Lymphatic vessels play a distinctive role in draining fluid, molecules and even cells from interstitial and serosal spaces back to the blood circulation. Lymph vessels of the gut, and especially those located in the villi (called lacteals), not only serve this primary function, but are also responsible for the transport of lipid moieties absorbed by the intestinal mucosa and serve as a second line of defence against possible bacterial infections.
- lymphatic vessel
- lacteal
- microbiota
1. Foreword
The lymphatic system is a fascinating and still partially undiscovered fluid transport system that lies in parallel with the blood circulation and complements it by returning the liquid filtered from the blood capillaries towards the interstitial spaces back to the blood stream. Its role is fundamental in maintaining a functional fluid volume and composition in various areas of the body, preventing organ failure. In this review, we will briefly discuss the general mechanisms of lymph drainage and propulsion, and then focus on the most recent findings that pertain to the exquisite, peculiar environment of the initial lymphatic vessels of the gut, the lacteals. They have recently been the site of extensive research because of the pivotal role that the close association between lacteals and microbiota exerts on the whole-body homeostasis.
2. General Overview of the Lymphatic System
The lymphatic network is widely distributed throughout the body, arising as lymphatic capillaries, thin-walled vessels devoid of lymphatic muscle (LM), connected to the extracellular matrix by anchoring filaments, forming primary valves[1]. Lymphatic capillaries then drain into progressively larger and converging collecting lymphatics, which are equipped with a LM layer owning unique features (as it displays skeletal, cardiac, and smooth muscle contractile elements[2]), and possess intraluminal valves[3], separating adjacent vessel segments named “lymphangions”, the functional contracting pump units of the lymphatic system. The proper function of the lymphatic system is critically related to the development of pressure gradients between the vessel’s segments and/or surrounding tissue. According to Starling’s Law[4], lymph formation depends upon the transmural pressure gradient (ΔPTM) between intraluminal (PLymph) and interstitial (Pint) hydraulic pressures (ΔPTM = PLymph − Pint). Lymph propulsion is due to the intraluminal hydraulic pressure gradient (ΔPLymph) across adjacent lymphangions (ΔPLymph = PL,1 − PL,2), acting against an overall opposite pressure gradient[5]. In most tissues’ lymphatic capillaries, PLymph is almost slightly subatmospheric[4], whereas in the venous system, the intraluminal pressure is ~10 cmH2O. However, exceeding the transvalve ΔPLymph (1–1.5 cmH2O) is enough to guarantee the proper lymph propulsion to the downstream lymphangion, against an adverse hydraulic pressure gradient and the force of gravity[5].
ΔPTM and ΔPLymph are deeply affected by different mechanisms, either involving the spontaneous contraction of the vessel itself (“intrinsic” mechanism) or mechanical stresses originating in the surrounding tissues (“extrinsic” mechanisms). The intrinsic mechanism is predominant in vessels located in soft tissues and body areas experiencing no significant tissue displacement, such as mesenteric lymphatics. It relies on spontaneous contractions of the vessel triggered by pacemaker cells in the LM layer[6][7] and then transmitted to electrically coupled LM cells in the vessel’s wall[8]. Different pacemaking mechanisms have been proposed, such as Spontaneous Transient Depolarisations (STDs)[9][10] induced by calcium-dependent chloride currents or If-like currents, due to hyperpolarisation-activated cyclic nucleotide (HCN) channels, similarly to what occurs in the heart sinoatrial node[11][12]. Hence, in analogy to the cardiac cycle, LM intrinsic activity generates phasic contractions, displaying an active systolic phase, which forces lymph propulsion to the adjacent vessel segment, and a passive diastolic phase, due to LM relaxation, which favours lymphangion fluid refilling. The whole mechanism can be described in terms of contraction frequency and ejection fraction or stroke volume[13][14].
Lymph flow dynamics and the surrounding microenvironment can deeply affect lymphatic spontaneous contractions. Changes in transmural and/or intraluminal pressures, lymph flow-induced wall shear stress, nitric oxide, histamine, fluid osmolarity, local tissue temperature and neuronal modulation by the autonomous nervous system can significantly alter contraction frequency (i.e., chronotropic effect) and/or contraction amplitude (i.e., inotropic effect), continuously modulating and adapting lymph drainage and transport to current needs[15][16][17][18][19][20][21][22][23][24][25][26]. Impaired intrinsic contractility, as well as lymphatic vessels obstruction, may lead to oedema development as a result of tissue fluid imbalance[4]. The extrinsic mechanism, on the other hand, is related to mechanical stresses arising in surrounding tissues then transmitted to the lymphatic vessels by means of fibrous elements of the extracellular matrix[1]. It typically involves vessels located in areas of the body which experience cyclical movements such as the heart or skeletal muscle, lymphatics undergoing cardiogenic activity or respiratory movements, intestinal motility, external compression and arteriolar vasomotion[17][27][28][29][30][31][32]. These mechanisms rhythmically exert external forces compressing and expanding lymphatic vessels, thus dramatically affecting primary and intraluminal valves dynamics and both ΔPTM and ΔPLymph .
