surgery (lobectomy or total/near-total thyroidectomy, with or without lymph node dissection), dependent on the tumor size, extrathyroidal extension, and metastatic potential. Very low-risk PTCs can be serially followed with active surveillance, using neck ultrasound (US) without surgical intervention, if the size remains stable over time [3].
Following surgery, all patients classified as high-risk for persistent/recurrent disease with evidence of radioactive iodine (RAI)-avid metastases, as well as patients classified as intermediate risk for persistence or recurrence, receive RAI treatment, as it has shown to decrease mortality and/or morbidity, respectively [4][3,4]. Metastatic RAI-non-avid disease carries a worse prognosis, as the five-year survival is estimated to be as low as 10% [3].
In contrast to DTC, all patients with MTC undergo total thyroidectomy with or without lymph node dissection. Patients with MTC do not benefit from RAI therapy, as the neuroendocrine tumors lack the sodium-iodide (NIS) symporter necessary to incorporate RAI within the cell[5] [5]. External beam radiation to the neck may be used for persistent or residual local disease [5]. The overall 10-year survival for MTC confined to the thyroid gland is 95.6% but is as low as 40% for patients with distant metastatic disease at the time of diagnosis [6].
Since standard RAI therapy for DTC is not effective in 5–22% of patients, and surgical treatment is not curative in patients with MTC presenting with metastatic disease, small molecules have been developed that target aberrant signaling pathways specifically found in thyroid cancer [3]. Therefore, understanding of the molecular landscape of thyroid cancer is crucial to provide individualized targeted therapies.
2. Targeted Therapies for Thyroid Cancer
- Targeted Therapies for Thyroid Cancer
2.1. Tyrosine Kinase Inhibitors
2.1.1. DTC
In November 2013, sorafenib was the first tyrosine kinase inhibitor (TKI) to be approved by the Food and Drug Administration (FDA), for the treatment of progressive metastatic DTC refractory to RAI treatment. Sorafenib targets VEGFR 1-3, PDGFR, RET, FLT, and c-kit. The approval was based on the results of the DECISION trial, which was a phase III, multicenter, double-blind, placebo-controlled trial conducted in 417 patients with progressive DTC which failed standard treatment. Sorafenib was associated with a significantly longer median progression-free survival (PFS) (10·8 months) when compared to the placebo (5.8 months). The most common side effects encountered in patients treated with sorafenib were hand–foot skin reaction (76.3%), diarrhea (68.6%), alopecia (67.1%), and rash or desquamation (50.2%) [7][8][79,80].
In February 2015, a second TKI lenvatinib, which targets VEGFR2, VEGFR3, EGFR, PDGFR, KIT, and RET, was approved for the treatment of progressive DTC refractory to RAI, based on the SELECT trial—A phase III, randomized, double-blind, multicenter study involving 261 patients with progressive DTC. Lenvatinib was associated with a longer median PFS of 18.3 months vs. 3.6 months in the placebo group. Of the 20 deaths that occurred on lenvatinib, six were attributed to the treatment itself, as TKIs lead to QT interval prolongation and fatal tachyarrhythmias [9][81]. Adverse reactions led to dose reductions in 68% of patients receiving lenvatinib and discontinuation in 18% of patients [10][82]. Given lack of permanent complete remissions after therapy with the FDA-approved agents, the use of alternative TKIs, as well as combination therapies involving TKIs and mTOR inhibitors or immune checkpoint inhibitors, is being evaluated in several ongoing clinical trials [11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][83–102] .
