Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Dong-Woo Cho.
Advances in three-dimensional (3D) printing techniques and the development of tailored biomaterials have facilitated the precise fabrication of biological components and complex 3D geometrics over the past few decades. Moreover, the notable growth of 3D printing has facilitated pharmaceutical applications, enabling the development of customized drug screening and drug delivery systems for individual patients, breaking away from conventional approaches that primarily rely on transgenic animal experiments and mass production.
- 3D printing
- 3D bioprinting
- disease modeling
- drug testing
- Pharmaceutical application
1. Introduction
Since its introduction in the 1980s, three-dimensional (3D) printing has become a representative adjunct manufacturing technology. This technology was first developed for rapid prototyping and is widely applied in various industrial fields, including automotive, home appliances, space, and consumer goods [1]. In particular, advances in 3D printing techniques and the advent of printable/biocompatible materials have facilitated the fabrication of customized products in recent years [2]. Since the early 2000s, 3D bioprinting using biological materials such as cells and biomolecules has been successfully applied in tissue engineering to directly create living structures that reproduce the behavior of natural living systems [3]. The multiple advantages of 3D printing and bioprinting have become a major driving force for the rapid development of pharmaceutical applications, including drug screening and drug delivery systems (Figure 1A).
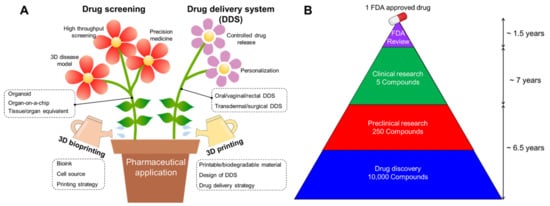
Figure 1. (A) Schematic illustration showing advances in pharmaceutical applications, including drug screening and drug delivery system (DDS) by employing 3D printing and bioprinting technology. (B) The average drug development timeline for one FDA-approved drug.
The development of new drugs is a high-cost and time-consuming endeavor, with high risks involved due to preclinical validation and clinical trials. Reportedly, an estimated 12–15 years are required for a single new drug candidate to undergo a series of evaluations before market availability (Figure 1B) [4]. For successful drug development, potential drugs are commonly identified and optimized through drug screening, followed by the selection of a candidate drug to progress to clinical trials. During the preclinical phase, drug candidates are screened for biological activity, toxicity, metabolism, pharmacological efficacy, and medicinal value using disease models for validating their efficacy and safety for further clinical trials. However, despite the prolonged evaluatory period, it has been reported that approximately 50% of failures can be attributed to unpredictable drug toxicity and inefficiency [5].
Over the past few decades, drug screening has conventionally depended on transgenic animals as disease models. However, the use of animal models raises serious ethical concerns, as well as confer inevitable limitations in precisely representing human tissue in the context of pathophysiology owing to genetic discrepancies [6]. Statistically, approximately half the drugs that have been proven safe in animal testing are found to be harmful to humans, while others remain nontoxic [7]. In an attempt to overcome the limitations of animal models, two-dimensional (2D)-based cell cultures have been employed as a screening platform for potential drug candidates. However, recent studies have revealed that cells cultured on a planar substrate differ not only in inherent phenotypes [8,9][8][9] but also in terms of cellular functions, such as migration [10], proliferation [11[11][12],12], and differentiation [13,14][13][14] when compared with cells cultured in 3D microenvironments. Accordingly, 3D printing improves the existing drug screening platforms by accurately depositing biomaterials containing patient-derived cells and can reproduce the natural environment of the diseased human body.
In addition to drug screening, the development of printable materials with various biodegradation profiles has facilitated the manufacture of drug delivery systems with individually controlled doses and customized medical devices that closely match the patient anatomical features [15]. A key objective of therapeutic administration is maintaining bloodstream drug levels between the maximum level that would cause toxicity and the minimum value that would be inefficacious in the body [16]. However, the plasma drug level rapidly increases after traditional dosing strategies, followed by a gradual decrease, necessitating successive dosing at regular intervals to maintain drug levels within an appropriate therapeutic window, thus resulting in patient inconvenience [17]. Moreover, most medical treatments are designed for the average patient as a one-size-fits-all approach, which might be successful in some but challenging to other patients. Furthermore, outcomes may differ among individuals in terms of drug efficacy or side effects. Traditional mass manufacture of drug delivery systems has failed to afford a cost-effective approach to address the diversity of therapeutic regimens caused by individual differences [18]. Therefore, current investigations assessing 3D printed drug delivery have focused on developing personalized drug delivery systems with controlled drug release profiles within the desired range, with minimal administration frequency.
