Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Tina Garofalo.
ER lipid raft-associated protein 1 (ERLIN1) and 2 (ERLIN2) are 40 kDa transmembrane glycoproteins belonging to the family of prohibitins, containing a PHB domain. They are generally localized in the endoplasmic reticulum (ER), where ERLIN1 forms a heteroligomeric complex with its closely related ERLIN2. Well-defined functions of ERLINS are promotion of ER-associated protein degradation, mediation of inositol 1,4,5-trisphosphate (IP3) receptors, processing and regulation of lipid metabolism.
- ERLINs
- lipid rafts
- MAMs
- autophagy
- apoptosis
1. Introduction
Preferential interactions between lipids and proteins lead to the organization of specialized sphingolipid-based microdomains on both plasma membrane and distinct organelle membranes in different cell types.
Indeed, evidence which has accumulated over the last two decades strongly supports the view that interactions between specific lipids, including cholesterol and sphingomyelin, leading to the formation of functionally important and relatively liquid-ordered (Lo) domains, termed lipid rafts, which move within a fluid bilayer of cellular membranes, allowing the recruitment of other lipids and proteins [1,2][1][2]. Although cholesterol and sphingolipids are important rafts components, also gangliosides, sialic-acid containing glycosphingolipids (GSLs), are highly enriched in these regions where interact with cholesterol [3]. Consistent with these data, GSLs have been proposed as a core component of lipid rafts and are therefore used as typical lipid raft markers [4,5,6][4][5][6].
Development of methodologies available for their investigation clarified that these domains are distributed not only in both the outer and the inner leaflets of an asymmetric cell membrane, but also coupled across leaflets [7,8][7][8] to form functional domains with distinct compositions and properties [9,10][9][10]. Moreover, GSLs are asymmetrically enriched in the outer leaflet of the plasma membrane, suggesting that clustering of GSLs could be further stabilized by the formation of lateral carbohydrate-carbohydrate interactions [11].
Thus, a major feature of raft domains is to segregate specific elements with the aim to regulate their interactions with other membrane components, i.e., lipids and proteins, and hence their activity [2,12,13][2][12][13]. As multimolecular platforms, lipid rafts perform important functions through carbohydrate-interactions with specific proteins characterized especially by saturated lipid anchors GPI-like or palmitoyl moieties [14,15][14][15]. Some proteins are only transiently confined within these domains, while others are completely excluded [16], establishing the concept of transient and dynamic structures [17]. Recently, it has become evident that a complex network of lipid–lipid and lipid–protein interactions contribute to the activation of a variety of signaling pathways able to influence cell homeostasis [2].
2. Raft-like Microdomains in Internal Membranes
Lipid rafts, more specifically known as lipid “raft-like microdomains”, are distributed on the membrane of subcellular organelles, including ER, Golgi apparatus, endosomes, lysosomes and lipid droplets, as recently summarized in the review by Wang et al. [19][18], but also mitochondria [20[19][20][21],21,22], and nuclei [23][22]. At these sites, key reactions can be catalyzed with a significant impact in the regulation of intracellular trafficking and sorting [24][23], cholesterol homeostasis [25][24], and cell fate, i.e., survival or death [26,27,28,29][25][26][27][28]. In this way, they contribute to diverse biological processes [30][29].
Depending on the specific organelle, raft-like microdomains are particularly enriched in specific proteins, in close association with a certain lipid assortment, including cholesterol, glycosphingolipids and cardiolipin [31,32][30][31]. Although the key role of these structures in signal transduction is depending on protein composition, the characterization of lipid molecules as key components within raft-like microdomains has gained special attention in recent years [33,34,35][32][33][34]. Therefore, we focused on specific lipids associated with raft-like microdomains of principal organelles, which mainly contribute to cell fate.
2.1. Rafts-like Microdomains in the Mitochondria
It is well known that, although the 90% of total cellular cholesterol content is located in plasma membranes, a relatively low content of this lipid (around 3%) is also present in internal membranes [36][35]; nevertheless, as well as for plasma membranes, in internal membranes cholesterol is considered the major lipid component of “raft-like” microdomains, responsible for stabilizing protein and lipid interactions, leading to the formation of dynamic lipid platforms for internal signal transduction. Moreover, GD3 ganglioside, which is normally confined to plasma membrane lipid rafts, can be redistributed to mitochondrial membranes by actin cytoskeleton vesicular trafficking [37,38][36][37] in response to death signals.
A well described example of this statement is the redistribution of GD3 in lymphoblastoid T cells upon pro-apoptotic triggering induced by CD95/Fas ligation from plasma membrane (and/or from trans Golgi network) to the mitochondria lipid microdomains [38,39,40,41][37][38][39][40]. In this way, GD3 could promote both morphogenetic changes of mitochondrial membrane (i.e., curvature and membrane viscosity), and lead to the formation of clusters of apoptotic signaling molecules (t-Bid and Bax), which represent key events for apoptosis execution [42,43][41][42]. Moreover, disruption of lipid microdomains in isolated mitochondria using cholesterol sequestering agents selectively rescues the mitochondria depolarization induced by GD3 with consequent impairment of apoptosis [20][19]. In addition, molecules involved in the fission processes are associate with these microdomains. Indeed, hFis is constitutively present in mitochondrial raft-like microdomains, whereas dynamin-like protein 1 is recruited following proapoptotic stimulus. Thus, recruitment of fission-associated molecules to raft-like microdomains play a role in the morphogenetic changes leading to organelle fission [39][38]. On the other hand, localization of MFN2 in lipid rafts, via its molecular interaction with the ganglioside GD3, is mandatory in mitochondria network organization, playing a role in mitochondria fusion [41][40].
