Polysaccharides are often the most abundant antigens found on the extracellular surfaces of bacterial cells. These polysaccharides play key roles in interactions with the outside world, and for many bacterial pathogens, they represent what is presented to the human immune system. As a result, many vaccines have been or currently are being developed against carbohydrate antigens. In this review, we explore the diversity of capsular polysaccharides (CPS) in Salmonella and other selected bacterial species and explain the classification and function of CPS as vaccine antigens. Despite many vaccines being developed using carbohydrate antigens, the low immunogenicity and the diversity of infecting strains and serovars present an antigen formulation challenge to manufacturers. Vaccines tend to focus on common serovars or have changing formulations over time, reflecting the trends in human infection, which can be costly and time-consuming. We summarize the approaches to generate carbohydrate-based vaccines for Salmonella, describe vaccines that are in development and emphasize the need for an effective vaccine against non-typhoidal Salmonella strains.
- carbohydrate
- bacterial vaccines
- typhoid fever
- gastroenteritis
- T-cell-independent antigen
- capsule
- capsular polysaccharide
1. Salmonellosis
Salmonellosis is one of the most common foodborne diseases worldwide [1]. Symptoms of Salmonellosis include abdominal cramps, diarrhea, nausea, vomiting, chills, fever, and headache, and typically develop 6–72 h after ingestion. These symptoms can last up to 2–14 days depending on the initial concentration of bacteria ingested, serotype, age and the immune status of the host [2][3]. Salmonellosis in humans is primarily caused by non-typhoidal Salmonella (NTS), consisting of >1000 different serovars, with serovars Enteritidis and Typhimurium being the most common [4]. NTS infections can cause bacteremia in rare cases (i.e., 3–10%), which can lead to further complications, such as endocarditis, septic shock, infection of the liver, spleen, biliary tract and urinary tract, mesenteric lymphadenitis, pneumonia, osteomyelitis, pulmonary and brain abscess, empyema, meningitis, CNS infections and death [3]. In addition, perforation of the gut and necrosis of Peyer’s patches can result in toxic encephalopathy [5]. The risk of bacteremia is higher in immunosuppressed individuals, infants, and the elderly, and the number of infections has risen in recent times in correlation with increased incidences of the human immunodeficiency virus (HIV) and malaria.
The global surveillance of Salmonellosis is primarily conducted by the US Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO). In the United States, Salmonella strains cause 1.35 million reported infections, 26,500 hospitalizations and 420 deaths annually [6]. According to the United States Department of Health and Human Services, Salmonella infections are the leading cause of hospitalizations and death among foodborne pathogens. For Canadians, travel to endemic regions was the most common factor for reported NTS infections [4], specifically, travel to Central and South America, the Caribbean, Mexico and the Dominican Republic have seen the highest rates of infection [4]. Between 2009 and 2013, a yearly average of 6500 infections was reported throughout Canada, and in 2018, 7300 infections were reported, accounting for 48% of all notifiable isolates reported within the year [4]. In developing countries, Salmonella is responsible for 20% of childhood diarrheal infections and the mortality rate can reach as high as 24%, with certain serotypes having higher mortality rates than others [3]. However, 60–80% of Salmonellosis cases are thought to be either not diagnosed at all or are classified as sporadic cases [2][4].
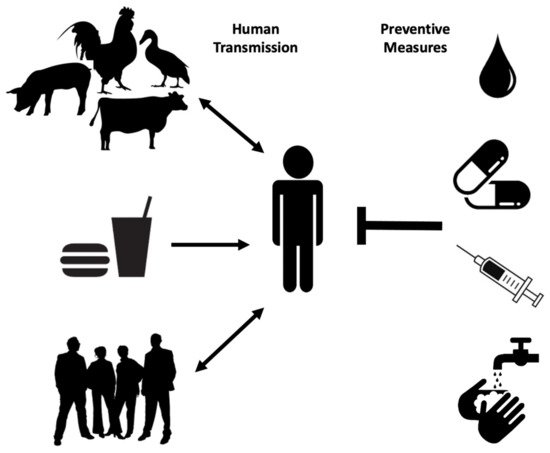
Human transmission occurs through ingestion of Salmonella -contaminated food (i.e., raw or undercooked poultry) or drinks, contact with infected animals, especially food animals and pets, and animal feed ( Figure 1 ). Person-to-person transmission through the fecal–oral route is also common and is facilitated by inadequate handwashing [3]. Preliminary diagnosis of salmonellosis in Canada is mainly based on symptoms and travel history and can be confirmed by serotyping (i.e., whole genome sequencing) each specimen from stool, rectal swabs, vomit, urine, deep tissue wounds, or sterile sites at the public health laboratories [4][7]. Treatment is based on symptoms and typically includes electrolyte replacement and rehydration, due to fluid loss that occurs through diarrhea and vomiting [2]. Commonly used first-line antibiotics include fluoroquinolones for adults and azithromycin for children. Ceftriaxone is used as an alternative first-line treatment [8]. However, antimicrobial therapy is usually reserved for immunocompromised patients, infants, children, the elderly and for severe cases of gastroenteritis, as unnecessary administration of antibiotics can select for resistant strains and contributes to prolonged shedding [2].
