Aspergillus species are filamentous fungi commonly observed in different environmental compartments such as soil, water and air, with an emphasis on decaying vegetation, seeds and grains, where they prosper as saprophytes. Aspergillus species are also found in different indoor environments, and some species are considered opportunistic pathogens for humans. Aspergillus conidia can be abundant in outdoor and indoor environments and are easily dispersed in the air depending on the developed activities. Since the conidia are very small, they are easily inhaled and may colonize the upper and lower respiratory tract of exposed individuals. Aspergillus section Fumigati is one of the Aspergillus sections more frequently related to respiratory symptoms due to the small size of the conidia, thermotolerance, its nutritional versatility, and several other virulence factors . Additionally, the development of resistance to antifungal drugs, mainly in this Aspergillus section, is a phenomenon with growing prevalence in Europe, being associated with therapeutic failure and high mortality rates.
- sampling approach
- culture-based methods
- molecular tools
- azole resistance profile
1. Aspergillus Section Fumigati Distribution
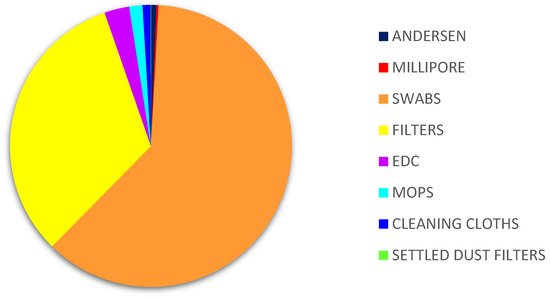
Sample |
MEA | DG18 | ||||
|---|---|---|---|---|---|---|
| Fungi | CFU. m−3/m−2/g | % | Fungi | CFU. m3 3/m2/g | % | |
Andersen |
Other species Aspergillus sp. |
405,281.8 486 |
99.9 0.1 |
Other species Aspergillus sp. |
67,915.2 1040 |
98.5 1.5 |
Millipore |
Other species Aspergillus sp. |
147,399.8 158.2 |
99.9 0.1 |
Other species Aspergillus sp |
50,624.64 393 |
99.2 0.8 |
| EDC * | Other species Aspergillus sp. |
391,345 743.1 |
99.8 0.2 |
Other species Aspergillus sp. |
210,245.9 4640.126 |
97.8 2.2 |
Cleaning cloths |
Other species Aspergillus sp. |
29,500 500 |
98.3 1.7 |
Other species Aspergillus sp. |
19,700 1500 |
92.9 7.1 |
Mops |
Other species Aspergillus sp. |
2600 - |
100 - |
Other species Aspergillus sp. |
13,500 2500 |
84.4 15.6 |
Identification badges |
Other species Aspergillus sp. |
45,500 - |
100 - |
Other species Aspergillus sp. |
32,500 - |
100 - |
Filters |
Other species Aspergillus sp. |
3,679,700 128,500 |
96.6 3.4 |
Other species Aspergillus sp. |
2,699,000 52,505 |
98.1 1.9 |
Settled dust |
Other species Aspergillus sp. |
6496.5 - |
100 - |
Other species Aspergillus sp. |
2983.9 27 |
99.1 0.9 |
Swabs |
Other species Aspergillus sp. |
4,562,500 10,000 |
99.8 0.2 |
Other species Aspergillus sp. |
4,131,556 100,000 |
97.6 2.4 |
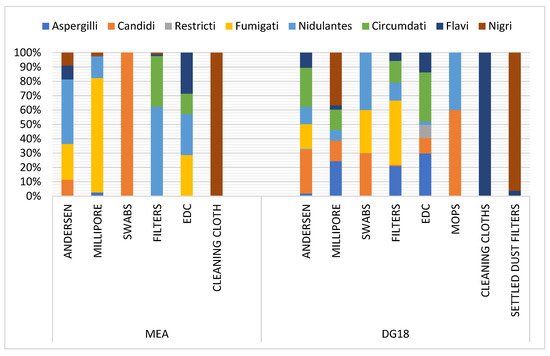
2. Screening of Azole Resistance
Matrices |
SDA | ITR | VOR | POS | ||||
|---|---|---|---|---|---|---|---|---|
| CFU.m² | % | CFU.m² | % | CFU.m² | % | CFU.m² | % | |
| Mops | 2500 | 100% | - | - | - | - | - | - |
| EDC * | 106 | 4.4% | 1062 | 100% | 3503 | 97.1% | - | - |