Intrinsic and extrinsic mechanisms may coexist according to area on the body: their relevance depends upon the sources of extrinsic forces ranging from blood vessels’ vasomotion caused by the pulsatile blood flow, to skeletal muscle fibres’ contraction. Indeed, in the rat diaphragmatic lymphatic network, both intrinsic and extrinsic mechanisms cooperate as the contraction of the skeletal muscle fibres is adequate to sustain lymph flow in vessels of the tendinous and medial muscle regions, but it is not sufficient in the muscular periphery adjacent to the chest wall, where intrinsic contractions are required to prevent fluid accumulation[33][34][35]. However, if extrinsically related mechanisms are sufficient to generate lymph flow-supportive pressure gradients for proper lymph propulsion, when flow rates are elevated, lymphatic vessels generally display their own flow-induced inhibition of the spontaneous contractions, and lymphatics behave like conduits[36].
3. The Lymphatic System of the Intestine and Mesentery
The organisation of the lymphatic network greatly varies among different body areas. In the intestine, a three-level distribution of lymphatic vessels can be identified: (a) in the small intestinal villi, (b) in the submucosa and (c) in the smooth muscle layer surrounding the mucosa[37]. The blind-ended lymphatic capillaries, also known as intestinal lacteals (Figure 1), are exclusively located in the centre of villi, normally reaching 60–70% of the villus length[38], which is, however, variable among different intestine tracts. Indeed, villi length decreases from the duodenum to the jejunum and distal ileum. As a result, according to the absorptive properties of the intestinal epithelium, lacteals are longest in the duodenum, where most nutrient uptake occurs. Lacteals merge at the villi basis, forming the submucosal network. Intestinal villi contain a blood vascular capillary network and 1–10 central lacteals, providing a route for absorbed nutrient distribution[39][40]. Water-soluble molecules enter blood vessels and are transported to the portal vein; conversely, lipids and other lipophilic molecules of large size such as chylomicrons enter lymphatic vessels, which then reach the blood circulatory system without passing through the liver. Such a privileged delivery route can also be used to enhance the bioavailability of oral lipophilic drugs, thus improving the efficacy of therapeutical strategies[41][42].
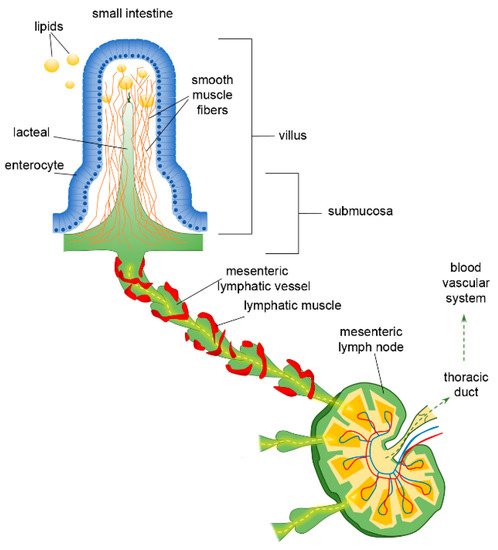
Figure 1. Functional organisation of lymphatic capillaries (lacteals), submucosal and mesenteric collecting vessels in the intestine. Dietary lipids are absorbed at the epithelial surface of the intestine, entering lacteals by paracellular and/or transcellular mechanisms. Lacteals are located at the centre of the intestinal villus, surrounded by villus smooth muscle fibres. They merge at the villus basis forming the submucosal network and then lymph is propelled along mesenteric collecting vessels endowed with a lymphatic muscle mesh. Lymph passes through mesenteric lymph nodes, ultimately reaching the venous circulatory system via the thoracic duct.
