Cortical visual impairment in childhood is a kind of visual damage congenitally sustained by children.
- cortical blindness
- cortical visual impairment
- vision
- blindsight
- Sprague Effect
- infant vision
1. Introduction
A principal reason for visual dysfunction in childhood in developed countries is CVI [1][2][3]. This has occurred as technology development has led to better visual treatment for other conditions such as congenital glaucoma, retinopathy of prematurity, and congenital cataracts as well as the increased survival of infants with central nervous system damage or disease.
The incidence of CVI has increased, with it now being a highly significant public health concern. Approximately 30–40% of children with visual impairments have CVI. The National Institutes of Health website cites a CVI prevalence of 10.5% of all children with developmental disabilities [4]. Generally, the prevalence of visual impairment in children under 16 years ranges between 10–22 per 10,000 births in developed countries and 40 per 10,000 births in developing countries [5][6].
In children with cerebral palsy, approximately two-thirds also demonstrate impaired visual acuity and/or field defects indicative of CVI [6]. In an African study, 47.7 percent of cerebral palsied children also demonstrated CVI [6][7], and in India, reports of 28 percent have been described [6][8].
An infant or child is said to’ have CVI if (a) the loss of functional vision cannot be explained completely by an eye examination; (b) has a history of neurological dysfunction even with brain imaging studies appear to be normal; (c) demonstrates an array of visual or behavioral features identified in medical, psychological, or educational research [9]. As CVI is a consequence of brain insult rather than ocular dysfunction, an understanding of the dynamic properties of neurological development of the infant and child can assist in planning and developing better treatment protocols that may influence the developing child’s functional vision. The process of neuroplasticity related to the development and function of the visual system will be discussed.