Moreover, cardiolipin, a mitochondrial phospholipid, was found as a crucial component within raft-like microdomains, specifically located at the contact sites formed between the inner and outer membranes [32][31], where acts as an activation platform for both caspase-8 recruitment, either contributing to regulate apoptosis, and for the recruitment of the multimolecular complex AMBRA1/BECN1/WIPI1 to regulate autophagy execution [44,45,46][43][44][45].
Thus, the inclusion of proteins into lipid rafts is tightly dependent on the lipid composition which becomes responsible for these preferential membrane sites, where membrane receptors are in close contact with target signaling molecules inducing the activation of signaling pathways of survival or death.
2.2. Rafts-like Microdomains in the Nuclei
For many years research focused on glycosphingolipids as lipid components of the inner nuclear membrane that influences the formation and stability of nuclear “rafts”. These structures represent essential platforms which strongly participate in maintaining the internal nuclear organization and function, influencing specific nuclear functions, including proliferation, differentiation, and apoptosis [47,48][46][47]. Lipid analysis of microdomains isolated from highly purified hepatocyte nuclei revealed a peculiar lipid composition characterized by high levels of phosphatidylcholine and sphingomyelin [23][22], partially linked with cholesterol. Cholesterol is considered essential to ensure lipid rafts formation on nuclear membrane, where it can exist in two principal pools: as sphingomyelin-free cholesterol without variation during cell proliferation and as sphingomyelin-linked cholesterol, which can be altered during the S-phase of the cell cycle when the nuclear-sphingomyelinase is activated [49][48].
These microdomains were proposed to act as platforms for transcription processes, as demonstrated by labeled (H3)-uridine incorporation during the S phase of the cell cycle [23][22]. Modification of nuclear microdomains induces a destruction of internal nuclear architecture with impairment of RNA transcription, suggesting a role for raft associated sphingomyelin in maintaining the nuclear integrity and function [50][49]. Moreover, exogenous cholesterol is also required for lipid accumulation and stabilization during cytokinesis [51,52][50][51].
Several lines of investigation indicate that control of cholesterol and sphingolipid metabolism is essential for regulation of signaling molecules associated to lipid rafts in mediating biological functions, such as cell survival and death [53][52]. In particular, studies in the recent past investigated the role of cholesterol in modulation of cell growth/proliferation and apoptosis [54][53]. Indeed, altered cholesterol metabolism occurs in a variety of cancers and contributes to tumor cell growth, since molecules derived from cholesterol, including steroid hormones, oxysterols and vitamin D can act as a ligand for estrogen-related receptor [55][54], with different and even opposite actions on cancer cells and tumor progression [56][55]. Depleting cholesterol from lipid rafts results in the disorganization of signal molecules and therefore increases the sensitivity of cancer cells to chemotherapy. Moreover, cholesterol depletion from lipid rafts in ovarian cancer cells, after increased cholesterol efflux due to specific transporters, is responsible for the phenotypic reprogramming of macrophages into tumor-associated macrophages, making them more responsive to pro-tumor signals, such as IL-4, and more resistant to the action of anti-tumor cytokines, such as interferon-gamma [53,54,55,56,57][52][53][54][55][56].
2.3. Rafts-like Microdomains in Golgi and ER
Endoplasmic reticulum (ER) stores only ~0.5–1% of the cell total cholesterol [58][57], comprising ∼3–5% of all ER lipids. However, functional raft-like microdomains have been described in the ER and in the Golgi apparatus under certain conditions [19][18].
In particular, the P24-P23 protein complex, which can bind to Sec24D, acting as a cargo receptor to mediate the export of GPI-anchored proteins like CD59 or the folate receptor may be associated with raft-like microdomains on the ER membrane. Cholesterol depletion disrupts the interaction between CD59 and P24-P23 and thus reduces the export of CD59 to Golgi, indicating that functional lipid rafts at the ER membrane can assist GPI-anchored proteins in transporting to Golgi [19][18].
In general, the subcellular distribution of lipid raft on internal membranes, including the Golgi apparatus or the ER, has a significant impact in the sorting of proteins and in the trafficking and overall exocytosis of viral proteins, which constitute fundamental steps in viral infection [59][58].
2.4. MAMs Raft-like Microdomains
Moreover, organelles communications have been reported to occur between ER and other organelles, such as mitochondria, through close physical contacts which are strongly modulated by lipid raft-like components. In this regard, emerging data support the existence of mitochondria-associated ER membranes (MAMs), which represent tethering sites between the membrane of ER and mitochondrial with 10–25 nm between them [60][59].
MAMs do not simply structurally link ER and mitochondria. An emerging role of MAMs is their ability in many signaling regulations, starting from the first discovery on the exchange of phospholipids [61][60], up to the control of metabolism and trafficking of different classes of lipids (i.e., cholesterol, sphingolipids, ceramide) [62][61] and proteins [63][62]. Thus, it is not surprising that the interplay between ER and mitochondria contributes during many circumstances to choose whether the cell should live or die.
Of interest, the biochemical analysis of purified MAM fractions revealed that they are characterized by the presence of specialized raft-like sub-domains enriched in cholesterol, which makes these membranes portions quite different from the remaining ones, allowing the membrane scrambling and contributing to the multiple functions of ER and mitochondria, respectively [64][63].