As other lymphatic capillaries, lacteals are non-contracting vessels, having no LM elements in their vessels’ walls nor intraluminal valves. Therefore, lymph drainage by intestinal lymphatics is deeply affected by extrinsic forces related to vasomotion and intestinal motility[43][44]. Indeed, the pulsatile activity of neighbouring arteries as well as villous motility may easily mechanically deform lymphatics. Lacteals are surrounded by villus smooth muscle fibres, organised in a tree-like structure (Figure 1), whose contractile activity exerts extrinsic forces contributing to enhance intestinal lymph and blood flow, propelling lymph at velocities up to 150 µm/s[45], with a positive effect on lipid absorption[44]. Lacteals’ periodic squeezing due to the contraction of those longitudinally oriented smooth muscle fibres is critically modulated by neurohormonal factors released by the autonomic nervous system. Thus, in the intestinal lymphatic network, neuromodulation may exert a mixed modulatory role by acting on both intrinsic and extrinsic mechanisms of drainage and propulsion[45]. Moreover, the contraction of smooth muscle layers in the intestinal wall gives rise to a compressive stress on lacteals and gut lymphatics, favouring vessel squeezing and lymph propulsion. On the contrary, when smooth muscle relaxes, lymphatics are stretched and a net ΔPTM and/or ΔPLymph favouring fluid entry is provided. Thus, intestinal lymph drainage and propulsion are pulsatile. Lymphatic vessels in the smooth muscle layers are anatomically segregated from submucosal ones; however, both networks merge into larger collectors next to the mesentery, where almost all the lymph is of intestinal origin[46]. Here, the collecting vessels are equipped with intraluminal valves and a proper LM mesh (Figure 1) so that intrinsic spontaneous contractions can be identified along the lymphangion chain, allowing lymph propulsion. In rat mesenteric lymphatics, spontaneous contractions arise in the smaller vessels and then propagate to the larger collecting lymphatics, generating progressively higher pressure oscillations from distal (2–4 cmH2O) to proximal vessels (up to 10–20 cmH2O)[5]. Those lymphatics display an intrinsic contraction frequency of about of 6.4 ± 0.6 cycles/min and an ejection fraction of about 67% of their resting diastolic volume[47]. Lymph propelled along the mesenteric lymphangions chain passes through mesenteric lymph nodes, then drains into the thoracic duct and, eventually, empties into the blood circulatory system at the level of the subclavian vein (Figure 1).
4. Maturation and Stability of Lacteals
To date, few mechanisms have been elucidated regarding the development and maintenance of a fully functional lacteal network in the adult subject, and, surprisingly, they all require the presence of a normal gut microbiota. Lymph drainage from the interstitial space of the villi represents a balanced mechanism of different needs: while a lymph drainage increase can improve the immune surveillance keeping pathogens under control[53], on the other hand, a lymph drainage reduction can prevent damages caused by the spread of pathogens and/or pro-inflammatory factors coming from nutrients hydrolysis closely in contact with a deteriorated intestinal epithelium[54][55][56].
Lacteals sprout into the villi around postnatal day 7 and continue to develop and remodel after weaning at P21 (in mice), into adult life[57]. The first evidence of the need of gut microbiota for proper lacteals development came from the findings that germ-free (GF) mice, which entirely lack an endogenous microbiota, have decreased lacteal length and a significantly lower number of lymphatic endothelial cells (Prox1+) in their villi and reduced VEGFR3 (Vascular endothelial growth factor receptor 3) expression, when compared to same-age mice grown in a controlled, specific pathogen-free condition[58]. Disruption of intestinal lymphatics, in adult mice, leads to immune homeostasis failure and results in rapid lethality, due to the lack of immune surveillance that lacteals and mesenteric lymph nodes are expected to deploy[38][59]. Interestingly, lymphatic regression only affects lacteals, since this phenomenon was not observed in other organs and tissues where lymphatic networks are present, such as diaphragm, skin and trachea.
