Phytosomes are an innovative lipid-based delivery system that have a liposomes-related structure and can be used for the entrapment of different types of polyphenolic-based phytoconstituents to improve their absorption when administrated. The first phytosomes were developed by Indena company (Milan, Italy) in the late 1980s, which aimed to increase the bioavailability of drugs by complexing them to phospholipids. The structure of phytosomes is composed of standardized polyphenolic plant extract incorporated into phospholipids, mainly phosphatidylcholine (PC).
- phytosomes
- nanocarriers
- skin barrier
- phytochemicals
- topical application
- drug delivery
1. Introduction
Phytochemicals are bioactive polyphenolic compounds naturally found in plants that have been studied extensively due to their potential medicinal and nutritional benefits to humans. They not only play a protective role for the plant but are responsible for its color, aroma, and flavor. These compounds have attracted the attention of scientists worldwide, owing to their potent bioactivity against different diseases, their low cytotoxicity and their ability to be utilized in the production of cosmetics and dietary supplements [1,2,3][1][2][3].
Nowadays, the skin is more likely to become infected as a result of changing environmental conditions and an increase in pollution levels. Consequently, there has been a significant increase in the demand for herbal medicine in both developed and developing countries owing to their potent biological efficacy, higher safety margins, and lower cost than synthetic agents [13][4]. Numerous herbal extracts originating from different plant species have been evaluated for their potential to treat skin conditions due to their medicinal benefits, which include antimicrobial and anti-inflammatory properties, their ability to promote blood clotting and wound healing, and to relieve burns and other skin diseases [14,15][5][6]. Several common skin conditions, including eczema, acne, urticaria, pruritus, psoriasis, and other bacterial and fungal skin diseases, can be treated efficiently using medicinal herbs [16,17][7][8]. The chemical structure of most plant extracts used in pharmaceutical and cosmeceutical applications is based on flavonoids or other polyphenol rings, which have high molecular weight and, hence, have poor solubility and are poorly absorbed through the skin [18,19][9][10].
The human skin is a remarkably complex and sophisticated organ that serves as a barrier to external exposure by enveloping the inner body components. It consists of three main layers—the epidermis, dermis and hypodermis—and each has different degrees of specialization. Along with its function as a waterproof barrier, the epidermis contains melanocytes, which determine the skin’s tone and pigmentation. The dermis is the skin layer underneath the epidermis that composed of connective tissue, sweat glands and hair follicles and helps to maintain the skin’s flexibility. In comparison to the other layers of the skin, the hypodermis is made up of fat and connective tissue and is responsible for shock absorption [20][11]. The stratum corneum (SC), or outer layer of the epidermis, is entirely made up of dead, keratinized epithelium cells and other metabolically inactive cells that cover the outer surface of the skin [21][12]. The SC and epidermis are regarded as the most effective barriers against hydrophilic and lipophilic compounds, respectively [22,23][13][14]. Furthermore, in terms of skin mechanical properties, elastic fibres are essential for skin elasticity and are directly responsible for skin performance and appearance [24][15]. Figure 1 shows a simplified structure of the skin and its barriers.
There are various types of nanotechnology-based materials that are currently used in the delivery of polyphenolic phytochemicals. Nanosized delivery systems can be classified into two main groups: Organic delivery systems (i.e., liposomes and polymeric nanoparticles) and inorganic delivery systems (i.e., silver, gold, and copper nanoparticles) [48][16]. Liposomes are one of the most commonly used nanoparticles that have been successfully used in the pharmaceutical and cosmetics fields [49][17]. The encapsulation of curcumin into liposomal nanoparticles showed potent activity against lung, pancreatic, and colorectal cancer at a lower dose in comparison to free curcumin [50][18]. Polymeric nanoparticles can also be used as an efficient nanocarrier of phytochemicals, and the encapsulation of curcumin extract into chitosan and polylactic-co-glycolic acid (PLGA) nanoparticles exhibited a significant improvement in its solubility profile compared to a conventional curcumin formulation [51][19].