2. Advent of 3D Printing for Pharmaceutical Application
3D printing is an additive manufacturing process for creating three-dimensional objects from a digital file. 3D bioprinting has been recognized as a promising technology for creating tissue-based platforms with high reproducibility and versatility by accurately positioning biomaterials together with cells and biomolecules. These features of 3D bioprinting are directly associated with requirements of drug screening and drug delivery systems, enabling the design of more advanced pharmaceutical applications. To meticulously design a physiologically functioning model/device, researchers should consider various aspects of 3D bioprinting, such as suitable biomaterials, cell sources, and printing strategies.
2.1. Bioink
The appropriate choice of printable biomaterials, commonly referred to as bioinks, is essential for building tissue architectures with desired biophysical and biochemical properties. Biomaterials currently employed as bioinks are predominantly natural or synthetic-based polymers, which should possess features of biocompatibility and printability. In selecting an appropriate bioink, the major features of each bioink should be considered.
Naturally derived biomaterials offer greater similarity to biophysical and biochemical constituents in native tissues, thereby closely recapitulating biological responses when compared with synthetic biomaterials. Numerous natural polymers isolated from animal or human tissues have been developed as bioinks and have revealed superior cell affinity to promote cellular functions such as migration, proliferation, and differentiation [47][19]. However, most natural bioinks, including collagen [48][20], gelatin [49][21], alginate [50][22], and hyaluronic acid [51][23] possess only a single protein component of the ECM and remain limited in representing intrinsic biophysical and biochemical elements such as growth factors, glycosaminoglycans, laminin, fibronectin, and elastin [52][24]. From this perspective, recent studies have highlighted the significance of decellularized ECM (dECM) as a promising bioink, allowing a niche microenvironment with synergistic effects on encapsulated cells [53,54][25][26]. Based on the differential proteomic analysis, Han et al. confirmed that a variety of inherent matrisome protein constituents were retained in each dECM bioink, playing a crucial role in inducing tissue-specific cellular behavior [55][27]. Although natural polymer-derived bioinks can provide better cell affinity, their weak mechanical properties hinder the construction of cellular architectures. To compensate for the innate limitations of naturally derived bioinks, several researchers have utilized synthetic polymers that can improve or modulate a wide range of features, including mechanical properties, degradation profiles, crosslinking mechanisms, and chemical compositions [56][28]. Huston et al., for instance, developed a photocrosslinkable composite hydrogel by incorporating poly(ethylene glycol) (PEG) and methacrylated gelatin (GelMA) (i.e., denatured collagen), and demonstrated that its biological and mechanical properties could be adjustable by altering the concentration of each component [57][29]. As an alternative approach, Pati et al. showed that 3D bioprinting using multiple bioinks can compensate for the weak mechanical properties of naturally derived polymers by first depositing synthetic polymers that serve as a supportive framework [58][30].
Selecting a proper bioink depends on various factors, such as the characteristics of the target tissue, printing strategy, and biological process. To effectively build a drug screening platform and drug delivery device, it is necessary to continue developing and optimizing physically and chemically tunable biomaterials.
2.2. Cell Source
Although most drug delivery devices are acellular, appropriate cell selection is a paramount factor in designing a drug screening platform to represent the physiological state and pathological process of the tissue of interest more precisely. Three types of cell sources (primary cells, cell lines, and stem cells) are commonly used to create a 3D bioprinted cellular model. To narrow the gap in biological responses due to genetic discrepancies between animals and humans, most in vitro platforms for disease modeling prefer utilizing cells derived from human tissue [59,60,61][31][32][33]. Among various cell types, primary cells directly isolated from the tissue have the advantage of reproducing tissue functions at specific points or stages [62][34]. However, several challenges concerning the limited lifetime and donor-to-donor variations need to be resolved [63][35]. Cells that can continuously propagate over a prolonged period are called cell lines and have homogeneous phenotypic and genotypic features. These immortalized cell lines can proliferate indefinitely through genetic mutations or artificial modifications. Compared with other cell types, cell lines can be purchased at a low price and allow convenient handling. Accordingly, cell lines can be ideal for testing cells to establish a new tissue fabrication platform. However, cell lines are less preferred as a biologically relevant option because they lose the inherent characteristics of the original tissue. Hence, cell lines are not preferably used for the development of personalized artificial tissues. The third type of cell commonly used in 3D bioprinting is stem cells, including mesenchymal stem cells, embryonic stem cells, and induced pluripotent stem cells (iPSCs). Stem cells characterized by self-renewal and differentiation potency are gradually attracting attention due to the unlimited potential for tissue regenerative medicine and in vitro human disease modeling. In particular, the ability to reprogram donor-specific properties enables an improved understanding of phenotypic variability and disease mechanisms, possibly providing an accurate solution focused on a specific patient [64][36]. Stem cells with different lineages and potencies have been widely utilized in 3D bioprinting for human disease modeling. For example, Dai et al. created a brain tumor model using glioma stem cells, known to be the primary cell type closely associated with high-grade gliomas [65][37]. The researchers verified that stem cells composing the brain tumor model maintained their intrinsic characteristics while affording differentiation potential during the entire in vitro culture period. However, the ability of stem cells to precisely regulate the differentiation pathways into desired lineages and the immature cell phenotype genetically similar to fetal cells needs to be addressed [62][34].