From a purely phenomenological view, the lacteals wall is not able to selectively avoid the drainage of pathogens, endotoxins and/or pro-inflammatory molecules present in the villi interstitial space. This is due to button-like junctions between adjacent lymphatic endothelial cells, which allow their free, overlapping ends to open and behave similar to unidirectional primary valves[60], favouring interstitial liquid (and all the dissolved and suspended particles) progression into the vessel lumen. Therefore, the prevention of lacteal-draining toxic gut-derived lymph to the rest of the body depends on the maintenance of mucus and epithelial cells’ integrity[61]. In healthy individuals, the gut microbiota produces short-chain fatty acids, which stimulate the epithelial cells to produce mucus and antimicrobial peptides, thus increasing the mucosal immune response. Mucus creates a favourable environment, which harbours commensal microbiota, protecting the intestine against colonisation by pathogenic agents[62], and a very hostile environment for pathogens, which are mostly excluded from reaching the epithelial layer[63][64]. Despite the healthy intestine being lined by a monolayer of epithelial cells, it represents a proper selective barrier, thus controlling the movement of different substances and macromolecules. This is due to tight junctions (TJs) and junctional adherens molecules (JAMs) between neighbouring cells, forming a strong seal which regulates the paracellular pathway and prevents the uncontrolled systemic spread of potentially toxic agents[65][66]. In critical illness, TJs homeostasis can be impaired by proinflammatory cytokines, pathogens and lipopolysaccharides, damaging the integrity of the intestinal epithelium. The increase in barrier permeability with the loss of functionality affects not only fat absorption, but also leads to dysbiosis and to an inflammatory-related alteration of immunosurveillance.
5. Closing Remarks
Lymph formation and propulsion are crucial to attain the correct fluid homeostasis of interstitial tissue and serosal cavities. In the peculiar gut microenvironment, this primary requirement is intertwined with the need of lipid transport associated with the absorption of dietary lipids, and the compartmentalised immunosurveillance exerted by dendritic cells (DCs) recirculating between the villi interstitial space and mesenteric lymph nodes. All these factors are mutually coordinated and any small imbalance, in the short or medium time frame, can cause severe illness due to oedema, reduced dietary lipids transport to the blood or even lack of immune surveillance. Most of the research in recent years has been focused on the primary site of potential translocation of bacteria, bacterial-derived or even tissue-derived toxins to the lymph, trying to unveil possible sites of intervention at the first step of this potentially life-threatening process.
Is the influence of the microbiota’s density and composition on lacteals development and stability a one-way relationship or is there a mutual exchange and effect by lacteals as well? While the DC-mediated transport of invading intestinal bacteria is well acknowledged, very few research studies are related to the possible alteration of the microbiota in response to a primitive impairment of lymphatic function. Among others, in chronic colitis mice, the supplementation of VEGFC causes an increase in lymph drainage from the small intestine, and this, in turn, alters the composition of the intestinal microbiota, causing a net reduction in its amount but not in its diversity. Overall, an increased Bacteroidetes/Firmicutes ratio caused by increased lymphatic drainage closed the gap towards a healthy microbiota profile, thus reducing colitis[67]. Despite the very small amount of data collected so far, it is envisaged that a more efficient lymphatic drainage might exert a positive effect on the composition of gut microbiota, potentially through a better immunological control on the phyla.
References
- L V Leak; J F Burke; Ultrastructural studies on the lymphatic anchoring filaments.. Journal of Cell Biology 1968, 36, 129-49.
- Mariappan Muthuchamy; Anatoliy Gashev; Niven Boswell; Nancy Dawson; David Zawieja; Molecular and functional analyses of the contractile apparatus in lymphatic muscle. The FASEB Journal 2003, 17, 1-25, 10.1096/fj.02-0626fje.
- Eleni Bazigou; Taija Makinen; Flow control in our vessels: vascular valves make sure there is no way back. Experientia 2012, 70, 1055-1066, 10.1007/s00018-012-1110-6.
- K. Aukland; Rolf K. Reed; Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiological Reviews 1993, 73, 1-78, 10.1152/physrev.1993.73.1.1.
- B W Zweifach; J W Prather; Micromanipulation of pressure in terminal lymphatics in the mesentery. American Journal of Physiology-Legacy Content 1975, 228, 1326-1335, 10.1152/ajplegacy.1975.228.5.1326.
- Eric A. Bridenbaugh; Anatoliy A. Gashev; David C. Zawieja; Lymphatic Muscle: A Review of Contractile Function. Lymphatic Research and Biology 2003, 1, 147-158, 10.1089/153968503321642633.
- Pierre-Yves von der Weid; David Zawieja; Lymphatic smooth muscle: the motor unit of lymph drainage. The International Journal of Biochemistry & Cell Biology 2004, 36, 1147-1153, 10.1016/j.biocel.2003.12.008.
- David Zawieja; K. L. Davis; R. Schuster; W. M. Hinds; H. J. Granger; Distribution, propagation, and coordination of contractile activity in lymphatics. American Journal of Physiology-Heart and Circulatory Physiology 1993, 264, H1283-H1291, 10.1152/ajpheart.1993.264.4.h1283.
- D F Van Helden; Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery.. The Journal of Physiology 1993, 471, 465-479, 10.1113/jphysiol.1993.sp019910.