Damage, insult, or dysfunction to the visual system during fetal, neonatal and infant development may well have long-term consequences that are, as we shall see, potentially more capable of alteration and restoration of function in the infant and child as compared to similar insult in adults [10].
2. Is Recovery of Normal Conscious Vision Possible?
Our visual perceptual abilities are dependent on the pathways represented in Figure 1 [11][12][13]. Guzzetta and associates [14][15] propose that three criteria are necessary for the restoration of vision that includes: (a) pathology of involvement of the geniculostriatal pathway, (b) specific loss of vision that is independent of any other functional abnormality, and (c) regaining the formerly impaired function with concomitant empirical change in brain state or electrophysiological activity.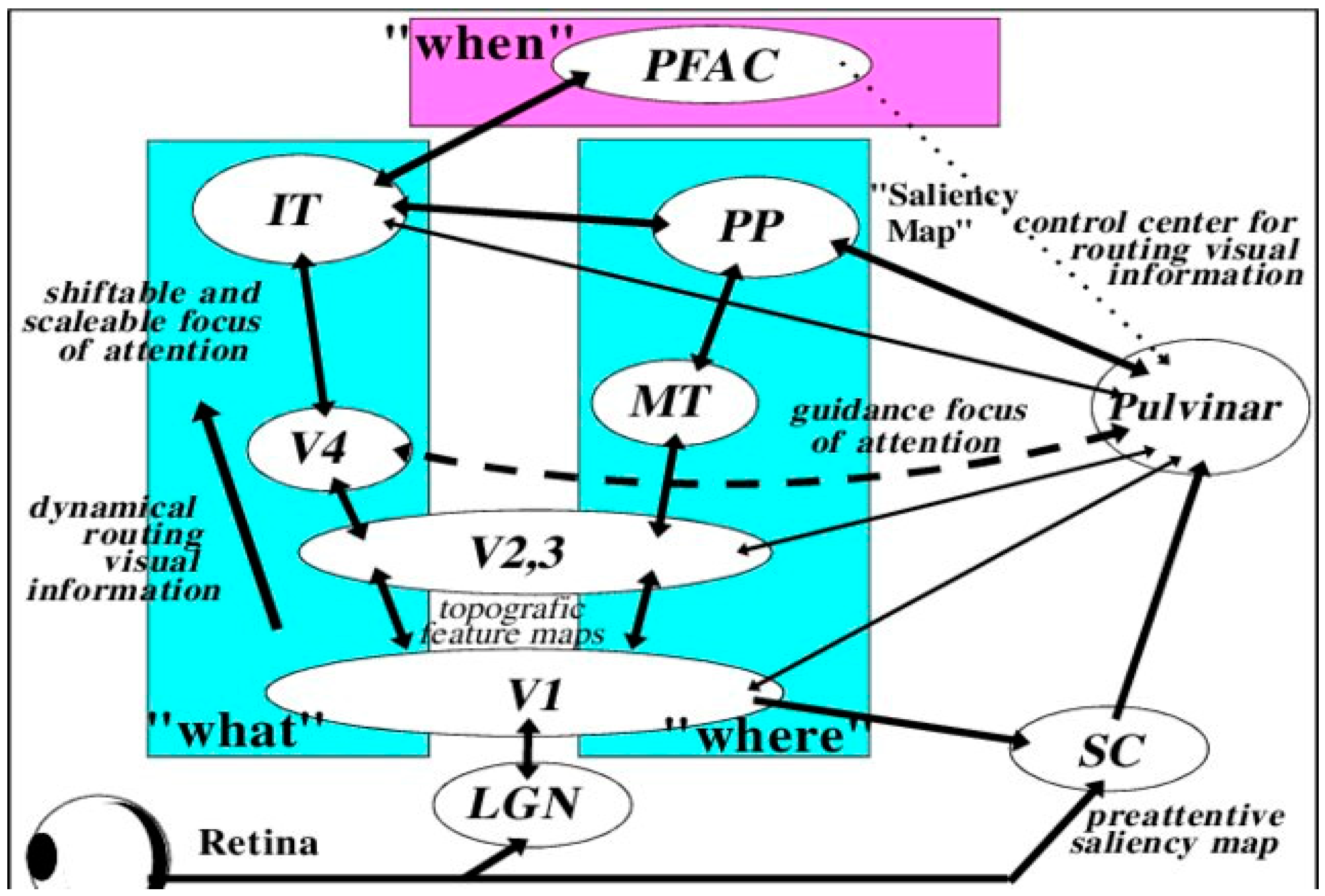
3. Neuroplasticity and Developmental Damage to the Primary Visual Cortex (V1)
4. Conclusions
References
- Chang, M.Y.; Borchert, M.S. Advances in the evaluation and management of cortical/cerebral visual impairment in children. Surv. Ophthalmol. 2020, 65, 708–724.
- Khan, R.I.; O’Keefe, M.; Kenny, D.; Nolan, L. Changing pattern of childhood blindness. Ir. Med. J. 2007, 100, 458–461.
- Kong, L.; Fry, M.; Al-Samarraie, M.; Gilbert, C.; Steinkuller, P.G. An update on progress and the changing epidemiology of causes of childhood blindness worldwide. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2012, 16, 501–507.
- National Institutes of Health. 2021. Available online: https://www.nei.nih.gov/about/news-and-events/news/vision-loss-children-whose-eyesight-may-be-2020-requires-new-diagnostic-and-teaching-strategies (accessed on 11 September 2021).
- Flanagan, N.M.; Jackson, A.J.; Hill, A.E. Visual impairment in childhood: Insights from a community-based survey. Child Care Health Dev. 2003, 29, 493–499.
- Philip, S.S.; Dutton, G.N. Identifying and characterising cerebral visual impairment in children: A review. Clin. Exp. Optom. 2014, 97, 196–208.
- Lagunju, I.A.; Oluleye, T.S. Ocular abnormalities in children with cerebral palsy. Afr. J. Med. Med. Sci. 2007, 36, 71–75.
- Katoch, S.; Devi, A.; Kulkarni, P. Ocular defects in cerebral palsy. Indian J. Ophthalmol. 2007, 55, 154–156.
- Dutton, G.; Bax, M. (Eds.) Visual Impairment in Children Due to Damage to the Brain; John Wiley & Sons: Hoboken, NJ, USA, 2010; Volume 186.