Multidrug-resistant Salmonella strains have emerged as far back as 1989 [3]. As of 1997, more than 70% of clinical isolates showed resistance to ampicillin, tetracycline, sulphonamides, streptomycin, and chloramphenicol, with few strains even resistant to nalidixic acid, trimethoprim, sulfamethoxazole, and kanamycin [9]. Ceftiofur resistance is considered significant because it is often correlated with resistance to other antibiotics commonly used, such as ceftriaxone, cefoxitin, amoxicillin-clavulanic acid, and ampicillin. As extra-intestinal Salmonellosis in young children and pregnant women is treated with Ceftriaxone, it is recognized as a drug of very high importance to human medicine. Thus, patients infected with even moderately Ceftriaxone-resistant Salmonella strains (i.e., Salmonella serovar Heidelberg) are at elevated risk of ceftriaxone therapy failure [10]. Proper sanitation, access to clean water supplies and treatment of infected individuals are among the best prophylactic measures to prevent the spread of Salmonellosis especially in endemic regions ( Figure 1 ) [3]. A vaccine against common NTS serovars would also represent a big step in reducing the global incidence of Salmonellosis and bacteremia.
2. Capsular Polysaccharides
During capsule biosynthesis, activated precursor molecules (monophosphate and diphosphate sugars) are assembled by inner membrane enzymes to form the growing polysaccharide chain. Following this, in the case of LPS assembly, the seven transport proteins Lpt A–G move the newly synthesized polysaccharide through the periplasm and across the outer membrane to the cell surface [11][12]. Studies have indicated the presence of transenvelope assembly complexes that coordinate simultaneous biosynthesis, export and translocation of the polysaccharide [13]. In some instances, identical translocation pathways are used for certain capsular polysaccharides and LPS O-Antigens [14].
During translocation, group 1 and 4 capsule assembly occurs at the periplasmic face of the plasma membrane and uses the Wzx pathway, while the assembly of group 2 and 3 capsules occurs at the cytoplasmic face of the plasma membrane and an ABC transporter is used for translocation. During the assembly of capsules from all four groups, the sequential action of glycosyltransferase enzymes joins individual repeat units together to elongate the polysaccharide [15].
As a major cell-surface component of many bacterial species, CPS can provide diverse functions. CPS often contribute to virulence, with innate and adaptive immune evasion; they also provide barrier protection, desiccation resistance, and act as lubricating agents and reduce friction over solid surfaces. For example, CPS facilitate swarming by Proteus mirabilis on solid surfaces [14]. It was observed that capsulated E. coli, Acinetobacter calcoaceticus, and Erwinia stewartii strains are more resistant to desiccation than non-capsulated strains [34]. In addition, CPS can allow bacterial adherence to host cells, surfaces, and to each other, thereby facilitating biofilm formation and colonization [35]. Biofilm formation is ubiquitous and can provide bacteria with nutritional advantages as well as protection from bacteriophage and phagocytic protozoa [12,36].
Studies have shown that pure carbohydrate antigens are T-cell-independent activators of B cells. Consequently, CPS antigens often induce immunoglobulin M (IgM) responses without a detectable level of IgG production. The absence of antibody class switching from IgM to IgG and the inability to produce higher titers of antibody upon subsequent exposure to the antigen are hallmarks of T-cell-independent antigens [16]. However, conjugation of polysaccharides to carrier proteins can lead to T-cell activation, memory B-cell production, and the development of polysaccharide-specific immune responses [37].