- Pierre-Yves von der Weid; Mozibur Rahman; Mohammad S. Imtiaz; Dirk F. van Helden; Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: pharmacology and implication for spontaneous contractility. American Journal of Physiology-Heart and Circulatory Physiology 2008, 295, H1989-H2000, 10.1152/ajpheart.00007.2008.
- K. D. McCloskey; H. M. Toland; Mark Hollywood; Keith Thornbury; N. G. McHale; Hyperpolarisation‐activated inward current in isolated sheep mesenteric lymphatic smooth muscle. The Journal of Physiology 1999, 521, 201-211, 10.1111/j.1469-7793.1999.00201.x.
- Daniela Negrini; Cristiana Marcozzi; Eleonora Solari; Elena Bossi; Raffaella Cinquetti; Marcella Reguzzoni; Andrea Moriondo; Hyperpolarization-activated cyclic nucleotide-gated channels in peripheral diaphragmatic lymphatics. American Journal of Physiology-Heart and Circulatory Physiology 2016, 311, H892-H903, 10.1152/ajpheart.00193.2016.
- Joshua P. Scallan; Scott D. Zawieja; Jorge Castorena-Gonzalez; Michael J. Davis; Lymphatic pumping: mechanics, mechanisms and malfunction. The Journal of Physiology 2016, 594, 5749-5768, 10.1113/jp272088.
- David C. Zawieja; Contractile Physiology of Lymphatics. Lymphatic Research and Biology 2009, 7, 87-96, 10.1089/lrb.2009.0007.
- Michael J. Davis; Joshua P. Scallan; John H. Wolpers; Mariappan Muthuchamy; Anatoliy A. Gashev; David C. Zawieja; Intrinsic increase in lymphangion muscle contractility in response to elevated afterload.. American Journal of Physiology-Heart and Circulatory Physiology 2012, 303, H795-808, 10.1152/ajpheart.01097.2011.
- N G McHale; I C Roddie; The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels.. The Journal of Physiology 1976, 261, 255-269, 10.1113/jphysiol.1976.sp011557.
- Eleonora Solari; Cristiana Marcozzi; Daniela Negrini; Andrea Moriondo; Lymphatic Vessels and Their Surroundings: How Local Physical Factors Affect Lymph Flow. Biology 2020, 9, 463, 10.3390/biology9120463.
- Eleonora Solari; Cristiana Marcozzi; Barbara Bartolini; Manuela Viola; Daniela Negrini; Andrea Moriondo; Acute Exposure of Collecting Lymphatic Vessels to Low-Density Lipoproteins Increases Both Contraction Frequency and Lymph Flow: AnIn VivoMechanical Insight. Lymphatic Research and Biology 2020, 18, 146-155, 10.1089/lrb.2019.0040.
- Anatoliy A. Gashev; Michael J. Davis; David C. Zawieja; Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. The Journal of Physiology 2002, 540, 1023-1037, 10.1111/j.1469-7793.2002.01023.x.
- Risuke Mizuno; Akos Koller; Gabor Kaley; Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 1998, 274, R790-R796, 10.1152/ajpregu.1998.274.3.r790.
- James L R Fox; Pierre-Yves Von Der Weid; Effects of histamine on the contractile and electrical activity in isolated lymphatic vessels of the guinea-pig mesentery. Journal of Cerebral Blood Flow & Metabolism 2002, 136, 1210-1218, 10.1038/sj.bjp.0704820.
- Eleonora Solari; Cristiana Marcozzi; Daniela Negrini; Andrea Moriondo; Fluid Osmolarity Acutely and Differentially Modulates Lymphatic Vessels Intrinsic Contractions and Lymph Flow. Frontiers in Physiology 2018, 9, 871, 10.3389/fphys.2018.00871.
- Eleonora Solari; Cristiana Marcozzi; Daniela Negrini; Andrea Moriondo; Temperature-dependent modulation of regional lymphatic contraction frequency and flow. American Journal of Physiology-Heart and Circulatory Physiology 2017, 313, H879-H889, 10.1152/ajpheart.00267.2017.
- Eleonora Solari; Cristiana Marcozzi; Michela Bistoletti; Andreina Baj; Cristina Giaroni; Daniela Negrini; Andrea Moriondo; TRPV4 channels’ dominant role in the temperature modulation of intrinsic contractility and lymph flow of rat diaphragmatic lymphatics. American Journal of Physiology-Heart and Circulatory Physiology 2020, 319, H507-H518, 10.1152/ajpheart.00175.2020.