Regardless of the nature of the cell source, there is a high possibility of variations between batches, attributed to various causes such as technical skills of users, differences in culture conditions [66][38], and inconsistent cell differentiation potency [67][39]. Therefore, it is essential to characterize cells through multiple assays and approaches to reduce inconsistencies and variations between experiments [68,69][40][41].
2.3. Printing Strategy
The 3D printing strategy for biomaterial deposition includes inkjet-based, extrusion-based, and laser-assisted printing. These approaches possess different features, including the printing mechanism, resolution, speed, and applicable biomaterials. Herein, we provide a brief overview of the respective printing strategies.
2.3.1. Inkjet-Based 3D Printing
Inkjet-based 3D printing is a non-contact approach that allows controlled volumes of liquid bioink to be precisely dispensed onto a planar substrate (Figure 2A). Inkjet printers employ thermal, piezoelectric, or electromagnetic methods to apply bioinks drop-wise from a nozzle. Thermal inkjet printers electrically heat the printing head up to 300 °C, inflating an air bubble to eject the droplet. It has been confirmed that markedly high temperatures do not have harmful effects on biological molecules or cells in the bioink because localized and momentary (~2 µs) heating increases the overall temperature to only 4–10 °C [70][42]. Piezoelectric inkjet printing creates droplets at regular intervals by generating pressure with an acoustic wave inside the printing head, whereas the electromagnetic approach uses electromagnetic forces such as Lorentz or permanent magnetic configurations to position the droplets.
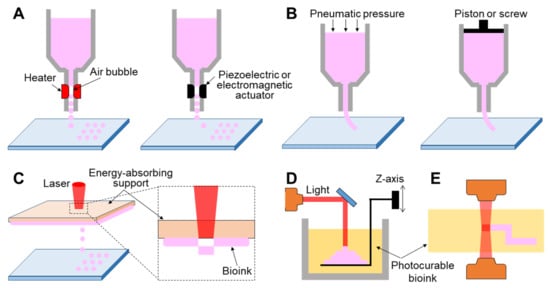

Figure 2. Schematic illustration showing printing strategy. (A) Inkjet-based 3D printing, (B) extrusion-based 3D printing, and light-assisted 3D printing, including (C) laser-induced forward transfer technique, (D) stereolithography, and (E) photon polymerization technique.
Inkjet printing can regulate the amount of bioink ejected with a high resolution of up to 20 μm [71,72][43][44]. Using a commercial inkjet printer, Roth et al. achieved highly precise cellular patterns in various shapes by depositing biologically active proteins, which modulate cell attachment [73][45]. In addition, Christensen et al. demonstrated the feasibility of inkjet bioprinting to build a cell structure similar to that of blood vessels with horizontal and vertical branches [74][46]. Inkjet bioprinters enable the fabrication of multiple tissues within a limited area. For example, Xu et al. successfully produced a complex heterogeneous construct composed of three different cell types [75][47]. Recent studies have demonstrated the potential of the inkjet printing technique in HTS for drug discovery, as it offers miniaturization, repeatability, low costs, high resolution, and generation of minimal waste and contamination [76][48]. Furthermore, the inkjet-based approach is widely applied for creating a plethora of microarrays for genomic and pharmacological profiling of a biochemical pathway of interest [76][48]. Hughes et al. developed a microarray system for gene expression profiling using oligonucleotides synthesized using an inkjet bioprinting-based approach [77][49].
Although inkjet bioprinting has gained momentum in recent decades owing to its versatile potential, there remain limitations that need to be solved in the near future. As only bioinks with low viscosity (~0.1 Pa∙s) can be applied in inkjet printers, the narrow range of printable bioinks has often been challenging in inkjet-based bioprinting [78][50]. In addition, its drop-by-drop biomaterial deposition might prolong the printing time, resulting in the increased likelihood of nozzle clogging [79][51].