There are two types of CPS found in Salmonella —the Vi capsule (also known as Vi antigen) produced by Salmonella serovar Typhi, and the O-Antigen capsule produced by NTS. The Vi antigen is composed of O-acetylated α-1,4-linked N -acetylgalactosaminuronic acid residues and is attached to the cell surface via a reducing terminal lipid structure [16]. The Vi capsule increases virulence and disease severity of S . Typhi infection by interfering with complement deposition and subsequent neutrophil chemotaxis and bacterial clearance [5][17]. Deletion of genes for Vi capsule biosynthesis can remarkably enhance neutrophil chemotaxis in vitro. In addition, the presence of the Vi capsule prevents complement activation through the alternative pathway, as C3b is unable to deposit on the capsule surface and promotes opsonophagocytosis [17][18]. It has also been demonstrated that the Vi capsule promotes immune evasion by masking LPS molecules and preventing recognition through pathogen recognition receptors (PRR). As a consequence, Vi-positive S. Typhi does not induce neutrophil influx and can disseminate systemically and cause a persistent infection [5]. Furthermore, the capsule-mediated suppression of TNF-α production by human monocytes leads to low serum concentrations of pyrogenic cytokines and thereby prevents the development of septic shock [19][20].
The O-Antigen capsule produced by NTS is co-regulated with cellulose and fimbriae to be part of the extracellular matrix (ECM). The group 4 O-Antigen capsules were shown to be important for attachment to surfaces, colonization, and desiccation resistance [21]. S . Typhimurium O-Antigen capsule was shown to be expressed both at lower and higher temperatures indicating that the capsule may play a role in bacterial survival inside and outside the host, as described for other bacterial species, such as Hyphomonas strain MHS-3 [22][23]. Salmonella O-Ag capsule was initially hypothesized to be a CPS that was distinct from very long chain LPS O-Ag and assembled by the yih operons [21]. However, in our recent study, we hypothesize that O-Ag capsule and very-long-chain LPS O-Ag are the same CPS and demonstrated that the yihUTSRQP operon is not directly involved in capsule assembly [23].
3. Polysaccharide Vaccines
Since the late 20th century, polysaccharide vaccines have helped save the lives of millions of people worldwide. However, there are several limitations associated with pure polysaccharide vaccines. Mainly these include hyporesponsiveness and poor immunogenicity in children under the age of two [24]. In addition, the response for polysaccharide antigens among two to five-year-old children, the elderly, and the immunocompromised individuals are not optimal, and these populations remain at an elevated risk of contracting bacterial diseases [25]. Furthermore, due to hyporesponsiveness, pure polysaccharide vaccines are unable to provide long-lasting herd immunity, which is a key element in the prevention of invasive diseases. Herd immunity is achieved when the majority of a community is immunized against a given infectious disease, such that even those who have not received immunization are protected as the spread of the disease is contained [26].
To address the concerns of T-cell-independent immunogenicity of pure polysaccharide vaccines, polysaccharide antigens were chemically conjugated to carrier proteins in the late 1980s, resulting in polysaccharide–protein conjugates that were T-cell dependent and highly immunogenic [27]. The first polysaccharide–protein conjugate vaccine was produced for H. influenzae type-b. A conjugate vaccine for S. pneumonia was produced in 2000 and for N. meningitides in 2005. When these vaccines were introduced to the immunization schedules of multiple countries, it resulted in a significant reduction in the disease burden associated with encapsulated bacteria [28][29]. Conjugate vaccines have the advantage of inducing memory B-cell production, induction of immunity in children under the age of 2, and the capacity to overcome hyporesponsiveness [29][30].
A pure polysaccharide vaccine for Haemophilus influenzae type b (Hib) was first used in the United States in 1985, but due to poor efficacy in children younger than 18 months of age and immunological variability in older children, the vaccine was withdrawn from use in 1988 [31]. The pure polysaccharide Hib vaccine elicited an age-dependent antibody response, where older children and adults developed protective levels of antibodies with long-lasting efficacy [32][33], while younger children generated protective, but lower levels of antibody titers that decreased after one year from initial immunization. In addition, infants, being the most susceptible to Hib infection, were non-responsive to the pure polysaccharide Hib vaccine [31][33]. In clinical trials, a reduction in the nasal carriage with vaccine-specific S. pneumoniae serotypes in children was also observed following immunization with two multivalent pneumococcal conjugate vaccines [25][34]. Furthermore, in older children and adults, even a single dose of a conjugate vaccine leads to highly persistent anti-capsule antibodies [35][36].