In this regard, since the major components of lipid microdomains reside within MAM subdomains [31[30][64],65], the role of gangliosides in regulating and influencing cellular activities through these subdomains has been investigated. In particular, gangliosides effect on cell fate could depend on structural characteristics and sugar modifications, as well as on their concentration [34,65][33][64]. GM1-ganglioside accumulation at MAMs can influence the activity of the inositol 1,4,5-trisphosphate (IP3) receptor (IP3R) by directly interacting with the channel. Consequently, this binding promotes an enhancement of Ca2+ transmission from the ER to the mitochondria, activating the mitochondrial apoptotic cascade [66][65].
Under stress conditions, cells can coordinate ER and mitochondrial functions to restore cellular homeostasis, involving MAMs raft-like microdomains, which contain many proteins that physically are able to make molecular bridges that regulate the close contact between the two organelles and that play important roles in lipid synthesis and Ca2+ transfer from the ER to mitochondria [67][66]. Both the mitochondria-shaping protein dynamin-related protein 1 (Drp1) [68][67] and Phosphofurin Acidic Cluster Sorting Protein 2 (PACS-2) [69][68], can have a function in regulating contacts between ER and mitochondria and both proteins were identified in lipid rafts. PACS-2, at ER level, regulates juxtaposition of the two compartments through BAP31-dependent fission and perinuclear clustering of mitochondria [70][69]. In the same way, Drp1 could alter tethering by causing fragmentation of mitochondria.
Proteomic analysis of raft-like microdomains within MAM revealed that the majority of the identified proteins are bona fide mitochondrial or ER proteins, according to the Gene Ontology annotation most of which have previously been noted as MAM-resident or -associated proteins. Furthermore, about 20% of the identified proteins have a documented association with lipid rafts. Most importantly, known internal lipid raft marker proteins (inositol 1,4,5-trisphosphate receptor type 3), ERLIN2, and voltage-dependent anion channel 1 (VDAC1) were detected in these domains, as well as most of the components of the mitochondrial/MAM-localized Ca2+ signaling complex [71][70]. Moreover, recently, Manganelli and colleagues defined a specific inter-organelle localization of ERLIN1 at MAM level [72][71].
3. ERLINs: Localization and Function
ER lipid raft-associated protein 1 (ERLIN1) is a transmembrane glycoprotein that forms a heteroligomeric complex with its closely related ERLIN2 towards the ER lumen. ERLIN1 and ERLIN2 belong to the prohibitin family of proteins since they contain a PHB domain. For this reason, they share many similar characteristics, including localization in cellular membranes, detergent insolubility, association with detergent resistant membranes and a propensity to form homo- and hetero-oligomers [73,74,75,76,77,78,79][72][73][74][75][76][77][78]. Furthermore, ERLIN1 and ERLIN2 are also known members of the Stomatin-prohibitin-flotillin-HflC/K (SPFH) domain–containing protein family, which includes stomatins, prohibitins, and flotillins [80][79]. In particular, members of the SPFH domain-containing proteins are associated to membranes of different intracellular compartments, including mitochondria (prohibitin), trans-Golgi network (flotillins), endosomes and plasma membrane (flotillin and stomatin) [79,81][78][80]. The association of these proteins with lipid raft–like microdomains [79][78] has led to the speculation that these proteins could bind specific classes of lipids. Several studies show, for example, that stomatin binds cholesterol [82][81], stomatin-like protein 2 binds cardiolipin [83][82], and prohibitin links to phosphatidylinositol (3,4,5)-trisphosphate PIP3 [84][83]. In fact, ERLINs not only bind cholesterol [85][84], but also associate to phosphatidylinositol 3-phosphate PI3P. ERLINs would provide a protein scaffold for the formation of specialized raft-like microdomains in the ER membrane, where they create a lipid microenvironment distinct from the rest of the ER membrane. Flotillins have been proposed to form “scaffolding microdomains” at the plasma membrane, which could provide platforms to include certain plasma membrane receptor signaling pathways [86][85] and a novel type of endocytosis [87][86]. Similarly, the “ERLIN microdomains” in the ER membrane could facilitate certain ER-associated processes, for example by clustering the proteins involved.
Since ERLIN1/2 complex seems to be localized exclusively at the ER [79,85,88][78][84][87] and ERLIN1/2 complex binds specifically to PI3P, it appears likely that the ERLIN1/2 complex–PI3P interaction may play a role in the ER functions.
By far, a well-defined function of ERLINS is to mediate IP3Rs processing [89][88] and regulation of lipid metabolism [85,90][84][89]. ERLINs promote ER-associated protein degradation (ERAD) of the activated IP3 receptor [88,91][87][90] and of 3-hydroxy-3-methylglutaryl- CoA reductase (HMGR) [92][91]. ER-associated protein degradation of several protein substrates has been shown to require ERLIN2 [91,93][90][92].
It has been shown that ERLIN2 is the dominant partner in the ERLIN1/2 complex and contains the determinants for binding to activated IP3Rs and PI3P. Interestingly, interaction of ERLIN2 with PI3P may be regulated by the Thr-65 region since the T65I mutation inhibits PI3P binding. Thus, some determinants of PI3P and activated IP3R are enriched in the same region of ERLIN2. It suggests, that PI3P may be a cofactor able to link activated IP3Rs to the ERLIN1/2 complex contributing to the retro-translocation of ubiquitinated IP3Rs from ER membrane [89][88].