- André, V.; Henry, S.; Lemasson, A.; Hausberger, M.; Durier, V. The human newborn’s umwelt: Unexplored pathways and perspectives. Psychon. Bull. Rev. 2018, 25, 350–369.
- Stoerig, P. Blindsight, conscious vision, and the role of primary visual cortex. Prog. Brain Res. 2006, 155, 217–234.
- Briscoe, R.; Schwenkler, J. Conscious vision in action. Cognit. Sci. 2015, 39, 1435–1467.
- Gross, H.M.; Heinke, D.; Boehme, H.J.; Braumann, U.D.; Pomierski, T. A behaviour-oriented approach to an implicit “object-understanding” in visual attention. In Proceedings of the ICNN’95—International Conference on Neural Networks (ICNN’95), Perth, Australia, 27 November–1 December 1995; Volume 1, pp. 657–662.
- Masuda, Y.; Dumoulin, S.O.; Nakadomari, S.; Wandell, B.A. V1 projection zone signals in human macular degeneration depend on task, not stimulus. Cereb. Cortex 2008, 18, 2483–2493.
- Guzzetta, A.; Cioni, G.; Cowan, F.; Mercuri, E. Visual disorders in children with brain lesions: 1. Maturation of visual func- tion in infants with neonatal brain lesions: Correlation with neuroimaging. Eur. J. Paediatr. Neurol. 2001, 5, 107–114.
- Huxlin, K.R. Perceptual plasticity in damaged adult visual systems. Vision Res. 2008, 48, 2154–2166.
- Crawford, L.B.; Golomb, M.R. Childhood Stroke and Vision: A Review of the Literature. Pediatr. Neurol. 2018, 81, 6–13.
- Zhang, X.; Kedar, S.; Lynn, M.J.; Newman, N.J.; Biousse, V. Natural history of homonymous hemianopia. Neurology 2006, 66, 901–905.
- Sabel, B.A.; Kasten, E. Restoration of vision by training of residual functions. Curr. Opin. Ophthalmol. 2000, 11, 430–436.
- Fontenot, J.L.; Bona, M.D.; Kaleem, M.A.; McLaughlin, W.M.; Morse, A.R.; Schwartz, T.L.; Shepherd, J.D.; Jackson, M.L. Vision rehabilitation preferred practice pattern. Ophthalmology 2018, 125, P228–P278.
- Bridge, H.; Thomas, O.; Jbabdi, S.; Cowey, A. Changes in connectivity after visual cortical brain damage underlie altered visual function. Brain 2008, 131, 1433–1444.
- Bridge, H. Loss of visual cortex and its consequences for residual vision. Curr. Opin. Physiol. 2020, 16, 21–26.
- Bouwmeester, L.; Heutink, J.; Lucas, C. The effect of visual training for patients with visual field defects due to brain damage: A systematic review. J. Neurol. Neurosurg. Psychiatry 2007, 78, 555–564.
- Waddington, J.; Hodgson, T. Review of rehabilitation and habilitation strategies for children and young people with homonymous visual field loss caused by cerebral vision impairment. Br. J. Vis. Impair. 2017, 35, 197–210.
- Moore, K.L.; Persaud TV, N.; Torchia, M.G. Before We Are Born E-Book: Essentials of Embryology and Birth Defects; Elsevier Health Sciences: Philadelphia, PA, USA, 2015.
- Walsh, V.; Butler, S.R.; Carden, D.; Kulikowski, J.J. The effects of V4 lesions on the visual abilities of macaques: Shape discrimination. Behav. Brain Res. 1992, 50, 115–126.
- Hovda, D.A.; Villablanca, J.R. Depth perception in cats after cerebral hemispherectomy: Comparisons between neonatal-and adult-lesioned animals. Behav. Brain Res. 1989, 32, 231–240.
- Zennou-Azogui, Y.; Xerri, C.; Leonard, J.; Tighilet, B. Vestibular compensation: Role of visual motion cues in the recovery of posturo-kinetic functions in the cat. Behav. Brain Res. 1996, 74, 65–77.