2. Advantages of Phytosomes in Topical Applications
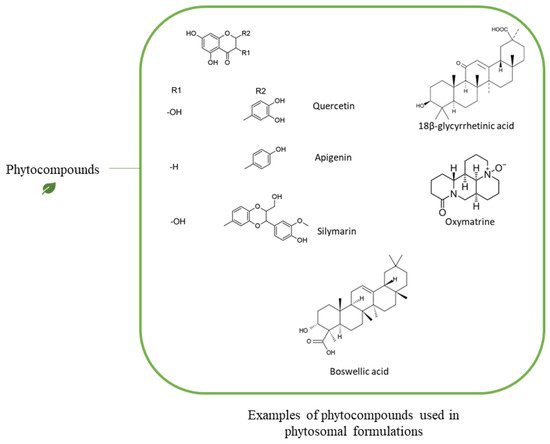
| Phytochemical | Type of Phytochemical | Type of Phospholipid | Chemical Interaction | Analysis Method | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Quercetin | Polyphenols | PC | H-bonds with the polar group of the phosphplipid | 1H-NMR, 31P-NMR, 13C-NMR | [127] | [41] | ||
| Polyphenols | DPPC | 1 | (PC) |
(1) Electrostatic interactions, (2) H-bonds with the polar group of the phosphplipid, (3) Hydrophobic interaction with fatty acyl chains |
1H-NMR, 31P-NMR, 13C-NMR | [128] | [42] | |
| Lycopene | Carotenoids (Terpenoid) | DPPC (PC) |
Hydrophobic interaction with the acyl fatty acid chain | X-Ray | [133] | [47] | ||
| β-carotene, Lycopene | Carotenoids (Terpenoid) | POPC | 2 | (PC) |
Hydrophobic interaction with the acyl fatty acid chain | X-Ray | [131] | [45] |
| Tyrosol, Verbascoside, Hydroxytyrosol | Polyphenols | PC | H-bonds with the polar group of the phosphplipid | 1H-NMR, 31P-NMR, 13C-NMR | [33] | [39] | ||
| Saponin | Triterpene glycosides | PC | H-bonds with the polar group of the phosphplipid | 1H-NMR, 31P-NMR, 13C-NMR | [15] | [6] | ||
| 18-β-glycyrrhetinic Acid | Triterpenoids | Soy lecithin (PC) | H-bonds with the polar group of the phosphplipid | DSC | [132] | [46] |
References
- Jasemi, S.V.; Khazaei, H.; Aneva, I.Y.; Farzaei, M.H.; Echeverría, J. Medicinal Plants and Phytochemicals for the Treatment of Pulmonary Hypertension. Front. Pharmacol. 2020, 11.
- Oveissi, V.; Ram, M.; Bahramsoltani, R.; Ebrahimi, F.; Rahimi, R.; Naseri, R.; Belwal, T.; Devkota, H.P.; Abbasabadi, Z.; Farzaei, M.H. Medicinal plants and their isolated phytochemicals for the management of chemotherapy-induced neuropathy: Therapeutic targets and clinical perspective. DARU J. Pharm. Sci. 2019, 27, 389–406.
- Kooti, W.; Servatyari, K.; Behzadifar, M.; Asadi-Samani, M.; Sadeghi, F.; Nouri, B.; Marzouni, H.Z. Effective Medicinal Plant in Cancer Treatment, Part 2: Review Study. J. Evid.-Based Integr. Med. 2017, 22, 982–995.
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177.
- Kim, J.-E. Molecular Targets of Phytochemicals for Skin Inflammation. Curr. Pharm. Des. 2018, 24, 1533–1550.
- Bahramsoltani, R.; Farzaei, M.H.; Rahimi, R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: An integrative review. Arch. Dermatol. Res. 2014, 306, 601–617.
- Dawid-Pac, R. Medicinal plants used in treatment of inflammatory skin diseases. Adv. Dermatol. Allergol. 2013, 3, 170–177.
- Laura, V.; Mattia, F.; Roberta, G.; Federico, I.; Emi, D.; Chiara, T.; Luca, B.; Elena, C. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169.
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747.