Furthermore, several works have identified ERLINs as novel ER regulators of sterol regulatory element-binding proteins (SREBPs) that are crucial for cholesterol homeostasis. Cellular cholesterol levels are regulated by endoplasmic reticulum (ER) sterol sensing proteins, which include SREBP cleavage-activating protein (Scap) and Insulin-induced gene 1 (Insig1). SREBPs are transcription factors that dimerize with Scap in the event of low cellular cholesterol level. Under conditions of cholesterol sufficiency, cholesterol-bound Scap associates with Insig, which promotes ER retention of the SREBP–Scap complex [94][93]. However, when ER cholesterol decreases below a critical value, Scap undergoes a conformational change that allows packaging of SREBP–Scap in COPII-coated vesicles for subsequent transport to the Golgi. In the Golgi, site-specific proteases release the cytosolic transcription factor domain of SREBPs, that activates genes for cholesterol and fatty acid biosynthesis [94][93]. When cholesterol levels are restored, the SREBP–Scap–Insig complex accumulates in the ER [95][94]. In this scenario, ERLIN1 and ERLIN2 may suppress cholesterol production by blocking the export of (SREBPs) from the ER to the Golgi under high cholesterol conditions [85][84]. Because ERLINs bind to cholesterol and physically interact with SREBP–Scap–Insig, they could directly promote ER retention of SREBP–Scap. Thus, ERLINs could promote stability of the SREBP–Scap–Insig complex and may contribute to the highly cooperative control of the SREBP machine [85][84]. Alternatively, cholesterol association with ERLINs might nucleate the formation of cholesterol-rich microdomains in the ER that increase the interaction of Insig with SREBP–Scap. A schematic drawing of ERLINs as novel regulators of SREBP machinery is shown in Figure 1.
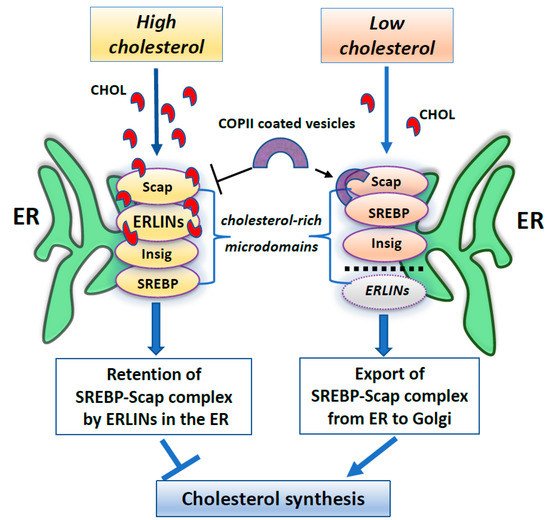

Figure 1. A summary scheme showing ERLINs as novel regulators of SREBP machinery. High levels of cholesterol promote the stability of the SREBP–Scap–Insig complex by ERLINs-cholesterol binding at ER cholesterol-rich microdomains. When cholesterol is depleted, COPII proteins coat clusters of SREBP-Scap complexes excluding ERLINs and facilitating their vesicular transport to the Golgi.
These data induce a reflection on the role that ERLIN could play as regards the structure and function of the lipid rafts, which may represent essential signaling platforms in the life-death balance of the cell [26,96][25][95].
Although ERLIN1 and ERLIN2 are well known as exclusively lipid raft-located proteins on ER membrane, little is known about their association with ER-MAM interface [97][96] and their involvement in autophagic initiation. In this concern, ERLIN contribution to the early phases of autophagosome formation was recently shown, suggesting that the interaction of ERLIN1 with autophagic proteins at lipid rafts is essential to promoting autophagy [72][71].
The article is from 10.3390/cells10092408
References
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50.
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell. Biol. 2017, 18, 361–374.
- Lozano, M.M.; Liu, Z.; Sunnick, E.; Janshoff, A.; Kumar, K.; Boxer, S.G. Colocalization of the ganglioside G(M1) and cholesterol detected by secondary ion mass spectrometry. J. Am. Chem. Soc. 2013, 135, 5620–5630.
- Sonnino, S.; Mauri, L.; Chigorno, V.; Prinetti, A. Gangliosides as components of lipid membrane domains. Glycobiology 2007, 17, 1R–13R.
- Sorice, M.; Garofalo, T.; Misasi, R.; Manganelli, V.; Vona, R.; Malorni, W. Ganglioside GD3 as a raft component in cell death regulation. Anticancer Agents Med. Chem. 2012, 12, 376–382.
- Perissinotto, F.; Rondelli, V.; Parisse, P.; Tormena, N.; Zunino, A.; Almásy, L.; Merkel, D.G.; Bottyán, L.; Sajti, S.; Casalis, L. GM1 Ganglioside role in the interaction of Alpha-synuclein with lipidmembranes: Morphology and structure. Biophys. Chem. 2019, 255, 106272.
- Kiessling, V.; Wan, C.; Tamm, L.K. Domain coupling in asymmetric lipid bilayers. Biochim. Biophys. Acta 2009, 1788, 64–71.
- Raghupathy, R.; Anilkumar, A.A.; Polley, A.; Singh, P.P.; Yadav, M.; Johnson, C.; Suryawanshi, S.; Saikam, V.; Sawant, S.D.; Panda, A.; et al. Transbilayer lipid interactions mediate nanoclustering of lipid-anchored proteins. Cell 2015, 161, 581–594.
- Barbat, C.; Trucy, M.; Sorice, M.; Garofalo, T.; Manganelli, V.; Fischer, A.; Mazerolles, F. p56lck, LFA-1 and PI3K but not SHP-2 interact with GM1- or GM3-enriched microdomains in a CD4-p56lck association-dependent manner. Biochem. J. 2007, 402, 471–481.
- Sonnino, S.; Prinetti, A. Membrane domains and the “lipid raft” concept. Curr. Med. Chem. 2013, 20, 4–21.