| Settled dust filters | 1500 |
3.7% |
1062 |
100% |
- |
- |
- |
- |
| Total | 4106 | 8.9% | 1562 | 100% | 3503 | 85.3% | - | - |
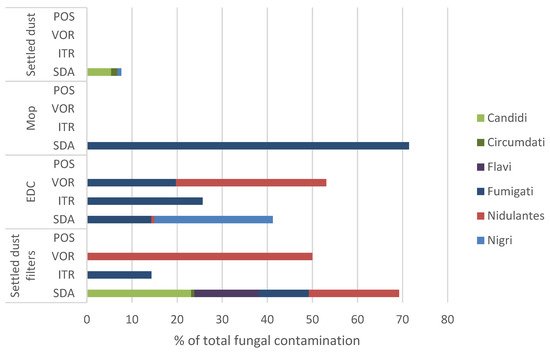
34. Discussion
References
- WHO. World Health Organisation Guidelines for Indoor Air Quality: Dampness and Mould. Available online: https://www.who.int/publications/i/item/9789289041683 (accessed on 1 September 2021).
- APA. Qualidade do Ar em Espaços Interiores Um Guia Técnico Agência Portuguesa do Ambiente. Available online: https://www.voltimum.pt/artigos/biblioteca-de-flipbooks/qualidade-do-ar-em (accessed on 1 September 2021).
- Viegas, C.; Aranha Caetano, L.; Viegas, S. Occupational exposure to Aspergillus section Fumigati: Tackling the knowledge gap in Portugal. Environ. Res. 2021, 194.
- Strachan, D.P. Damp housing and childhood asthma: Validation of reporting of symptoms. BMJ 1988, 297, 1223–1226.
- Platt, S.D.; Martin, C.J.; Hunt, S.M.; Lewis, C.W. Damp housing, mould growth, and symptomatic health state. BMJ 1989, 298, 1673–1678.
- Dales, R.E.; Burnett, R.; Zwanenburg, H. Adverse health effects among adults exposed to home dampness and molds. Am. Rev. Respir. Dis. 1991, 143, 505–509.
- Mendell, M.J.; Mirer, A.G.; Cheung, K.; Tong, M.; Douwes, J. Respiratory and Allergic Health Effects of Dampness, Mold, and Dampness-Related Agents: A Review of the Epidemiologic Evidence. Environ. Health. Perspect. 2011, 119, 748–756.
- Kanchongkittiphon, W.; Mendell, M.J.; Gaffin, J.M.; Wang, G.; Phipatanakul, W. Indoor environmental exposures and exacerbation of asthma: An update to the 2000 review by the Institute of Medicine. Environ. Health Perspect. 2015, 123, 6–20.
- Adams, R.; Leppänen, H.; Karvonen, A.; Jacobs, J.; Borràs-Santos, A.; Valkonen, M.; Krop, E.; Haverinen-Shaughnessy, U.; Huttunen, K.; Zock, J.-P.; et al. Microbial exposures in moisture-damaged schools and associations with respiratory symptoms in students: A multi-country environmental exposure study. Indoor Air 2021.
- Rajasekar, A.; Balasubramanian, R. Assessment of airborne bacteria and fungi in food courts. Build. Environ. 2011, 46, 2081–2087.
- Alberti, C.; Bouakline, A.; Ribaud, P.; Lacroix, C.; Rousselot, P.; Leblanc, T.; Derouin, F.; Aspergillus group. Relationship between environmental fungal contamination and the incidence of invasive aspergillosis in haematology patients. J. Hosp. Infect. 2001, 48, 198–206.
- Ramos, C.A.; Viegas, C.; Verde, S.C.; Wolterbeek, H.T.; Almeida, S.M. Characterizing the fungal and bacterial microflora and concentrations in fitness centres. Indoor Built Environ. 2016, 25, 872–882.
- Reponen, T. Sampling for Microbial Determinations. In Exposure to Microbiological Agents in Indoor and Occupational Environments, 1st ed.; Viegas, C., Viegas, S., Gomes, A.Q., Täubel, M., Sabino, R., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 85–96.