- Kidd, P.M. Bioavailability and activity of phytosome complexes from botanical polyphenols: The silymarin, curcumin, green tea, and grape seed extracts. Altern. Med. Rev. J. Clin. Ther. 2009, 14, 226–246.
- Wong, R.; Geyer, S.; Weninger, W.J.; Guimberteau, J.-C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2015, 25, 92–98.
- Prausnitz, M.R.; Elias, P.M.; Franz, T.J.; Schmuth, M.; Tsai, J.-C.; Menon, G.K.; Holleran, W.M.; Feingold, K.R. Skin barrier and transdermal drug delivery. Dermatology 2012, 32, 760–769.
- Schnittger, S.; Sinha, M. The Materials Science of Cosmetics. MRS Bull. 2007, 32, 760–769.
- Andrews, S.N.; Jeong, E.; Prausnitz, M.R. Transdermal Delivery of Molecules is Limited by Full Epidermis, Not Just Stratum Corneum. Pharm. Res. 2012, 30, 1099–1109.
- Sherratt, M.J. Tissue elasticity and the ageing elastic fibre. AGE 2009, 31, 305–325.
- Khalid, K.; Tan, X.; Zaid, H.F.M.; Tao, Y.; Chew, C.L.; Chu, D.-T.; Lam, M.K.; Ho, Y.-C.; Lim, J.W.; Wei, L.C. Advanced in developmental organic and inorganic nanomaterial: A review. Bioengineered 2020, 11, 328–355.
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102.
- Lin, Y.-L.; Liu, Y.-K.; Tsai, N.-M.; Hsieh, J.-H.; Chen, C.-H.; Lin, C.-M.; Liao, K.-W. A Lipo-PEG-PEI complex for encapsulating curcumin that enhances its antitumor effects on curcumin-sensitive and curcumin-resistance cells. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 318–327.
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 153–160.
- Dini, I.; Laneri, S. The New Challenge of Green Cosmetics: Natural Food Ingredients for Cosmetic Formulations. Molecules 2021, 26, 3921.
- Gupta, A.; Ashawat, M.; Saraf, S.; Saraf, S. Phytosome: A novel approach towards functional cosmetics. J. Plant Sci. 2007, 2, 644–649.
- Chanchal, D.; Swarnlata, S. Novel approaches in herbal cosmetics. J. Cosmet. Dermatol. 2008, 7, 89–95.
- Tessema, E.N.; Gebre-Mariam, T.; Neubert, R.H.; Wohlrab, J. Potential Applications of Phyto-Derived Ceramides in Improving Epidermal Barrier Function. Skin Pharmacol. Physiol. 2017, 30, 115–138.
- Haque, T.; Talukder, M.U. Chemical Enhancer: A Simplistic Way to Modulate Barrier Function of the Stratum Corneum. Adv. Pharm. Bull. 2018, 8, 169–179.
- Patzelt, A.; Antoniou, C.; Sterry, W.; Lademann, J. Skin penetration from the inside to the outside: A review. Drug Discov. Today Dis. Mech. 2008, 5, e229–e235.
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal delivery systems in cosmetics. Biomed. Dermatol. 2020, 4, 1–12.
- Lu, M.; Qiu, Q.; Luo, X.; Liu, X.; Sun, J.; Wang, C.; Lin, X.; Deng, Y.; Song, Y. Phyto-phospholipid complexes (phytosomes): A novel strategy to improve the bioavailability of active constituents. Asian J. Pharm. Sci. 2018, 14, 265–274.
- Bombardelli, E.; Spelta, M. Phospholipid-polyphenol complexes: A new concept in skin care ingredients. Cosmet. Toilet. 1991, 106, 69–76.
- Tripathy, S.; Patel, D.K.; Barob, L.; Naira, S.K. A review on phytosomes, their characterization, advancement & potential for transdermal application. J. Drug Deliv. Ther. 2013, 3, 147–152.
- Droy-Lefaix, M.T. Effect of the antioxidant action of Ginkgo biloba extract (EGb 761) on aging and oxidative stress. Age 1997, 20, 141–149.