- Sonnino, S.; Prinetti, A. Lipids and membrane lateral organization. Front. Physiol. 2010, 1, 153.
- Levental, I.; Grzybek, M.; Simons, K. Raft domains of variable properties and compositions in plasma membrane vesicles. Proc. Natl. Acad. Sci. USA 2011, 108, 11411–11416.
- Lorent, J.H.; Diaz-Rohrer, B.; Lin, X.; Spring, K.; Gorfe, A.A.; Levental, K.R.; Levental, I. Structural determinants and functional consequences of protein affinity for membrane rafts. Nat. Commun. 2017, 8, 1219.
- Varma, R.; Mayor, S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature 1998, 394, 798–801.
- Komura, N.; Suzuki, K.G.; Ando, H.; Konishi, M.; Koikeda, M.; Imamura, A.; Chadda, R.; Fujiwara, T.K.; Tsuboi, H.; Sheng, R.; et al. Raft-based interactions of gangliosides with a GPI-anchored receptor. Nat. Chem. Biol. 2016, 12, 402–410.
- Lin, X.; Gorfe, A.A.; Levental, I. Protein partitioning into ordered membrane domains: Insights from simulations. Biophys. J. 2018, 114, 1936–1944.
- Jacobson, K.; Liu, P.; Lagerholm, B.C. The lateral organization and mobility of plasma membrane components. Cell 2019, 177, 806–819.
- Wang, H.Y.; Bharti, D.; Levental, I. Membrane heterogeneity beyond the plasma membrane. Front. Cell Dev. Biol. 2020, 8, 580814.
- Garofalo, T.; Giammarioli, A.M.; Misasi, R.; Tinari, A.; Manganelli, V.; Gambardella, L.; Pavan, A.; Malorni, W.; Sorice, M. Lipid microdomains contribute to apoptosis-associated modifications of mitochondria in T cells. Cell. Death Differ. 2005, 12, 1378–1389.
- Garofalo, T.; Manganelli, V.; Grasso, M.; Mattei, V.; Ferri, A.; Misasi, R.; Sorice, M. Role of mitochondrial raft-like microdomains in the regulation of cell apoptosis. Apoptosis 2015, 20, 621–634.
- Ciarlo, L.; Manganelli, V.; Matarrese, P.; Garofalo, T.; Tinari, A.; Gambardella, L.; Marconi, M.; Grasso, M.; Misasi, R.; Sorice, M.; et al. Raft-like microdomains play a key role in mitochondrial impairment in lymphoid cells from patients with Huntington’s disease. J. Lipid. Res. 2012, 53, 2057–2068.
- Cascianelli, G.; Villani, M.; Tosti, M.; Marini, F.; Bartoccini, E.; Magni, M.V.; Albi, E. Lipid microdomains in cell nucleus. Mol. Biol. Cell. 2008, 19, 5289–5295.
- Helms, J.B.; Zurzolo, C. Lipids as targeting signals: Lipid rafts and intracellular trafficking. Traffic 2004, 5, 247–254.
- Ouweneel, A.B.; Thomas, M.J.; Sorci-Thomas, M.G. The ins and outs of lipid rafts: Functions in intracellular cholesterol homeostasis, microparticles, and cell membranes: Thematic Review Series: Biology of Lipid Rafts. J. Lipid Res. 2020, 61, 676–686.
- Young, M.M.; Kester, M.; Wang, H.G. Sphingolipids: Regulators of crosstalk between apoptosis and autophagy. J. Lipid Res. 2013, 54, 5–19.
- Matarrese, P.; Garofalo, T.; Manganelli, V.; Gambardella, L.; Marconi, M.; Grasso, M.; Tinari, A.; Misasi, R.; Malorni, W.; Sorice, M. Evidence for the involvement of GD3 ganglioside in autophagosome formation and maturation. Autophagy 2014, 10, 50–65.
- Garcia-Ruiz, C.; Morales, A.; Fernández-Checa, J.C. Glycosphingolipids and cell death: One aim, many ways. Apoptosis 2015, 20, 607–620.
- Garofalo, T.; Ferri, A.; Sorice, M.; Azmoon, P.; Grasso, M.; Mattei, V.; Capozzi, A.; Manganelli, V.; Misasi, R. Neuroglobin overexpression plays a pivotal role in neuroprotection through mitochondrial raft-like microdomains in neuroblastoma SK-N-BE2 cells. Mol. Cell. Neurosci. 2018, 88, 167–176.
- Santos, A.L.; Preta, G. Lipids in the cell: Organisation regulates function. Cell. Mol. Life Sci. 2018, 75, 1909–1927.
- Fujimoto, M.; Hayashi, T.; Su, T.P. The role of cholesterol in the association of endoplasmic reticulum membranes with mitochondria. Biochem. Biophys. Res. Commun. 2012, 417, 635–639.
- Sorice, M.; Manganelli, V.; Matarrese, P.; Tinari, A.; Misasi, R.; Malorni, W.; Garofalo, T. Cardiolipin-enriched raft-like microdomains are essential activating platforms for apoptotic signals on mitochondria. FEBS Lett. 2009, 583, 2447–2450.
- Vance, J.E. MAM (mitochondria-associated membranes) in mammalian cells: Lipids and beyond. Biochim. Biophys. Acta. 2014, 1841, 595–609.
- Annunziata, I.; Sano, R.; d’Azzo, A. Mitochondria-associated ER membranes (MAMs) and lysosomal storage diseases. Cell Death Dis. 2018, 9, 328.