2.3.2. Extrusion-Based 3D Printing
Extrusion-based 3D printing continuously deposits a stream of bioink on a substrate driven by pneumatic [72,80,81][44][52][53] or mechanical [82,83,84][54][55][56] dispensing systems (Figure 2B). The pneumatic system is the most commonly utilized extrusion method owing to its low cost and ease of use. It uses compressed air to push the bioink, while the mechanical method employs a piston or screw to force the materials to be extruded through a nozzle. However, a mechanically driven approach can precisely regulate the amount of extruded material when compared with the pneumatic system, causing a delay time due to the compressed air volume. Unlike inkjet-based 3D printing that requires low-viscosity bioink, both extrusion mechanisms can employ a wide range of high-viscosity bioinks (0.03–6 104 Pa∙s), including dECM, gelatin, alginate solution, and thermally or chemically molten synthetic polymers [85,86,87,88][57][58][59][60]. Undoubtedly, the use of bioink that has high mechanical strength affords the possibility of fabricating a final tissue architecture with more robust mechanical properties.
Extrusion-based 3D printing allows the selective printing of multiple types of bioinks in a predefined location [89[61][62],90], which also contributes to simplifying the fabrication process by simultaneously printing cellular components and structural support [91][63]. More recently, extrusion-based 3D printing was shown to achieve fiber-shaped tubular constructs using a coaxial nozzle [92,93][64][65], which simultaneously extrudes alginate-laden bioink and Ca2+-laden fugitive bioink via the shell and core of a coaxial nozzle, respectively.
It is essential to consider the cell damage inflicted from shear stress during nozzle passage in extrusion-based 3D printing [94][66]. Accordingly, to protect cells from stress during passage through the nozzle, bioinks should exhibit shear-thinning behavior, reducing viscosity under shear strain. This property decreases the stress inflicted on cells and maintains the printed shape [53][25]. Furthermore, although low pressure and a large nozzle size probably maintain high cell viability, the trade-off is a limited resolution and printing speed. Hence, researchers should establish diverse printing conditions that barely influence cell viability and additional functionalities before producing the final tissue product.
32.3.3. Light-Assisted 3D Printing
Light-assisted 3D printing is divided into laser-induced forward transfer (LIFT) and stereolithography (STL) techniques. First, LIFT consists of a pulsed laser, an energy-absorbing support, and a substrate where the bioinks are deposited (Figure 2C). The energy-absorbing support was coated with a cell-laden hydrogel at the lower surface. This technology provides a high printing resolution by controlling the droplet transfer to the receiving substrate. When the laser is focused on the energy-absorbing layer, the energy absorption generates a vapor pocket or mechanical wave that forces the droplet to be separated from the support. The resolution of light-assisted printing is generally known to range between 20 and 30 μm and is associated with several parameters such as energy per surface, viscosity of the bioink, surface tension, and the thickness of the energy-absorbing support [95][67]. Unlike inkjet- or extrusion-based approaches, as this strategy does not require any printing nozzles, it can avoid the issue of nozzle clogging with biological materials. Despite its superior capability in affording high cell viability and resolution, this system is limited due to the operational costs of laser-assisted printers and preparation of printing components such as the energy-absorbing layer and collecting substrate [96][68].
STL, one of the oldest 3D printing technologies, enables the creation of complex geometry in a layer-by-layer fashion by selectively solidifying liquid photopolymers with ultraviolet (UV), infrared, or visible light (Figure 2D) [97][69]. This technology projects a 2D slice containing cross-sectional information of a 3D model from biomedical information, such as magnetic resonance imaging (MRI) or CT, onto a photopolymer reservoir, thereby allowing the fabrication of a volumetric and arbitrary structure at a rapid speed when compared with a biomaterial deposition-based approach through a printing nozzle. The focal size of the light source determines its printing resolution, which is usually at the microscale level [98][70]. High shape fidelity with rapid fabrication speed has enabled researchers to produce highly elaborate structures useful for biomedical applications. More recently, the two-photon polymerization-based approach, a direct-writing technique, has been introduced as a promising new technology (Figure 2E). When the laser is focused at a single point in the photosensitive monomer, it begins to polymerize by simultaneously absorbing two photons. The high feature resolution due to the unique polymerization mechanism has allowed the fabrication of biomedical devices at micro/nanoscale sizes [99][71]. Gittard et al. successfully developed microneedle array templates for the transdermal delivery of protein-based pharmacological agents, and the dimensions of each microneedle were hundreds of micrometers [100,101][72][73]. Initially, as photocurable materials in STL were unsuitable for use with living cells, they were utilized to build patient-specific models of interest as surgical guides to reduce time and potential risks in the operating theater [102,103][74][75]. However, biocompatible/photosensitive materials such as collagen type I [104][76], laminin [105][77], streptavidin [106][78], and PEG-based hydrogels [107][79] have been successfully developed in recent years. Many researchers have created implantable and anatomically patient-specific devices for the skin, heart valves [108][80], aortas [109][81], and nasal implants [110][82] using the STL-based approach.