- Loggia, R.d.; Sosa, S.; Tubaro, A.; Morazzoni, P.; Bombardelli, E.; Griffini, A. Anti-inflammatory activity of some Ginkgo biloba constituents and of their phospholipid-complexes. Fitoterapia 1996, 67, 257–264.
- Chen, Z.-P.; Sun, J.; Chen, H.-X.; Xiao, Y.-Y.; Liu, D.; Chen, J.; Cai, H.; Cai, B.-C. Comparative pharmacokinetics and bioavailability studies of quercetin, kaempferol and isorhamnetin after oral administration of Ginkgo biloba extracts, Ginkgo biloba extract phospholipid complexes and Ginkgo biloba extract solid dispersions in rats. Fitoterapia 2010, 81, 1045–1052.
- Kennedy, D.; Haskell, C.F.; Mauri, P.; Scholey, A.B. Acute cognitive effects of standardised Ginkgo biloba extract complexed with phosphatidylserine. Hum. Psychopharmacol. Clin. Exp. 2007, 22, 199–210.
- Maramaldi, G.; Togni, S.; Pagin, I.; Giacomelli, L.; Cattaneo, R.; Eggenhöffner, R.; Burastero, S.E. Clin. CosmetSoothing and anti-itch effect of quercetin phytosome in human subjects: A single-blind study. Clin. Cosmet. Investig. Dermatol. 2016, 9, 55–62.
- El-Fattah, A.I.A.; Fathy, M.M.; Ali, Z.Y.; El-Garawany, A.E.-R.A.; Mohamed, E.K. Enhanced therapeutic benefit of quercetin-loaded phytosome nanoparticles in ovariectomized rats. Chem. Interact. 2017, 271, 30–38.
- Okonogi, S.; Riangjanapatee, P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formulations for topical administration. Int. J. Pharm. 2015, 478, 726–735.
- Sharma, P.K.S.P.; Saxena, P.; Jaswanth, A.; Chalamaiah, M.; Tekade, K.R.; Balasubramaniam, A. Novel encapsulation of lycopene in niosomes and assessment of its anticancer activity. J. Bioequivalence Bioavailab. 2016, 8, 224–232.
- Ghazi, A.M.; Al-Bayati, M.A. Anti-proliferative of the phytosome propolis, phytosome lycopene and synergistic effect on the benign prostatic hyperplasia cells in-vitro. Plant Arch. 2020, 20, 6579–6589.
- Semalty, A.; Semalty, M.; Rawat, M.S.M.; Franceschi, F. Supramolecular phospholipids–polyphenolics interactions: The PHYTOSOME® strategy to improve the bioavailability of phytochemicals. Fitoterapia 2010, 81, 306–314.
- Singh, A.; Saharan, V.A.; Singh, M.; Bhandari, A. Phytosome: Drug Delivery System for Polyphenolic Phytoconstituents. Iran. J. Pharm. Sci. 2011, 7, 209–219.
- De Granada-Flor, A.; Sousa, C.; Filipe, H.A.L.; Santos, M.S.C.S.; De Almeida, R.F.M. Quercetin dual interaction at the membrane level. Chem. Commun. 2019, 55, 1750–1753.
- Pawlikowska-Pawlęga, B.; Gruszecki, W.I.; Misiak, L.; Paduch, R.; Piersiak, T.; Zarzyka, B.; Pawelec, J.; Gawron, A. Modification of membranes by quercetin, a naturally occurring flavonoid, via its incorporation in the polar head group. Biochim. Biophys. Acta Biomembr. 2007, 1768, 2195–2204.
- Emiliano, A.; Veronica, B.; Walter, V.; Elena Del, B.; Marzia, C. Oral Curcumin (Meriva) Is Effective as an Adjuvant Treatment and Is Able to Reduce IL-22 Serum Levels in Patients with Psoriasis Vulgaris. BioMed Res. Int. 2015, 3, 1–7.
- Djekic, L.; Krajišnik, D.; Mićic, Z.; Čalija, B. Formulation and physicochemical characterization of hydrogels with 18β-glycyrrhetinic acid/phospholipid complex phytosomes. J. Drug Deliv. Sci. Technol. 2016, 35, 81–90.