- Mattei, V.; Matarrese, P.; Garofalo, T.; Tinari, A.; Gambardella, L.; Ciarlo, L.; Manganelli, V.; Tasciotti, V.; Misasi, R.; Malorni, W.; et al. Recruitment of cellular prion protein to mitochondrial raft-like microdomains contributes to apoptosis execution. Mol. Biol. Cell. 2011, 22, 4842–4853.
- Maxfield, F.R.; van Meer, G. Cholesterol, the central lipid of mammalian cells. Curr. Opin. Cell Biol. 2010, 22, 422–429.
- García-Ruiz, C.; Colell, A.; Morales, A.; Calvo, M.; Enrich, C.; Fernández-Checa, J.C. Trafficking of ganglioside GD3 to mitochondria by tumor necrosis factor-alpha. J. Biol. Chem. 2002, 277, 36443–36448.
- Sorice, M.; Matarrese, P.; Manganelli, V.; Tinari, A.; Giammarioli, A.M.; Mattei, V.; Misasi, R.; Garofalo, T.; Malorni, W. Role of GD3-CLIPR-59 association in lymphoblastoid T cell apoptosis triggered by CD95/Fas. PLoS ONE 2010, 5, e8567.
- Ciarlo, L.; Manganelli, V.; Garofalo, T.; Matarrese, P.; Tinari, A.; Misasi, R.; Malorni, W.; Sorice, M. Association of fission proteins with mitochondrial raft-like domains. Cell Death Differ. 2010, 17, 1047–1058.
- Matarrese, P.; Manganelli, V.; Garofalo, T.; Tinari, A.; Gambardella, L.; Ndebele, K.; Khosravi-Far, R.; Sorice, M.; Esposti, M.D.; Malorni, W. Endosomal compartment contributes to the propagation of CD95/Fas-mediated signals in type II cells. Biochem. J. 2008, 413, 467–478.
- Ciarlo, L.; Vona, R.; Manganelli, V.; Gambardella, L.; Raggi, C.; Marconi, M.; Malorni, W.; Sorice, M.; Garofalo, T.; Matarrese, P. Recruitment of mitofusin 2 into "lipid rafts" drives mitochondria fusion induced by Mdivi-1. Oncotarget 2018, 9, 18869–18884.
- Scorrano, L.; Petronilli, V.; Di Lisa, F.; Bernardi, P. Commitment to apoptosis by GD3 ganglioside depends on opening of the mitochondrial permeability transition pore. J. Biol. Chem. 1999, 274, 22581–22585.
- Sorice, M.; Matarrese, P.; Tinari, A.; Giammarioli, A.M.; Garofalo, T.; Manganelli, V.; Ciarlo, L.; Gambardella, L.; Maccari, G.; Botta, M.; et al. Raft component GD3 associates with tubulin following CD95/Fas ligation. FASEB J. 2009, 23, 3298–3308.
- Malorni, W.; Giammarioli, A.M.; Garofalo, T.; Sorice, M. Dynamics of lipid raft components during lymphocyte apoptosis: The paradigmatic role of GD3. Apoptosis 2007, 12, 941–949.
- Scorrano, L. Caspase-8 goes cardiolipin: A new platform to provide mitochondria with microdomains of apoptotic signals? J. Cell Biol. 2008, 183, 579–581.
- Manganelli, V.; Capozzi, A.; Recalchi, S.; Riitano, G.; Mattei, V.; Longo, A.; Misasi, R.; Garofalo, T.; Sorice, M. The role of cardiolipin as a scaffold mitochondrial phospholipid in autophagosome formation: In vitro evidence. Biomolecules 2021, 11, 222.
- Sun, J.; Xu, H.; Negi, S.; Subramony, S.H.; Hebert, M.D. Differential effects of polyglutamine proteins on nuclear organization and artificial reporter splicing. J. Neurosci. Res. 2007, 85, 2306–2317.
- Manfiolli, A.O.; Maragno, A.L.; Baqui, M.M.; Yokoo, S.; Teixeira, F.R.; Oliveira, E.B.; Gomes, M.D. FBXO25-associated nuclear domains: A novel subnuclear structure. Mol. Biol. Cell. 2008, 19, 1848–1861.
- Albi, E.; Cataldi, S.; Rossi, G.; Magni, M.V. A possible role of cholesterol-sphingomyelin/phosphatidylcholine in nuclear matrix during rat liver regeneration. J. Hepatol. 2003, 38, 623–628.
- Scassellati, C.; Albi, E.; Cmarko, D.; Tiberi, C.; Cmarkova, J.; Bouchet-Marquis, C.; Verschure, P.J.; van Driel, R.; Magni, M.V.; Fakan, S. Intranuclear sphingomyelin is associated with transcriptionally active chromatin and plays a role in nuclear integrity. Biol. Cell. 2010, 102, 361–375.
- Abe, M.; Kobayashi, T. Dynamics of sphingomyelin- and cholesterol-enriched lipid domains during cytokinesis. Methods Cell Biol. 2017, 137, 15–24.
- Ouled-Haddou, H.; Messaoudi, K.; Demont, Y.; Lopes Dos Santos, R.; Carola, C.; Caulier, A.; Vong, P.; Jankovsky, N.; Lebon, D.; Willaume, A.; et al. A new role of glutathione peroxidase 4 during human erythroblast enucleation. Blood Adv. 2020, 4, 5666–5680.
- Galbiati, F.; Razani, B.; Lisanti, M.P. Emerging themes in lipid rafts and caveolae. Cell 2001, 106, 403–411.
- Wang, Y.; Liu, C.; Hu, L. Cholesterol regulates cell proliferation and apoptosis of colorectal cancer by modulating miR-33a-PIM3 pathway. Biochem. Biophys. Res. Commun. 2019, 511, 685–692.