Light-assisted 3D printing has a significant advantage in constructing complex structures rapidly, but it remains challenging to build a heterogeneous construct consisting of multiple materials. Thus, the selection of an appropriate printing strategy should be considered based on the characteristics of each printing technology.
This review provides a summary and brief classification of pharmaceutical applications applied to 3D printing technology. We first discuss the requirements of drug screening and drug delivery systems for advanced pharmaceuticals, which could be met by 3D printing. Furthermore, we describe the current methodological approaches of 3D printing applicable to the pharmaceutical field. Lastly, based on the strategy of 3D printing for more advanced drug screening and drug delivery systems, we introduce the recent pharmaceutical applications of 3D printing.
References
- Alhnan, M.A.; Okwuosa, T.C.; Sadia, M.; Wan, K.-W.; Ahmed, W.; Arafat, B. Emergence of 3D printed dosage forms: Opportunities and challenges. Pharm. Res. 2016, 33, 1817–1832.
- Park, B.J.; Choi, H.J.; Moon, S.J.; Kim, S.J.; Bajracharya, R.; Min, J.Y.; Han, H.-K. Pharmaceutical applications of 3D printing technology: Current understanding and future perspectives. J. Pharm. Investig. 2019, 49, 575–585.
- Vijayavenkataraman, S.; Fuh, J.Y.; Lu, W.F. 3D printing and 3D bioprinting in pediatrics. Bioengineering 2017, 4, 63.
- Corr, P.; Williams, D. The pathway from idea to regulatory approval: Examples for drug development. In Conflict of Interest in Medical Research Education and Practice; National Academies Press: Washingtom, DC, USA, 2009.
- Takebe, T.; Imai, R.; Ono, S. The current status of drug discovery and development as originated in United States academia: The influence of industrial and academic collaboration on drug discovery and development. Clin. Transl. Sci. 2018, 11, 597–606.
- Vanderburgh, J.; Sterling, J.A.; Guelcher, S.A. 3D printing of tissue engineered constructs for in vitro modeling of disease progression and drug screening. Ann. Biomed. Eng. 2017, 45, 164–179.
- Hansen, K.; Khanna, C. Spontaneous and genetically engineered animal models: Use in preclinical cancer drug development. Eur. J. Cancer 2004, 40, 858–880.
- Fischbach, C.; Kong, H.J.; Hsiong, S.X.; Evangelista, M.B.; Yuen, W.; Mooney, D.J. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc. Natl. Acad. Sci. USA 2009, 106, 399–404.
- Pickl, M.; Ries, C. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene 2009, 28, 461–468.
- West, J.L.; Hubbell, J.A. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules 1999, 32, 241–244.
- Ng, K.W.; Leong, D.T.; Hutmacher, D.W. The challenge to measure cell proliferation in two and three dimensions. Tissue Eng. 2005, 11, 182–191.
- Li, D.-W.; He, F.-L.; He, J.; Deng, X.; Liu, Y.-L.; Liu, Y.-Y.; Ye, Y.-J.; Yin, D.-C. From 2D to 3D: The morphology, proliferation and differentiation of MC3T3-E1 on silk fibroin/chitosan matrices. Carbohydr. Polym. 2017, 178, 69–77.
- Baharvand, H.; Hashemi, S.M.; Ashtiani, S.K.; Farrokhi, A. Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro. Int. J. Dev. Biol. 2004, 50, 645–652.
- Salinas, C.N.; Anseth, K.S. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials 2008, 29, 2370–2377.
- Wu, W.; Zheng, Q.; Guo, X.; Sun, J.; Liu, Y. A programmed release multi-drug implant fabricated by three-dimensional printing technology for bone tuberculosis therapy. Biomed. Mater. 2009, 4, 065005.
- Siegel, R.A.; Rathbone, M.J. Overview of controlled release mechanisms. In Fundamentals and Applications of Controlled Release Drug Delivery; Springer: New York, NY, USA, 2012; pp. 19–43.