- McNulty, H.P.; Byun, J.; Lockwood, S.F.; Jacob, R.F.; Mason, R.P. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim. Biophys. Acta Biomembr. 2007, 1768, 167–174.
- Khan, J.; Alexander, A.; Uddin, A.; Saraf, S.; Saraf, S. Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profile of plant actives. J. Control. Release 2013, 168, 50–60.
- Hidalgo, P.; Strzalka, K.; Kostecka-Gugala, A. Comparative X-Ray Studies on the Interaction of Carotenoids with a Model Phosphatidylcholine Membrane. Z. Nat. C 2002, 57, 129–134.
- Djekic, L.; Krajisnik, D.; Micic, Z. Polyphenolics-Phospholipid Complexes as Natural Cosmetic Ingredients: Properties and Application. Tenside Surfactants Deterg. 2015, 52, 186–192.
- Cao, F.-H.; Ouyang, W.-Q.; Wang, Y.-P.; Yue, P.-F.; Li, S.-P. A combination of a microemulsion and a phospholipid complex for topical delivery of oxymatrine. Arch. Pharmacal Res. 2011, 34, 551–562.
- Ma, A.; Yang, Y.; Wang, Q.; Wang, Y.; Wen, J.; Zhang, Y. Anti-inflammatory effects of oxymatrine on rheumatoid arthritis in rats via regulating the imbalance between Treg and Th17 cells. Mol. Med. Rep. 2017, 15, 3615–3622.
- Iram, F.; Khan, S.; Husain, A. Phytochemistry and potential therapeutic actions of Boswellic acids: A mini-review. Asian Pac. J. Trop. Biomed. 2017, 7, 513–523.
- Hüsch, J.; Gerbeth, K.; Fricker, G.; Setzer, C.; Zirkel, J.; Rebmann, H.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Effect of Phospholipid-Based Formulations of Boswellia serrataExtract on the Solubility, Permeability, and Absorption of the Individual Boswellic Acid Constituents Present. J. Nat. Prod. 2012, 75, 1675–1682.
- Sharma, A.; Gupta, N.K.; Dixit, V.K. Complexation with phosphatidyl choline as a strategy for absorption enhancement of boswellic acid. Drug Deliv. 2010, 17, 587–595.
- Ho, P.J.; Sung, J.J.; Cheon, K.K.; Tae, H.J. Anti-inflammatory effect of Centella asiatica phytosome in a mouse model of phthalic anhydride-induced atopic dermatitis. Phytomedicine 2018, 43, 110–119.
- Gray, N.E.; Magana, A.A.; Lak, P.; Wright, K.M.; Quinn, J.; Stevens, J.F.; Maier, C.S.; Soumyanath, A. Centella asiatica: Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem. Rev. 2017, 17, 161–194.
- Bombardelli, E.; Cristoni, A.; Morazzoni, P. Phytosome® s in functional cosmetics. Fitoterapia 1994, 65, 387–401.
- Darvishi, B.; Manoochehri, S.; Kamalinia, G.; Samadi, N.; Amini, M.; Mostafavi, S.H.; Maghazei, S.; Atyabi, F.; Dinarvand, R. Preparation and Antibacterial Activity Evaluation of 18-β-glycyrrhetinic Acid Loaded PLGA Nanoparticles. Iran. J. Pharm. Res. 2015, 14, 373–383.
- Anitha, V.; Reddy, P.D.; Ramkanth, S. Phytosomes: A promising technology in novel herbal drug delivery system. PharmaTutor 2019, 7, 18–25.
- Agarwal, A.; Chakraborty, P.; Chakraborty, D.D.; Saharan, V.A.S. Phytosomes: Complexation, Utilisation and Commerical Status. J. Biol. Act. Prod. Nat. 2012, 2, 65–77.
- Babazadeh, A.; Zeinali, M.; Hamishehkar, H. Nano-Phytosome: A Developing Platform for Herbal Anti-Cancer Agents in Cancer Therapy. Curr. Drug Targets 2018, 19, 170–180.