- Niklas Telinius; Ulrik Baandrup; Jüri Rumessen; Hans Pilegaard; Vibeke Hjortdal; Christian Aalkjaer; Donna Boedtkjer; The human thoracic duct is functionally innervated by adrenergic nerves. American Journal of Physiology-Heart and Circulatory Physiology 2014, 306, H206-H213, 10.1152/ajpheart.00517.2013.
- Samia B. Bachmann; Denise Gsponer; Javier Montoya; Martin Schneider; Felix Scholkmann; Carlotta Tacconi; Simon F. Noerrelykke; Steven T. Proulx; Michael Detmar; A Distinct Role of the Autonomic Nervous System in Modulating the Function of Lymphatic Vessels under Physiological and Tumor-Draining Conditions. Cell Reports 2019, 27, 3305-3314.e13, 10.1016/j.celrep.2019.05.050.
- M. C. Mazzoni; T. C. Skalak; G. W. Schmid-Schonbein; Effects of skeletal muscle fiber deformation on lymphatic volumes. American Journal of Physiology-Heart and Circulatory Physiology 1990, 259, H1860-H1868, 10.1152/ajpheart.1990.259.6.h1860.
- G. W. Schmid-Schonbein; Microlymphatics and lymph flow. Physiological Reviews 1990, 70, 987-1028, 10.1152/physrev.1990.70.4.987.
- Daniela Negrini; Andrea Moriondo; Sylvain Mukenge; Transmural Pressure During Cardiogenic Oscillations in Rodent Diaphragmatic Lymphatic Vessels. Lymphatic Research and Biology 2004, 2, 69-81, 10.1089/lrb.2004.2.69.
- Andrea Moriondo; Sylvain Mukenge; Daniela Negrini; Transmural pressure in rat initial subpleural lymphatics during spontaneous or mechanical ventilation. American Journal of Physiology-Heart and Circulatory Physiology 2005, 289, H263-H269, 10.1152/ajpheart.00060.2005.
- J G McGeown; N G McHale; Keith Thornbury; The role of external compression and movement in lymph propulsion in the sheep hind limb.. The Journal of Physiology 1987, 387, 83-93, 10.1113/jphysiol.1987.sp016564.
- T.C. Skalak; G.W. Schmid-Schönbein; B.W. Zweifach; New morphological evidence for a mechanism of lymph formation in skeletal muscle. Microvascular Research 1984, 28, 95-112, 10.1016/0026-2862(84)90032-3.
- Andrea Moriondo; Eleonora Solari; Cristiana Marcozzi; Daniela Negrini; Diaphragmatic lymphatic vessel behavior during local skeletal muscle contraction. American Journal of Physiology-Heart and Circulatory Physiology 2015, 308, H193-H205, 10.1152/ajpheart.00701.2014.
- Andrea Moriondo; Eleonora Solari; Cristiana Marcozzi; Daniela Negrini; Spontaneous activity in peripheral diaphragmatic lymphatic loops. American Journal of Physiology-Heart and Circulatory Physiology 2013, 305, H987-H995, 10.1152/ajpheart.00418.2013.
- Andrea Moriondo; Eleonora Solari; Cristiana Marcozzi; Daniela Negrini; Lymph flow pattern in pleural diaphragmatic lymphatics during intrinsic and extrinsic isotonic contraction. American Journal of Physiology-Heart and Circulatory Physiology 2016, 310, H60-H70, 10.1152/ajpheart.00640.2015.
- Christopher M. Quick; Arun M. Venugopal; Anatoliy A. Gashev; David Zawieja; Randolph H. Stewart; Intrinsic pump-conduit behavior of lymphangions. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2007, 292, R1510-R1518, 10.1152/ajpregu.00258.2006.
- Jeremiah Bernier-Latmani; Tatiana V. Petrova; Intestinal lymphatic vasculature: structure, mechanisms and functions. Nature Reviews Gastroenterology & Hepatology 2017, 14, 510-526, 10.1038/nrgastro.2017.79.
- Jeremiah Bernier-Latmani; Christophe Cisarovsky; Cansaran Saygili Demir; Marine Bruand; Muriel Jaquet; Suzel Davanture; Simone Ragusa; Stefanie Siegert; Olivier Dormond; Rui Benedito; et al.Freddy RadtkeSanjiv LutherTatiana V. Petrova DLL4 promotes continuous adult intestinal lacteal regeneration and dietary fat transport. Journal of Clinical Investigation 2015, 125, 4572-4586, 10.1172/jci82045.