Since BRAFV600E is one of the most common mutations in PTC, BRAF inhibitors such as vemurafenib or dabrafenib have been implemented in the management of thyroid cancer. Interestingly, a basket trial of vemurafenib in BRAFV600E mutation-positive tumors showed significant responses in patients with ATC [31][105]. However, thyroid cancers exhibit primary resistance to RAF kinase inhibition due to reduced negative feedback, and hence a combination of RAF and MEK kinase inhibitors is necessary to effect MAPK pathway inhibition [115] (Figure 1). An open-label phase II study of 16 patients with BRAFV600E-mutant ATC treated with dabrafenib in combination with the MEK inhibitor trametinib showed a remarkable overall response rate of 69% in patients with ATC, with one patient achieving complete response[32] [106]. The 12-month duration of response, PFS, and overall survival were estimated at 90%, 79%, and 80%, respectively, a phenomenon not previously seen in ATC, which is usually characterized by overall survival of 3–6 months post-diagnosis [116]. This observation has revolutionized the management of this very aggressive cancer and led to the FDA approval of combination therapy with dabrafenib and trametinib in BRAFV600E-mutant ATC in May 2018 [32][106].
2.1.2. MTC
Two additional TKIs—vandetanib, which targets VEGFR2, VEGFR3, EGFR, KIT, and RET, and cabozantinib, which targets VEGFR2, RET, MET, FLT3, and AXL (AXL Receptor Tyrosine Kinase)—have been FDA-approved for the treatment of metastatic progressive MTC. The treatment with the first one was associated with a longer median PFS of 22.6 compared with 16.4 months in individuals exposed to placebo, while the latter led to a PFS of 11.2 months in the treated group vs. four months in the placebo arm in multicenter, randomized, double-blind phase III clinical trials [33][34][103,104,117,118]. Again, the side effect profile was similar to other TKIs and included QT prolongation and arrhythmias, severe hypertension leading to reversible posterior leukoencephalopathy syndrome, headaches, hand–foot syndrome, fistula formation, profound fatigue, decreased appetite, nausea, diarrhea, and abdominal pain [104]. In addition, interstitial lung disease and Stevens Johnson syndrome have been associated with use of vandetanib [104].
The large number of severe side effects of TKIs result from its ability to target multiple kinases (Figure 1) which play a role not only in cancer progression but also in many important physiological processes. Therefore, more targeted therapeutic strategies have been recently developed and include inhibition of RET-only in RET-mutated MTCs and PTCs and inhibition of BRAF-only in BRAFV600E-mutated tumors [35][106,115].
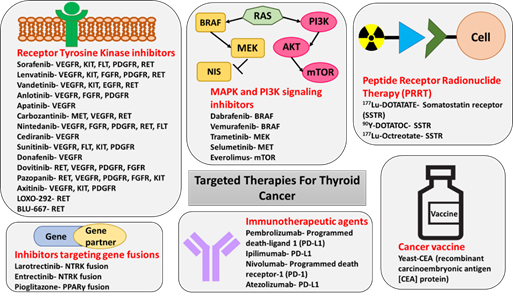
Figure 1. Targeted therapies for the treatment of thyroid cancer. The figure shows inhibitor drug molecules targeting various RTKs, components of MAPK and PI3K signaling pathways, and gene fusions in thyroid cancer. The immunotherapeutic agents, PRRT molecules, and a vaccine with the potential for the treatment of thyroid cancer are also included in the figure.
There are two RET inhibitors currently implemented in the management of RET mutation-positive tumors in the clinical trial setting—BLU-667 and selpercatinib (LOXO-292) [36][37][38][39][119–122]. BLU-667 inhibits the protein product of RETM918T, as well as RETV804L/M gatekeeper mutations conferring resistance to TKIs, while LOXO-292 is a highly selective RET kinase inhibitor with nanomolar potency against the canonical RET MTC drivers, RET gatekeeper mutations, and RET fusions [115]. Preliminary data from the ongoing clinical trials documented minimal side effects, excellent tolerability, and high efficacy, with objective response rates (complete and partial responses) ranging from 47% to 62% [36][37][38][39][115,119–122].