In 1989, the pure Hib polysaccharide vaccine was replaced by a polysaccharide–protein conjugate vaccine linking the Hib polysaccharide polyribosylribitol phosphate to diphtheria toxoid, tetanus toxoid, or meningococcal outer membrane protein [37]. Subsequent immunizations with pure polysaccharide Hib or Hib conjugate vaccines do not generate booster responses in adults. However, multiple immunizations with Hib conjugate vaccine elicit booster responses in infants with elevated antibody titers [31][38][39]. The United Kingdom introduced the Hib conjugate vaccine to the infant immunization schedule in 1992 [37]. These vaccines are administered either alone or in combination with other vaccines for the protection of preschool children [37]. As a result, meningitis and other infections caused by Hib have been eliminated in countries that have incorporated the Hib conjugate vaccines into the infant immunization schedules [31][40].
4. Non-Typhoidal Salmonella Vaccines
There are several different types of NTS vaccine candidates currently being tested, which fall into three main categories: glycoconjugate, live attenuated, and subunit vaccines ( Table 1 ). Glycoconjugate vaccines covalently link a poorly immunogenic antigen, such as a bacterial surface polysaccharide, to a protein carrier molecule to activate CD4 + T cells. For glycoconjugation, most Salmonella vaccine strategies use the common carrier proteins tetanus toxoid, diphtheria toxoid, nontoxic recombinant diphtheria toxoid, or P. aeruginosa exoprotein A (rEPA) [41]. In one study, S . Typhimurium O-Antigens 4 and 12 covalently linked to bovine serum albumin (BSA) were administered with Freund’s adjuvant, leading to the production of high O4 specific antibody titers in rabbits, comparable to those elicited by immunization with heat-killed bacteria [42].
| Description | Developer | Academic/Commercial | Phase |
|---|---|---|---|
| Vaccines Licensed for S. Typhi | |||
| Neotyf, Typhoral, Vivotif (Ty21a live attenuated) | Johnson & Johnson | Commercial | Marketed |
| Bio Typh (Vi capsular polysaccharide) | BioMed | Commercial | Marketed |
| Typhim Vi, Typhyvax (Vi capsular polysaccharide) | Sanofi (Pasteur Merieux) | Commercial | Marketed |
| Typherix (Vi capsular polysaccharide) | GlaxoSmithKline | Commercial | Marketed |
| Peda-Typh (conjugate) | BioMed | Commercial | Marketed |
| Typbar-TCV (conjugate) | Bharat Biotech | Commercial | Marketed |
| Typhoid vaccine | Microgen | Commercial | Marketed |
| Vi Polysaccharide typhoid vaccine | China National Pharmaceutical (Beijing Tiantan Biological) | Commercial | Marketed |
| Vi-rEPA (Vi conjugate) | Lanzhou Institute (China) | Commercial | Marketed |
| Tyvax VI plus | VHB Life Sciences | Commercial | Marketed |
| Typhoid vaccine | Zydus Cadila (Zydus Vaccicare) | Commercial | Marketed |
| Tyrix Vi | SK Holdings | Commercial | Marketed |
| Shantyph | Sanofi (Shantha Biotechnics) | Commercial | Marketed |
| Typhobox, Typhovax | Green Cross | Commercial | Marketed |
| Zerotyph | Boryung | Commercial | Marketed |
| vax-TyVi | Finlay Institute | Commercial | Marketed |
| Typhoid-Kovax | Sanofi | Commercial | Marketed |
| Hepatyrix | GlaxoSmithKline | Commercial | Marketed |
| VIVAXIM | Sanofi | Commercial | Marketed |
| Biovac Typhoid | Wockhardt | Commercial | Marketed |
| Typho-Vi | Bio-Med | Commercial | Marketed |
| Vaccines in Development in Recent Years for S. Typhi | |||
| Vi-DT typhoid conjugate vaccine | Bio Farma | Commercial | Phase I |
| Typhetec | Prokarium | Commercial | Phase I |
| OmpC and OmpF | Institute Mexicano del Seguro Social | Academic | Phase 1 in Mexico |
| Enteric fever vaccine | Prokarium | Commercial | Phase II |
| M01ZH09 (live attenuated) | Emergent BioSolutions | Commercial | Phase II |
| CVD 909 (live attenuated) | University of Maryland | Academic | Phase II |