- Cox, J.; Mbareche, H.; Lindsley, W.G.; Duchaine, C. Field sampling of indoor bioaerosols. Aerosol Sci. Technol. 2020, 54, 572–584.
- Gonçalves, P.; Melo, A.; Dias, M.; Almeida, B.; Caetano, L.A.; Veríssimo, C.; Viegas, C.; Sabino, R. Azole-resistant Aspergillus fumigatus harboring the tr34/l98h mutation: First report in Portugal in environmental samples. Microorganisms 2021, 9, 57.
- Viegas, C.; Almeida, B.; Gomes, A.Q.; Carolino, E.; Caetano, L.A. Aspergillus spp. prevalence in Primary Health Care Centres: Assessment by a novel multi-approach sampling protocol. Environ Res. 2019, 175, 133–141.
- Viegas, C.; Almeida, B.; Monteiro, A.; Aranha Caetano, L.; Carolino, E.; Quintal-Gomes, A.; Twarużek, M.; Kosicki, R.; Marchand, G.; Viegas, S. Bioburden in healthcare centers: Is the compliance with Portuguese legislation enough to prevent and control infection? Build. Environ. 2019, 160.
- Viegas, C.; Almeida, B.; Monteiro, A.; Paciência, I.; Rufo, J.; Aguiar, L.; Lage, B.; Gonçalves, L.M.D.; Aranha Caetano, L.; Carolino, E.; et al. Exposure assessment in one central Hospital: A multi-approach protocol to achieve an accurate risk characterization. Environ. Res. 2019, 181, 108947.
- Viegas, C.; Faria, T.; Caetano, L.A.; Carolino, E.; Quintal-Gomes, A.; Twaruzek, M.; Kosicki, R.; Viegas, S. Characterization of Occupational Exposure to Fungal Burden in Portuguese Bakeries. Microorganisms 2019, 7, 234.
- Viegas, C.; Dias, M.; Almeida, B.; Carolino, E.; Quintal Gomes, A.; Viegas, S. Aspergillus spp. burden on filtering respiratory protective devices. Is there an occupational health concern? Air Qual. Atmos. Health 2020, 13.
- Viegas, C.; Sousa, P.; Dias, M.; Caetano, L.A.; Ribeiro, E.; Carolino, E.; Twarużek, M.; Kosicki, R.; Viegas, S. Bioburden contamination and Staphylococcus aureus colonization associated with firefighter’s ambulances. Environ. Res. 2021, 197.
- Viegas, C.; Dias, M.; Almeida, B.; Vicente, E.; Candeias, C.; Aranha Caetano, L.; Carolino, E.; Alves, C. Loading Rates of Dust and Bioburden in Dwellings in an Inland City of Southern Europe. Atmosphere 2021, 12, 378.
- Viegas, C.; Faria, T.; Aranha Caetano, L.; Carolino, E.; Quintal Gomes, A.; Viegas, S. Aspergillus spp. prevalence in different occupational settings. J. Occup. Environ. Hyg. 2017, 14, 771–785.
- Viegas, C.; Almeida, B.; Monteiro, A.; Paciência, I.; Cavaleiro, J.R.; Carolino, E.; Quintal-Gomes, A.; Twarużek, M.; Kosicki, R.; Marchand, G.; et al. Settled dust assessment in clinical environment: Useful for the evaluation of a wider bioburden spectrum. Int. J. Environ. Health Res. 2019, 26, 1–19.
- Viegas, C.; Almeida, B.; Caetano, L.A.; Afanou, A.; Straumfors, A.; Veríssimo, C.; Gonçalves, P.; Sabino, R. Algorithm to assess the presence of Aspergillus fumigatus resistant strains: The case of Norwegian sawmills. Int. J. Environ. Health Res. 2020, 19, 1–9.
- Viegas, C.; Caetano, L.A.; Cox, J.; Korkalainen, M.; Haines, S.R.; Dannemiller, K.C.; Viegas, S.; Reponen, T. The effects of waste sorting in environmental microbiome, THP-1 cell viability and inflammatory responses. Environ. Res. 2020, 185.