- Bhowmik, D.; Gopinath, H.; Kumar, B.P.; Duraivel, S.; Kumar, K.S. Controlled release drug delivery systems. Pharma Innov. 2012, 1, 24–32.
- Scoutaris, N.; Alexander, M.R.; Gellert, P.R.; Roberts, C.J. Inkjet printing as a novel medicine formulation technique. J. Control. Release 2011, 156, 179–185.
- Keane, T.J.; Badylak, S.F. Biomaterials for tissue engineering applications. In Seminars in Pediatric Surgery; Saunders: Philadelphia, PA, USA, 2014; pp. 112–118.
- Drzewiecki, K.E.; Malavade, J.N.; Ahmed, I.; Lowe, C.J.; Shreiber, D.I. A thermoreversible, photocrosslinkable collagen bio-ink for free-form fabrication of scaffolds for regenerative medicine. Technology 2017, 5, 185–195.
- Irmak, G.; Demirtaş, T.T.; Gumusderelioglu, M. Highly methacrylated gelatin bioink for bone tissue engineering. ACS Biomater. Sci. Eng. 2018, 5, 831–845.
- Jia, J.; Richards, D.J.; Pollard, S.; Tan, Y.; Rodriguez, J.; Visconti, R.P.; Trusk, T.C.; Yost, M.J.; Yao, H.; Markwald, R.R. Engineering alginate as bioink for bioprinting. Acta Biomater. 2014, 10, 4323–4331.
- Noh, I.; Kim, N.; Tran, H.N.; Lee, J.; Lee, C. 3D printable hyaluronic acid-based hydrogel for its potential application as a bioink in tissue engineering. Biomater. Res. 2019, 23, 3.
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243.
- Pati, F.; Jang, J.; Ha, D.-H.; Kim, S.W.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935.
- Choudhury, D.; Tun, H.W.; Wang, T.; Naing, M.W. Organ-derived decellularized extracellular matrix: A game changer for bioink manufacturing? Trends Biotechnol. 2018, 36, 787–805.
- Han, W.; Singh, N.K.; Kim, J.J.; Kim, H.; Kim, B.S.; Park, J.Y.; Jang, J.; Cho, D.-W. Directed differential behaviors of multipotent adult stem cells from decellularized tissue/organ extracellular matrix bioinks. Biomaterials 2019, 224, 119496.
- Zhu, J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656.
- Hutson, C.B.; Nichol, J.W.; Aubin, H.; Bae, H.; Yamanlar, S.; Al-Haque, S.; Koshy, S.T.; Khademhosseini, A. Synthesis and characterization of tunable poly (ethylene glycol): Gelatin methacrylate composite hydrogels. Tissue Eng. Part A 2011, 17, 1713–1723.
- Pati, F.; Ha, D.-H.; Jang, J.; Han, H.H.; Rhie, J.-W.; Cho, D.-W. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials 2015, 62, 164–175.
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224.
- Pampaloni, F.; Stelzer, E.H.; Masotti, A. Three-dimensional tissue models for drug discovery and toxicology. Recent Pat. Biotechnol. 2009, 3, 103–117.
- Maltman, D.J.; Przyborski, S.A. Developments in three-dimensional cell culture technology aimed at improving the accuracy of in vitro analyses. Biochem. Soc. Trans. 2010, 38, 1072–1075.
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue engineering approaches in the design of healthy and pathological in vitro tissue models. Front. Bioeng. Biotechnol. 2017, 5, 40.
- Benam, K.H.; Dauth, S.; Hassell, B.; Herland, A.; Jain, A.; Jang, K.-J.; Karalis, K.; Kim, H.J.; MacQueen, L.; Mahmoodian, R. Engineered in vitro disease models. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 195–262.
- Ong, C.S.; Yesantharao, P.; Huang, C.Y.; Mattson, G.; Boktor, J.; Fukunishi, T.; Zhang, H.; Hibino, N. 3D bioprinting using stem cells. Pediatric Res. 2018, 83, 223–231.
- Dai, X.; Ma, C.; Lan, Q.; Xu, T. 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication 2016, 8, 045005.
- Yao, T.; Asayama, Y. Animal-cell culture media: History, characteristics, and current issues. Reprod. Med. Biol. 2017, 16, 99–117.
- Morrison, G.; Liu, C.; Wing, C.; Delaney, S.M.; Zhang, W.; Dolan, M.E. Evaluation of inter-batch differences in stem-cell derived neurons. Stem Cell Res. 2016, 16, 140–148.
- Carmen, J.; Burger, S.R.; McCaman, M.; Rowley, J.A. Developing assays to address identity, potency, purity and safety: Cell characterization in cell therapy process development. Regen. Med. 2012, 7, 85–100.