- Leh, S.E.; Johansen-Berg, H.; Ptito, A. Unconscious vision: New insights into the neuronal correlate of blindsight using diffusion tractography. Brain 2006, 129, 1822–1832.
- Teuber, H.-L. Recovery of function after brain injury in man. In Ciba Foundation Symposium; Elsevier: Amsterdam, The Netherlands, 1975; Volume 34, pp. 159–190.
- Leisman, G.; Koch, P. Networks of conscious experience: Computational neuroscience in understanding life, death, and consciousness. Rev. Neurosci. 2009, 20, 151–176.
- Perenin, M.T. Visual function within the hemianopic field following early cerebral hemidecortication in man—II. Pattern discrimination. Neuropsychologia 1978, 16, 697–708.
- Perenin, M.T.; Jeannerod, M. Visual function within the hemianopic field following early cerebral hemidecortication in man—I. Spatial localization. Neuropsychologia 1978, 16, 1–13.
- Knyazeva, M.G.; Maeder, P.; Kiper, D.C.; Deonna, T.; Innocenti, G.M. Vision after early-onset lesions of the occipital cortex: II. Physiological studies. Neural Plast. 2002, 9, 27–40.
- Kiper, D.C.; Zesiger, P.; Maeder, P.; Deonna, T.; Innocenti, G.M. Vision after early-onset lesions of the occipital cortex: I. Neuropsychological and psychophysical studies. Neural Plast. 2002, 9, 1–25.
- Werth, R. Visual functions without the occipital lobe or after cerebral hemispherectomy in infancy. Eur. J. Neurosci. 2006, 24, 2932–2944.
- Cornwell, P.; Payne, B. Visual discrimination by cats given lesions of visual cortex in one or two stages in infancy or in one stage in adulthood. Behav. Neurosci. 1989, 103, 1191.
- Cornwell, P.; Herbein, S.; Corso, C.; Eskew, R.; Warren, J.M.; Payne, B. Selective sparing after lesions of visual cortex in newborn kittens. Behav. Neurosci. 1989, 103, 1176.
- Murmu, M.S.; Salomon, S.; Biala, Y.; Weinstock, M.; Braun, K.; Bock, J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur. J. Neurosci. 2006, 24, 1477–1487.
- Atapour, N.; Worthy, K.H.; Lui, L.L.; Yu, H.H.; Rosa, M.G. Neuronal degeneration in the dorsal lateral geniculate nucleus following lesions of primary visual cortex: Comparison of young adult and geriatric marmoset monkeys. Brain Struct. Funct. 2017, 7, 3283–3293.
- Atapour, N.; Worthy, K.H.; Rosa, M.G. Neurochemical changes in the primate lateral geniculate nucleus following lesions of striate cortex in infancy and adulthood: Implications for residual vision and blindsight. Brain Struct. Funct. 2021, 1–13.
- Moore, T.; Rodman, H.R.; Repp, A.B.; Gross, C.G.; Mezrich, R.S. Greater residual vision in monkeys after striate cortex damage in infancy. J. Neurophysiol. 1996, 76, 3928–3933.
- Moore, T.; Rodman, H.R.; Gross, C.G. Direction of motion discrimination after early lesions of striate cortex (V1) of the macaque monkey. Proc. Natl. Acad. Sci. USA 2001, 98, 325–330.
- Mercuri, E.; Atkinson, J.; Braddick, O.; Anker, S.; Nokes, L.; Cowan, F.; Rutherford, M.; Pennock, J.; Dubowitz, L. Visual function and perinatal focal cerebral infarction. Arch. Dis. Child. Fetal Neonatal Ed. 1996, 75, F76–F81.
- Burneo, J.G.; Kuzniecky, R.I.; Bebin, M.; Knowlton, R.C. Cortical reorganization in malformations of cortical development: A magnetoencephalographic study. Neurology 2004, 63, 1818–1824.