- Osamu Ohtani; Three-dimensional organization of lymphatics and its relationship to blood vessels in rat small intestine. Cell and Tissue Research 1987, 248, 365-374, 10.1007/bf00218204.
- J. L. Unthank; H. G. Bohlen; Lymphatic pathways and role of valves in lymph propulsion from small intestine. American Journal of Physiology-Gastrointestinal and Liver Physiology 1988, 254, G389-G398, 10.1152/ajpgi.1988.254.3.g389.
- Natalie Trevaskis; Lisa Kaminskas; Christopher Porter; From sewer to saviour — targeting the lymphatic system to promote drug exposure and activity. Nature Reviews Drug Discovery 2015, 14, 781-803, 10.1038/nrd4608.
- Yuling Mao; Shuang Feng; Shuai Li; Qinfu Zhao; Donghua Di; Yanfeng Liu; Siling Wang; Chylomicron-pretended nano-bio self-assembling vehicle to promote lymphatic transport and GALTs target of oral drugs. Biomaterials 2018, 188, 173-186, 10.1016/j.biomaterials.2018.10.012.
- Osamu Ohtan; Aiji Ohtsuka; Three-Dimensional Organization of Lymphatics and their Relationship to Blood Vessels in Rabbit Small Intestine. A Scanning Electron Microscopic Study of Corrosion Casts. Archivum histologicum japonicum 1985, 48, 255-268, 10.1679/aohc.48.255.
- Christopher P. Gayer; Marc D. Basson; The effects of mechanical forces on intestinal physiology and pathology. Cellular Signalling 2009, 21, 1237-1244, 10.1016/j.cellsig.2009.02.011.
- Kibaek Choe; Jeon Yeob Jang; Intae Park; Yeseul Kim; Soyeon Ahn; Dae-Young Park; Young-Kwon Hong; Kari Alitalo; Gou Young Koh; Pilhan Kim; et al. Intravital imaging of intestinal lacteals unveils lipid drainage through contractility. Journal of Clinical Investigation 2015, 125, 4042-4052, 10.1172/jci76509.
- D. C. Zawieja; B. J. Barber; Lymph protein concentration in initial and collecting lymphatics of the rat. American Journal of Physiology-Gastrointestinal and Liver Physiology 1987, 252, G602-G606, 10.1152/ajpgi.1987.252.5.g602.
- J. N. Benoit; David Zawieja; A. H. Goodman; H. J. Granger; Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. American Journal of Physiology-Heart and Circulatory Physiology 1989, 257, H2059-H2069, 10.1152/ajpheart.1989.257.6.h2059.
- Jeffrey Wigle; Guillermo Oliver; Prox1 Function Is Required for the Development of the Murine Lymphatic System. Cell 1999, 98, 769-778, 10.1016/s0092-8674(00)81511-1.
- Natasha Harvey; R Sathish Srinivasan; Miriam E Dillard; Nicole C Johnson; Marlys H Witte; Kelli Boyd; Mark W Sleeman; Guillermo Oliver; Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nature Genetics 2005, 37, 1072-1081, 10.1038/ng1642.
- Noelia Escobedo; Steven T. Proulx; Sinem Karaman; Miriam E. Dillard; Nicole Johnson; Michael Detmar; Guillermo Oliver; Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight 2016, 1, e85096, 10.1172/jci.insight.85096.
- Harri Nurmi; Pipsa Saharinen; Georgia Zarkada; Wei Zheng; Marius R Robciuc; Kari Alitalo; VEGF ‐C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Molecular Medicine 2015, 7, 1418-1425, 10.15252/emmm.201505731.
- Marika J. Karkkainen; Anne Saaristo; Lotta Jussila; Kaisa A. Karila; Elizabeth C. Lawrence; Katri Pajusola; Hansruedi Bueler; Anne Eichmann; Risto Kauppinen; Mikko Kettunen; et al.Seppo Ylä-HerttualaDavid FinegoldRobert E. FerrellKari Alitalo A model for gene therapy of human hereditary lymphedema. Proceedings of the National Academy of Sciences 2001, 98, 12677-12682, 10.1073/pnas.221449198.
- Silvia D’Alessio; Carmen Correale; Carlotta Tacconi; Alessandro Gandelli; Giovanni Pietrogrande; Stefania Vetrano; Marco Genua; Vincenzo Arena; Antonino Spinelli; Laurent Peyrin-Biroulet; et al.Claudio FiocchiSilvio Danese VEGF-C–dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. Journal of Clinical Investigation 2014, 124, 3863-3878, 10.1172/jci72189.