- Chimento, A.; Casaburi, I.; Avena, P.; Trotta, F.; De Luca, A.; Rago, V.; Pezzi, V.; Sirianni, R. Cholesterol and its metabolites in tumor growth: Therapeutic potential of statins in cancer treatment. Front. Endocrinol. 2019, 9, 807.
- Silvente-Poirot, S.; Poirot, M. Cancer. Cholesterol and cancer, in the balance. Science 2014, 343, 1445–1446.
- Goossens, P.; Rodriguez-Vita, J.; Etzerodt, A.; Masse, M.; Rastoin, O.; Gouirand, V.; Ulas, T.; Papantonopoulou, O.; Van Eck, M.; Auphan-Anezin, N.; et al. Membrane cholesterol efflux drives tumor-associated macrophage reprogramming and tumor progression. Cell Metab. 2019, 29, 1376–1389.e4.
- Lange, Y.; Ye, J.; Rigney, M.; Steck, T.L. Regulation of endoplasmic reticulum cholesterol by plasma membrane cholesterol. J. Lipid Res. 1999, 40, 2264–2270.
- Von Blume, J.; Hausser, A. Lipid-dependent coupling of secretory cargo sorting and trafficking at the trans-Golgi network. FEBS Lett. 2019, 593, 2412–2427.
- Csordás, G.; Renken, C.; Várnai, P.; Walter, L.; Weaver, D.; Buttle, K.F.; Balla, T.; Mannella, C.A.; Hajnóczky, G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell. Biol. 2006, 174, 915–921.
- Vance, J.E. Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 1990, 265, 7248–7256.
- Stiban, J.; Caputo, L.; Colombini, M. Ceramide synthesis in the endoplasmic reticulum can permeabilize mitochondria to proapoptotic proteins. J. Lipid Res. 2008, 49, 625–634.
- Lynes, E.M.; Bui, M.; Yap, M.C.; Benson, M.D.; Schneider, B.; Ellgaard, L.; Berthiaume, L.G.; Simmen, T. Palmitoylated TMX and calnexin target to the mitochondria-associated membrane. EMBO J. 2012, 31, 457–470.
- Raturi, A.; Simmen, T. Where the endoplasmic reticulum and the mitochondrion tie the knot: The mitochondria-associated membrane (MAM). Biochim. Biophys. Acta. 2013, 1833, 213–224.
- Garofalo, T.; Matarrese, P.; Manganelli, V.; Marconi, M.; Tinari, A.; Gambardella, L.; Faggioni, A.; Misasi, R.; Sorice, M.; Malorni, W. Evidence for the involvement of lipid rafts localized at the ER-mitochondria associated membranes in autophagosome formation. Autophagy 2016, 12, 917–935.
- Sano, R.; Annunziata, I.; Patterson, A.; Moshiach, S.; Gomero, E.; Opferman, J.; Forte, M.; d’Azzo, A. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol. Cell. 2009, 36, 500–511.
- Lan, B.; He, Y.; Sun, H.; Zheng, X.; Gao, Y.; Li, N. The roles of mitochondria-associated membranes in mitochondrial quality control under endoplasmic reticulum stress. Life Sci. 2019, 231, 116587.
- Pitts, K.R.; Yoon, Y.; Krueger, E.W.; McNiven, M.A. The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol. Biol. Cell. 1999, 10, 4403–4417.
- Myhill, N.; Lynes, E.M.; Nanji, J.A.; Blagoveshchenskaya, A.D.; Fei, H.; Carmine Simmen, K.; Cooper, T.J.; Thomas, G.; Simmen, T. The subcellular distribution of calnexin is mediated by PACS-2. Mol. Biol. Cell. 2008, 19, 2777–2788.
- Simmen, T.; Aslan, J.E.; Blagoveshchenskaya, A.D.; Thomas, L.; Wan, L.; Xiang, Y.; Feliciangeli, S.F.; Hung, C.H.; Crump, C.M.; Thomas, G. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J. 2005, 24, 717–729.
- Poston, C.N.; Duong, E.; Cao, Y.; Bazemore-Walker, C.R. Proteomic analysis of lipid raft-enriched membranes isolated from internal organelles. Biochem. Biophys. Res. Commun. 2011, 415, 355–360.
- Manganelli, V.; Matarrese, P.; Antonioli, M.; Gambardella, L.; Vescovo, T.; Gretzmeier, C.; Longo, A.; Capozzi, A.; Recalchi, S.; Riitano, G.; et al. Raft-like lipid microdomains drive autophagy initiation via AMBRA1-ERLIN1 molecular association within MAMs. Autophagy 2020, 1–21.
- Mairhofer, M.; Steiner, M.; Mosgoeller, W.; Prohaska, R.; Salzer, U. Stomatin is a major lipid-raft component of platelet alpha granules. Blood 2002, 100, 897–904.
- Neumann-Giesen, C.; Falkenbach, B.; Beicht, P.; Claasen, S.; Luers, G.; Stuermer, C.A.; Herzog, V.; Tikkanen, R. Membrane and raft association of reggie- 1/flotillin-2: Role of myristoylation, palmitoylation and oligomerization and induction of filopodia by overexpression. Biochem. J. 2004, 378, 509–518.
- Nijtmans, L.G.; Artal, S.M.; Grivell, L.A.; Coates, P.J. The mitochondrial PHB complex: Roles in mitochondrial respiratory complex assembly, ageing and degenerative disease. Cell Mol. Life Sci. 2002, 59, 143–155.