- Wang, L.; Xu, M.-E.; Luo, L.; Zhou, Y.; Si, P. Iterative feedback bio-printing-derived cell-laden hydrogel scaffolds with optimal geometrical fidelity and cellular controllability. Sci. Rep. 2018, 8, 2802.
- Cui, X.; Dean, D.; Ruggeri, Z.M.; Boland, T. Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol. Bioeng. 2010, 106, 963–969.
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112.
- Chang, C.C.; Boland, E.D.; Williams, S.K.; Hoying, J.B. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98, 160–170.
- Roth, E.A.; Xu, T.; Das, M.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing for high-throughput cell patterning. Biomaterials 2004, 25, 3707–3715.
- Christensen, K.; Xu, C.; Chai, W.; Zhang, Z.; Fu, J.; Huang, Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol. Bioeng. 2015, 112, 1047–1055.
- Xu, T.; Zhao, W.; Zhu, J.-M.; Albanna, M.Z.; Yoo, J.J.; Atala, A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials 2013, 34, 130–139.
- Scoutaris, N.; Ross, S.; Douroumis, D. Current trends on medical and pharmaceutical applications of inkjet printing technology. Pharm. Res. 2016, 33, 1799–1816.
- Hughes, T.R.; Mao, M.; Jones, A.R.; Burchard, J.; Marton, M.J.; Shannon, K.W.; Lefkowitz, S.M.; Ziman, M.; Schelter, J.M.; Meyer, M.R. Expression profiling using microarrays fabricated by an ink-jet oligonucleotide synthesizer. Nat. Biotechnol. 2001, 19, 342–347.
- Jang, J.; Yi, H.-G.; Cho, D.-W. 3D printed tissue models: Present and future. ACS Biomater. Sci. Eng. 2016, 2, 1722–1731.
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785.
- Chang, R.; Nam, J.; Sun, W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication–based direct cell writing. Tissue Eng. Part A 2008, 14, 41–48.
- Khalil, S.; Sun, W. Biopolymer deposition for freeform fabrication of hydrogel tissue constructs. Mater. Sci. Eng. C 2007, 27, 469–478.
- Visser, J.; Peters, B.; Burger, T.J.; Boomstra, J.; Dhert, W.J.; Melchels, F.P.; Malda, J. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication 2013, 5, 035007.
- Jakab, K.; Damon, B.; Neagu, A.; Kachurin, A.; Forgacs, G. Three-dimensional tissue constructs built by bioprinting. Biorheology 2006, 43, 509–513.
- Cohen, D.L.; Malone, E.; Lipson, H.; Bonassar, L.J. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng. 2006, 12, 1325–1335.
- Li, X.; Wang, Y.; Wang, Z.; Qi, Y.; Li, L.; Zhang, P.; Chen, X.; Huang, Y. Composite PLA/PEG/nHA/dexamethasone scaffold prepared by 3D printing for bone regeneration. Macromol. Biosci. 2018, 18, 1800068.
- Cho, W.W.; Kim, B.S.; Ahn, M.; Ryu, Y.H.; Ha, D.H.; Kong, J.S.; Rhie, J.W.; Cho, D.W. Flexible Adipose-Vascular Tissue Assembly Using Combinational 3D Printing for Volume-Stable Soft Tissue Reconstruction. Adv. Healthc. Mater. 2021, 10, 2001693.
- Ha, D.-H.; Chae, S.; Lee, J.Y.; Kim, J.Y.; Yoon, J.; Sen, T.; Lee, S.-W.; Kim, H.J.; Cho, J.H.; Cho, D.-W. Therapeutic effect of decellularized extracellular matrix-based hydrogel for radiation esophagitis by 3D printed esophageal stent. Biomaterials 2021, 266, 120477.
- Kim, B.S.; Das, S.; Jang, J.; Cho, D.-W. Decellularized extracellular matrix-based bioinks for engineering tissue-and organ-specific microenvironments. Chem. Rev. 2020, 120, 10608–10661.
- Shim, J.-H.; Kim, J.Y.; Park, M.; Park, J.; Cho, D.-W. Development of a hybrid scaffold with synthetic biomaterials and hydrogel using solid freeform fabrication technology. Biofabrication 2011, 3, 034102.
- Lee, J.-S.; Kim, B.S.; Seo, D.; Park, J.H.; Cho, D.-W. Three-dimensional cell printing of large-volume tissues: Application to ear regeneration. Tissue Eng. Part C Methods 2017, 23, 136–145.