- Viegas, C.; Faria, T.; Cebola de Oliveira, A.; Aranha Caetano, L.; Carolino, E.; Quintal-Gomes, A.; Twarużek, M.; Kosicki, R.; Soszczyńska, E.; Viegas, S. A new approach to assess fungal contamination and mycotoxins occupational exposure in forklifts drivers from waste sorting. Mycotoxin Res. 2017, 33, 285–295.
- Chowdhary, A.; Kathuria, S.; Xu, J.; Meis, J.F. Emergence of Azole-Resistant Aspergillus fumigatus Strains due to Agricultural Azole Use Creates an Increasing Threat to Human Health. PLoS Pathog. 2013, 9.
- Mao, Y.; Ding, P.; Wang, Y.; Ding, C.; Wu, L.; Zheng, P.; Zhang, X.; Lia, X.; Wang, L.; Suna, Z. Comparison of culturable antibiotic-resistant bacteria in polluted and non-polluted air in Beijing, China. Environ. Int. 2019, 131, 104936.
- Andersen, A. New sampler for the collection, sizing and enumeration of viable airborne particles. J. Bacteriol. 1958, 76, 471–484.
- Richardson, M.; Bowyer, P.; Sabino, R. The human lung and Aspergillus: You are what you breathe in? Med. Mycol. 2019, 57, S145–S154.
- Viegas, C.; Faria, T.; dos Santos, M.; Carolino, E.; Gomes, A.Q.; Sabino, R.; Viegas, S. Fungal burden in waste industry: An occupational risk to be solved. Environ. Monit. Assess. 2015, 187.
- Franklin, R.B.; Garland, J.L.; Bolster, C.H.; Mills, A.L. Impact of dilution on microbial community structure and functional potential: Comparison of numerical simulations and batch culture experiments. Appl. Environ. Microbiol. 2001, 67, 702–712.
- Black, W.D. A comparison of several media types and basic techniques used to assess outdoor airborne fungi in Melbourne. Australia. PLoS ONE 2020, 15.
- Nagano, Y.; Millar, B.C.; Goldsmith, C.E.; Walker, J.M.; Elborn, J.S.; Rendall, J.; Moore, J.E. Development of selective media for the isolation of yeasts and filamentous fungi from the sputum of adult patients with cystic fibrosis (CF). J. Cyst Fibros. 2008, 7, 566–572.
- Viegas, C.; Dias, M.; Carolino, E.; Sabino, R. Culture Media and Sampling Collection Method for Aspergillus spp. Assessment: Tackling the Gap between Recommendations and the Scientific Evidence. Atmosphere 2021, 12, 23.
- Sabino, R. Exposure to Fungi in Health Care Facilities. Ref. Modul. Life Sci. 2020, 1–10.
- Stop neglecting fungi. Nat. Microbiol. 2017, 2.
- Bush, R.K.; Portnoy, J.M.; Saxon, A.; Terr, A.I.; Wood, R.A. The medical effects of mold exposure. J. Allergy Clin. Immunol. 2006, 117, 326–333.
- Franchitti, E.; Pascale, E.; Fea, E.; Anedda, E.; Traversi, D. Methods for bioaerosol characterization: Limits and perspectives for human health risk assessment in organicwaste treatment. Atmosphere 2020, 11, 452.
- McDevitt, J.J.; Lees, P.S.J.; Merz, W.G.; Schwab, K.J. Inhibition of quantitative PCR analysis of fungal conidia associated with indoor air particulate matter. Aerobiologia 2007, 23, 35–45.
- Mensah-Attipoe, J.; Taubel, M. Analysis Approaches for Fungi in Indoor Environmental Assessments. In Exposure to Microbiological Agents in Indoor and Occupational Environments, 1st ed.; Viegas, C., Viegas, S., Gomes, A.Q., Täubel, M., Sabino, R., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 109–126.
- Yoo, K.; Lee, T.K.; Choi, E.J.; Yang, J.; Shukla, S.K.; Hwang, S.; Park, J. Molecular approaches for the detection and monitoring of microbial communities in bioaerosols: A review. J. Environ. Sci. 2016, 51, 234–247.
- Hung, L.L.; Miller, J.D.; Dillon, K.H. Field Guide for the Determination of Biological Contaminants in Environmental Samples, 2nd ed.; AIHA: Fairfax, VA, USA, 2005.