- Mercuri, E.; Anker, S.; Guzzetta, A.; Barnett, A.; Haataja, L.; Rutherford, M.; Cowan, F.; Dubowitz, L.; Braddick, O.; Atkinson, J. Neonatal cerebral infarction and visual function at school age. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, F487–F491.
- Artzi, M.; Shiran, S.I.; Weinstein, M.; Myers, V.; Tarrasch, R.; Schertz, M.; Fattal-Valevski, A.; Miller, E.; Gordon, A.M.; Green, D.; et al. Cortical reorganization following injury early in life. Neural Plast. 2016, 2016, 8615872.
- Liu, T.T.; Nestor, A.; Vida, M.D.; Pyles, J.A.; Patterson, C.; Yang, Y.; Yang, F.N.; Freud, E.; Behrmann, M. Successful reorganization of category-selective visual cortex following occipito-temporal lobectomy in childhood. Cell Rep. 2018, 24, 1113–1122.
- Yates, T.S.; Ellis, C.T.; Turk-Browne, N.B. Emergence and organization of adult brain function throughout child development. Neuroimage 2021, 226, 117606.
- Hagberg, H.; Edwards, A.D.; Groenendaal, F. Perinatal brain damage: The term infant. Neurobiol. Dis. 2016, 92, 102–112.
- Blume, W.T.; Whiting, S.E.; Girvin, J.P. Epilepsy surgery in the posterior cortex. Ann. Neurol. 1991, 29, 638–645.
- Innocenti, G.M.; Maeder, P.; Knyazeva, M.G.; Fornari, E.; Deonna, T. Functional activation of microgyric visual cortex in a human. Ann. Neurol. 2001, 50, 672–676.
- Kujala, T.; Alho, K.; Näätänen, R. Cross-modal reorganization of human cortical functions. Trends Neurosci. 2000, 23, 115–120.
- Melnick, M.D.; Tadin, D.; Huxlin, K.R. Relearning to see in cortical blindness. Neuroscientist 2016, 22, 199–212.
- Chokron, S.; Perez, C.; Peyrin, C. Behavioral consequences and cortical reorganization in homonymous hemianopia. Front. Sys. Neurosci. 2016, 10, 57.
- Jamal, Y.A.; Dilks, D.D. Rapid topographic reorganization in adult human primary visual cortex (V1) during noninvasive and reversible deprivation. Proc. Natl. Acad. Sci. USA 2020, 117, 11059–11067.
- Castaldi, E.; Lunghi, C.; Morrone, M.C. Neuroplasticity in adult human visual cortex. Neurosci. Biobehav. Rev. 2020, 112, 542–552.
- Coullon, G.S.; Jiang, F.; Fine, I.; Watkins, K.E.; Bridge, H. Subcortical functional reorganization due to early blindness. J. Neurophysiol. 2015, 113, 2889–2899.
- Hasson, U.; Andric, M.; Atilgan, H.; Collignon, O. Congenital blindness is associated with large-scale reorganization of anatomical networks. Neuroimage 2016, 128, 362–372.
- Leisman, G.; Mualem, R.; Mughrabi, S.K. The neurological development of the child with the educational enrichment in mind. Psicol. Educ. 2015, 21, 79–96.
- Melillo, R.; Leisman, G. Neurobehavioral Disorders of Childhood: An Evolutionary Approach; Springer: New York, NY, USA, 2010.
- Leisman, G.; Merrick, J. Neuroplasticity in Learning and Rehabilitation; Nova Science Publishers: Hauppauge, NY, USA, 2016.
- Reid, V.M.; Dunn, K.; Young, R.J.; Amu, J.; Donovan, T.; Reissland, N. The human fetus preferentially engages with face-like visual stimuli. Curr. Biol. 2017, 27, 1825–1828.
- Thomas, C.; Baker, C.I. Remodeling human cortex through training: Comment on May. Architecture 2012, 12, 1370–1371.
- Wandell, B.A.; Smirnakis, S.M. Plasticity and stability of visual field maps in adult primary visual cortex. Nat. Rev. Neurosci. 2009, 10, 873–884.
- Sprague, J.M. Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science 1966, 153, 1544–1547.