- Lee, H.; Cho, D.-W. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip 2016, 16, 2618–2625.
- Onoe, H.; Okitsu, T.; Itou, A.; Kato-Negishi, M.; Gojo, R.; Kiriya, D.; Sato, K.; Miura, S.; Iwanaga, S.; Kuribayashi-Shigetomi, K. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 2013, 12, 584–590.
- Gao, G.; Park, J.Y.; Kim, B.S.; Jang, J.; Cho, D.W. Coaxial cell printing of freestanding, perfusable, and functional in vitro vascular models for recapitulation of native vascular endothelium pathophysiology. Adv. Healthc. Mater. 2018, 7, 1801102.
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343.
- Guillemot, F.; Souquet, A.; Catros, S.; Guillotin, B. Laser-assisted cell printing: Principle, physical parameters versus cell fate and perspectives in tissue engineering. Nanomedicine 2010, 5, 507–515.
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434.
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent Application No. 638,905, 8 August 1984.
- Ma, X.; Liu, J.; Zhu, W.; Tang, M.; Lawrence, N.; Yu, C.; Gou, M.; Chen, S. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv. Drug Deliv. Rev. 2018, 132, 235–251.
- Park, S.H.; Yang, D.Y.; Lee, K.S. Two-photon stereolithography for realizing ultraprecise three-dimensional nano/microdevices. Laser Photonics Rev. 2009, 3, 1–11.
- Gittard, S.; Narayan, R.; Jin, C.; Ovsianikov, A.; Chichkov, B.; Monteiro-Riviere, N.; Stafslien, S.; Chisholm, B. Pulsed laser deposition of antimicrobial silver coating on Ormocer® microneedles. Biofabrication 2009, 1, 041001.
- Gittard, S.D.; Ovsianikov, A.; Chichkov, B.N.; Doraiswamy, A.; Narayan, R.J. Two-photon polymerization of microneedles for transdermal drug delivery. Expert Opin. Drug Deliv. 2010, 7, 513–533.
- Sarment, D.P.; Al-Shammari, K.; Kazor, C.E. Stereolithographic surgical templates for placement of dental implants in complex cases. Int. J. Periodontics Restor. Dent. 2003, 23, 287–296.
- Binder, T.M.; Moertl, D.; Mundigler, G.; Rehak, G.; Franke, M.; Delle-Karth, G.; Mohl, W.; Baumgartner, H.; Maurer, G. Stereolithographic biomodeling to create tangible hard copies of cardiac structures from echocardiographic data: In vitro and in vivo validation. J. Am. Coll. Cardiol. 2000, 35, 230–237.
- Bell, A.; Kofron, M.; Nistor, V. Multiphoton crosslinking for biocompatible 3D printing of type I collagen. Biofabrication 2015, 7, 035007.
- Su, P.-J.; Tran, Q.A.; Fong, J.J.; Eliceiri, K.W.; Ogle, B.M.; Campagnola, P.J. Mesenchymal stem cell interactions with 3D ECM modules fabricated via multiphoton excited photochemistry. Biomacromolecules 2012, 13, 2917–2925.
- Wylie, R.G.; Ahsan, S.; Aizawa, Y.; Maxwell, K.L.; Morshead, C.M.; Shoichet, M.S. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat. Mater. 2011, 10, 799–806.
- Zhang, W.; Soman, P.; Meggs, K.; Qu, X.; Chen, S. Tuning the poisson’s ratio of biomaterials for investigating cellular response. Adv. Funct. Mater. 2013, 23, 3226–3232.
- Sodian, R.; Loebe, M.; Hein, A.; Martin, D.P.; Hoerstrup, S.P.; Potapov, E.V.; Hausmann, H.; Lueth, T.; Hetzer, R. Application of stereolithography for scaffold fabrication for tissue engineered heart valves. ASAIO J. 2002, 48, 12–16.
- Sodian, R.; Fu, P.; Lueders, C.; Szymanski, D.; Fritsche, C.; Gutberlet, M.; Hoerstrup, S.; Hausmann, H.; Lueth, T.; Hetzer, R. Tissue engineering of vascular conduits: Fabrication of custom-made scaffolds using rapid prototyping techniques. Thorac. Cardiovasc. Surg. 2005, 53, 144–149.
- Jung, J.W.; Park, J.H.; Hong, J.M.; Kang, H.-W.; Cho, D.-W. Octahedron pore architecture to enhance flexibility of nasal implant-shaped scaffold for rhinoplasty. Int. J. Precis. Eng. Manuf. 2014, 15, 2611–2616.
More