- Edwin A. Deitch; Gut lymph and lymphatics: a source of factors leading to organ injury and dysfunction. Annals of the New York Academy of Sciences 2010, 1207, E103-E111, 10.1111/j.1749-6632.2010.05713.x.
- Edwin A. Deitch; Gut-origin sepsis: Evolution of a concept. The Surgeon 2012, 10, 350-356, 10.1016/j.surge.2012.03.003.
- Chirag D. Badami; Maheswari Senthil; Francis J. Caputo; Bobby J. Rupani; Danielle Doucet; Vadim Pisarenko; Da-Zhong Xu; Qi Lu; Rena Feinman; Edwin A. Deitch; et al. MESENTERIC LYMPH DUCT LIGATION IMPROVES SURVIVAL IN A LETHAL SHOCK MODEL. Shock 2008, 30, 680-685, 10.1097/shk.0b013e318173edd1.
- Kyung Eun Kim; Hoon-Ki Sung; Gou Young Koh; Lymphatic development in mouse small intestine. Developmental Dynamics 2007, 236, 2020-2025, 10.1002/dvdy.21200.
- Sang Heon Suh; Kibaek Choe; Seon Pyo Hong; Seung‐Hwan Jeong; Taija Makinen; Kwang Soon Kim; Kari Alitalo; Charles D Surh; Gou Young Koh; Joo‐Hye Song; et al. Gut microbiota regulates lacteal integrity by inducing VEGF‐C in intestinal villus macrophages. EMBO reports 2019, 20, e46927, 10.15252/embr.201846927.
- Jeon Yeob Jang; Young Jun Koh; Seung-Hun Lee; Junyeop Lee; Kyoo Hyun Kim; Daesoo Kim; Gou Young Koh; Ook Joon Yoo; Conditional ablation of LYVE-1+ cells unveils defensive roles of lymphatic vessels in intestine and lymph nodes. Blood 2013, 122, 2151-2161, 10.1182/blood-2013-01-478941.
- Peter Baluk; Jonas Fuxe; Hiroya Hashizume; Talia Romano; Erin Lashnits; Stefan Butz; Dietmar Vestweber; Monica Corada; Cinzia Molendini; Elisabetta Dejana; et al.Donald M. McDonald Functionally specialized junctions between endothelial cells of lymphatic vessels. Journal of Experimental Medicine 2007, 204, 2349-2362, 10.1084/jem.20062596.
- Marisol Chang; Tom Alsaigh; Erik Kistler; Geert W. Schmid-Schönbein; Breakdown of Mucin as Barrier to Digestive Enzymes in the Ischemic Rat Small Intestine. PLoS ONE 2012, 7, e40087, 10.1371/journal.pone.0040087.
- Nobuhiko Kamada; Sang-Uk Seo; Grace Y. Chen; Gabriel Núñez; Role of the gut microbiota in immunity and inflammatory disease. Nature Reviews Immunology 2013, 13, 321-335, 10.1038/nri3430.
- Rohit Mittal; Craig M. Coopersmith; Redefining the gut as the motor of critical illness. Trends in Molecular Medicine 2013, 20, 214-223, 10.1016/j.molmed.2013.08.004.
- Schmid-Schonbein Geert W; DeLano Frank A; Penn Alexander H; Kistler Erik; An elementary analysis of physiologic shock and multi-organ failure: The Autodigestion Hypothesis. Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 18 August-1 September 2012 2012, 2012, 114-3115.
- Bonggi Lee; Kyoung Mi Moon; Choon Young Kim; Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. Journal of Immunology Research 2018, 2018, 2645465, 10.1155/2018/2645465.
- Feng Zhang; Georgia Zarkada; Jinah Han; Jinyu Li; Alexandre Dubrac; Roxana Ola; Gael Genet; Kevin Boyé; Pauline Michon; Steffen E. Künzel; et al.Joao Paulo CamporezAbhishek K. SinghGuo-Hua FongMichael SimonsPatrick TsoCarlos Fernández-HernandoGerald I. ShulmanWilliam C. SessaAnne Eichmann Lacteal junction zippering protects against diet-induced obesity. Science 2018, 361, 599-603, 10.1126/science.aap9331.
- Wang Xiaolei; Zhao Jin; Qin Li; VEGF-C mediated enhancement of lymphatic drainage reduces intestinal inflammation by regulating IL-9/IL-17 balance and improving gut microbiota in experimental chronic colitis. American Journal of Translational Research 2017, 9, 4772-4784.