2.2. Therapies Targeting Gene Fusions
An additional development in the management of thyroid cancer which has been proven highly effective in preliminary clinical trials is the use of therapy aimed at targeting NTRK and ALK gene fusions Larotrectinib is a selective inhibitor of tropomyosin receptor kinase (TRKA), TRKB, and TRKC which has been approved by the FDA for treatment of solid tumors with NTRK fusion [107,123]. The objective response rate in phase I and II clinical trials was remarkably high (80%) with 63% of patients with NTRK-positive tumors experiencing partial response and 13% complete response [40][107,123]. Most of the adverse events reported in the primary analysis were grade 1 and grade 2, with the most common being elevated ALT and AST (42%), fatigue (36%), vomiting (33%), dizziness (31%), nausea (31%), diarrhea (29%), and anemia (29%). (Figure 1).
Entrectinib is another selective inhibitor of TRKA, TRKB, and TRKC that also inhibits ALK and ROS1 tyrosine kinases. In August 2019, it received FDA approval for treatment of TRK-positive solid tumors, based on results of phase 1 and 2 clinical trials documenting an objective response rate of 57%, including a partial response in 50% and complete response in 7% [108] (Figure 1). The most common adverse events included dysgeusia (43%), dizziness (33%), constipation (33%), diarrhea (28%), and weight increase (26%) [108].
Unfortunately, at the moment, there are no clinical trials targeting THADA or other described above fusions in thyroid cancer, such as PAX8/PPARγ, apart from a one case report describing application of pioglitazone in PPARγ fusion protein positive metastatic HTC and resulting in the reduction of tumor size and improved pain control [41][124].
2.3. Restoration of RAI Uptake via MEK and BRAF Inhibition
Another important strategy implemented in the management of de-differentiated DTC and PDTC relies on upregulation of sodium iodide symporter NIS via inhibition of its negative regulators MEK and RAF signaling. This strategy re-enables incorporation of iodine within the cancer cell and thus consists of the pretreatment with MEK and BRAF inhibitors, followed by RAI therapy [42][43][125,126].
The landmark study by Ho et al. has proven the principle that the inhibition of MEK1 and MEK2 by selumetinib induces RAI uptake in RAI-non-avid DTC [109]. Furthermore, an individualized approach consisting of evaluation of the amount of restored RAI uptake, as measured by 124I positron emission tomography/computed tomography (PET/CT)-based tumor dosimetry, has identified patients meeting the tumor accumulation threshold warranting RAI therapy. Among eight patients subsequently treated with RAI, five had a partial response and three had stable disease [109]. Interestingly, these preliminary data suggest a particularly high efficacy of this therapeutic approach in NRAS-mutated tumors. Given these promising results, there are several ongoing clinical trials utilizing selumetinib as an adjunct to RAI therapy in larger patients populations [44][127]. The second-generation MEK inhibitor trametinib is being evaluated in an ongoing phase II trial implementing pretreatment with trametinib, followed by 124I PET/CT-based lesional dosimetry and RAI therapy in patients with sufficiently restored RAI uptake (clinicaltrials.gov identifier NCT02152995) .
Additional clinical trials utilize individualized approaches to patients with RAS-mutated and BRAF-mutated tumors. Patients with tumors harboring an NRAS mutations are treated with the MEK inhibitor trametinib, while those with tumors characterized by BRAFV600E mutation are treated with combination therapies, including BRAF inhibitors such as dabrafenib and vemurafenib and MEK inhibitors. Preliminary data suggest high efficacy of such an approach with partial response to RAI therapy at three months’ follow-up observed in all patients who achieved sufficient restoration of RAI uptake [110]. A similar concept is being utilized in another ongoing multicentric prospective non-randomized phase II trial with two independent arms studying the use of trametinib for NRAS mutation-harboring tumors and dabrafenib for BRAFV600E-harboring tumors (clinicaltrials.gov identifier NCT03244956).
2.4. Peptide Receptor Radionuclide Therapy in Thyroid Cancer
Another therapeutic concept utilizing radiolabeled agents for treatment of metastatic thyroid cancer had been based on a subset of thyroid cancer expresses somatostatin receptors (SSTR) that could be targeted with peptide receptor radionuclide therapy (PRRT) [45][46][128,129].