- Salzer, U.; Prohaska, R. Stomatin, flotillin-1, and flotillin-2 are major integral proteins of erythrocyte lipid rafts. Blood 2001, 97, 1141–1143.
- Snyers, L.; Umlauf, E.; Prohaska, R. Oligomeric nature of the integral membrane protein stomatin. J. Biol. Chem. 1998, 273, 17221–17226.
- Browman, D.T.; Resek, M.E.; Zajchowski, L.D.; Robbins, S.M. Erlin-1 and erlin-2 are novel members of the prohibitin family of proteins that define lipid-raft-like domains of the ER. J. Cell. Sci. 2006, 119, 3149–3160.
- Browman, D.T.; Hoegg, M.B.; Robbins, S.M. The SPFHdomain-containing proteins: More than lipid raft markers. Trends Cell. Biol. 2007, 17, 394–402.
- Hoegg, M.B.; Duncan, T.B.; Resek, M.E.; Stephen, M.; Robbins, S.M. Distinct regions within the erlins are required for oligomerization and association with high molecular weight complexes. J. Biol. Chem. 2009, 284, 7766–7776.
- Morrow, I.C.; Parton, R.G. Flotillins and the PHB domain protein family: Rafts, worms and anaesthetics. Traffic 2005, 6, 725–740.
- Rungaldier, S.; Umlauf, E.; Mairhofer, M.; Salzer, U.; Thiele, C.; Prohaska, R. Structure-function analysis of human stomatin: A mutation study. PLoS ONE 2017, 12, e0178646.
- Christie, D.A.; Lemke, C.D.; Elias, I.M.; Chau, L.A.; Kirchhof, M.G.; Li, B.; Ball, E.H.; Dunn, S.D.; Hatch, G.M.; Madrenas, J. Stomatin-like protein 2 binds cardiolipin and regulates mitochondrial biogenesisand function. Mol. Cell. Biol. 2011, 31, 3845–3856.
- Ande, S.R.; Mishra, S. Prohibitin interacts with phosphatidy-linositol 3,4,5-trisphosphate (PIP3) and modulates insulin signaling. Biochem. Biophys. Res. Commun. 2009, 390, 1023–1028.
- Huber, M.D.; Vesely, P.W.; Datta, K.; Gerace, L. Erlins restrictSREBP activation in the ER and regulate cholesterol homeostasis. J. Cell Biol. 2013, 203, 427–436.
- Langhorst, M.F.; Reuter, A.; Stuermer, C.A. Scaffolding microdomains and beyond: The function of reggie/flotillin proteins. Cell. Mol. Life Sci. 2005, 62, 2228–2240.
- Glebov, O.O.; Bright, N.A.; Nichols, B.J. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat. Cell Biol. 2006, 8, 46–54.
- Pearce, M.M.; Wormer, D.B.; Wilkens, S.; Wojcikiewicz, R.J. An endoplasmic reticulum (ER) membrane complex composed of SPFH1 and SPFH2 mediates the ER-associated degradation of inositol 1,4,5-tris- phosphate receptors. J. Biol. Chem. 2009, 284, 10433–10445.
- Wright, F.A.; Caden, G.; Bonzerato, C.G.; Danielle, A.; Sliter, D.A.; Richard, J.H.; Wojcikiewicz, R.J.H. The erlin2 T65I mutation inhibits erlin1/2 complex–mediatedinositol 1,4,5-trisphosphate receptor ubiquitination and phosphatidylinositol 3-phosphate binding. J. Biol. Chem. 2018, 293, 15706–15714.
- Wang, G.; Zhang, X.; Lee, J.S.; Wang, X.; Yang, Z.Q.; Zhang, K. Endoplasmic reticulum factor erlin2 regulates cytosolic lipid con-tent in cancer cells. Biochem. J. 2012, 446, 415–425.
- Pearce, M.M.; Wang, Y.; Kelley, G.G.; Wojcikiewicz, R.J. SPFH2 mediates the endoplasmic reticulum-associated degradation of inositol 1,4,5-trisphosphate receptors and other substrates in mammalian cells. J. Biol. Chem. 2007, 282, 20104–20115.
- Jo, Y.; Sguigna, P.V.; DeBose-Boyd, R.A. Membrane-associated ubiquitin ligase complex containing gp78 mediates sterol-accelerated degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J. Biol. Chem. 2011, 286, 15022–15031.
- Stevenson, J.; Huang, E.Y.; Olzmann, J.A. Endoplasmic reticulum-associated degradation and lipid homeostasis. Annu. Rev. Nutr. 2016, 36, 511–542.
- Radhakrishnan, A.; Goldstein, J.L.; McDonald, J.G.; Brown, M.S. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: A delicate balance. Cell Metab. 2008, 8, 512–521.
- Howe, V.; Sharpe, L.J.; Alexopoulos, S.J.; Kunze, S.V.; Chua, N.K.; Li, D.; Brown, A.J. Cholesterol homeostasis: How do cells sense sterol excess? Chem. Phys. Lipids 2016, 199, 170–178.
- Iessi, E.; Marconi, M.; Manganelli, V.; Sorice, M.; Malorni, W.; Garofalo, T.; Matarrese, P. On the role of sphingolipids in cell survival and death. Int. Rev. Cell. Mol. Biol. 2020, 351, 149–195.
- Guardia-Laguarta, C.; Area-Gomez, E.; Rüb, C.; Liu, Y.; Magrané, J.; Becker, D.; Voos, W.; Schon, E.A.; Przedborski, S. α-Synuclein is localized to mitochondria-associated ER membranes. J. Neurosci. 2014, 34, 249–259.
More