Radiolabeled somatostatin receptor (SSTR) analogs, such as 68Ga-DOTATATE, which recognize predominantly SSTR type 2, are utilized for positron emission tomography/computed tomography (PET/CT) imaging. Of note, 68Ga-DOTATATE was FDA approved in June 2016 for clinical use for imaging of patients with neuroendocrine tumors, including MTC. Identification of patients with RAI-non-avid DTC and metastatic MTC characterized by positive 68Ga-DOTATATE uptake enables identification of individuals who may benefit from PRRT. There were small pilot studies performed in Europe that documented the utility of treatment of progressive RAI-non-avid metastatic DTC and MTC with SSTR agonists radiolabeled with 177Lutetium or 86Ytrium. The overall response rate was similar to current standard-of-care therapy with TKIs, while the quality of life was better and complication rate was lower after the therapy with PRRT [47][48][49][111–113,130–132].
2.5. Immunotherapy in Thyroid Cancer
2.5.1. DTC
The tumor microenvironment has been acknowledged as the major player in cancer progression and response to therapy. Therefore, the immune landscape of the thyroid cancer has emerged as a potential therapeutic target. Immune checkpoints, such as programmed cell death protein 1 (PD1) and its ligand-PDL-1, as well as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors, exhibit antitumor effects by altering the interaction between the immune system cells and tumor cells [50][133]. The expression of PD1/PDL1 in thyroid cancer has been extensively studied for both diagnostic and prognostic purposes [51][134]. In one meta-analysis by Aghajani et al., the expression of PDL1 in thyroid cancer was associated with tumor recurrence and poor survival [52][135]. Based upon analysis of the TCGA database, a higher level of PD-L1 mRNA was associated with lymph node metastasis, extrathyroidal invasion, and shorter disease-specific survival [53][136]. The latter was further supported by two independent cohort studies [54][89,137]. Nevertheless, DTC is thought to be poorly immunogenic due to a relatively low mutation burden. Consistently, published studies have shown that thyroid cancer has relatively poor response to immunotherapy with checkpoint inhibitors[55] [114,138] . In order to enhance the efficacy of immunotherapy, combination treatments are being tested in several clinical trials in solid tumors, including thyroid cancer . The efficacy of these therapies might be better particularly in de-differentiated tumors, such as widely invasive HTC and ATC, as they are characterized by high mutation burden and higher likelihood of introducing immunogenicity [56][10,139].
2.5.2. MTC
Another interesting concept utilizing the ability of MTC cells to produce carcinoembryonic antigen (CEA) consisted of the introduction of dendritic cell vaccination with anti-CEA vaccine [57][58][59][140–142]. Yeast-CEA (GI-6207) is a therapeutic cancer vaccine genetically modified to express recombinant carcinoembryonic antigen (CEA) protein. In a phase 1 trial involving 25 patients with CEA expressing cancers, the treatment with Yeast-CEA showed stabilization of disease and its biochemical biomarkers. It was also well tolerated, with the most common adverse effect being grade 1/2 injection-site reaction [59][142]. A phase II trial was designed at the National Cancer Institute to evaluate the use of GI-6207 in recurrent medullary thyroid cancer and documented biochemical response without a significant tumor response [60][143].
3. Conclusions
- Conclusions
Several novel targeted therapies have recently been approved by the FDA for use in advanced thyroid cancer. There are many ongoing national and multi-national studies implementing targeted therapies in thyroid cancer. There is a shift in the management paradigm, with the molecular landscape rather than histology/morphology driving an individualized treatment approach. Even amongst the same histological groups of thyroid cancer, the response to therapy is different based on genetic and immune signature of the tumor, further confirming that “one size does not fit all”. The use of the therapeutic agents should be individualized and based on shared decision-making after informing the patient about the possible benefits and disadvantages of the therapy. Encouraged by the results of individualized therapies documenting even complete responses when targeting driver mutations, the aim of future research should focus on finding efficacious treatments that will have a long-lasting curative effect.