| Ty800 (live attenuated) | Avant Immunotherapeutics | Commercial | Phase II |
| Vi-rEPA (Vi conjugate) | National Health Institute | Academic | Phase III |
| Vi-CRM (Vi conjugate) | Biological E | Commercial | Phase III |
| Vi conjugated to fusion protein PsaA-PdT | Harvard Medical School | Academic | Preclinical |
| O:9-DT (conjugate) | International Vaccine Institute (IVI) | Academic | Preclinical |
| Salmonella typhi + paratyphi vaccine | Prokarium | Commercial | Research/Preclinical |
| Salmonella Vaccine Project | Affinivax | Commercial | Research/Preclinical |
| Vi conjugated to fusion protein PsaA-PdT | Harvard Medical School | Academic | Research/Preclinical |
| Ty21a typhoid vaccine expressing Shigella LPS | Protein Potential LLC. | Commercial | Research/Preclinical |
| O:2,12-DT + Vi-DT | International Vaccine Institute (IVI) | Academic | Research/Preclinical |
| O:2,12-CRM197 + Vi-CRM197 | Biological E | Commercial | Research/Preclinical |
| Vaccines in Development in Recent Years for S. Paratyphi | |||
| CVD 1902 (live attenuated) | University of Maryland, Bharat Biotech | Commercial | Phase I |
| Paratyphoid A conjugate vaccine | Lanzhou Institute (China) | Academic | Phase II |
| O:2-TT (conjugate) | Technology transfer from NIH to Lanzhou Institute (China) | Commercial | Phase II |
| O:2,12-DT + Vi-DT | International Vaccine Institute (IVI) | Academic | Research/Preclinical |
| O:2,12-CRM197 + Vi-CRM197 | Biological E | Commercial | Research/Preclinical |
| O:2-TT (conjugate) | Technology transfer from NIH to Chengdu Institute (China) | Academic | Preclinical |
| O:2-TT (conjugate) | Changchun Institute of Biological Products | Commercial | Preclinical |
| NTS Vaccines in Development in Recent Years | |||
| WT05—aroC and ssaV mutants (live attenuated) | Microscience, Wokingham Berkshire | Academic | Phase 1 |
| NTS Vaccine | University of Maryland | Academic | Research/Preclinical |
| CVD 1921 and CVD 1941 NTS Project (live attenuated) | University of Maryland, Bharat Biotec, Wellcome Trust | Commercial | Research/Preclinical |
| Bivalent iNTS-GMMA | GlaxoSmithKline (Novartis Vaccine Institute for Global Health; NVGH) | Commercial | Research/Preclinical |
| Bivalent Conjugate (O:1,4[5],12-CRM197 + O:1,9,12-CRM197) | GlaxoSmithLine (Novartis Vaccine Institute for Global Health; NVGH NVGH) | Commercial | Research/Preclinical |
| OmpD | University of Birmingham | Academic | Research/Preclinical |
| O:4,5/O:9-flagellin (conjugate) | University of Maryland | Academic | Preclinical |
| O:4, 12-TT (conjugate) | National Institutes for Health NIH) | Academic | Preclinical |
| Os-po (O:4 porin conjugate) | National Bacteriology Laboratory, Stockholm | Preclinical | |
| S. Typhimurium ruvB mutant (live attenuated) | Seoul National University | Academic | Preclinical |
| Salmonella hfq deletion mutant (live attenuated) | Indian Institute of Science Bangalore | Academic | Preclinical |
| SA186 (live attenuated) | Istituto Superiore di Sanita Roma | Academic | Preclinical |
| MT13 (live attenuated) | KIIT University Odisha | Academic | Preclinical |
| DNA adenine methylase mutants (live attenuated) | University of California, Santa Barbara | Academic | Preclinical |
| live attenuated, regulated delayed attenuation | Arizona State University | Academic | Preclinical |
a This is not a comprehensive list of all the vaccine candidates that are being tested, especially since this list does not reflect vaccine candidates tested in research studies. This information was compiled from the Boston Consulting Group—Vaccine pipeline information web page (https://vaccinesforamr.org/review-of-pathogens/vaccine-pipeline-information/#Salmonella_non-typhoidal, accessed on April 2021), National Institute of Health—U.S. National Library of Medicine ClinicalTrials.gov web page (https://clinicaltrials.gov/ct2/home, accessed on April 2021), and the MacLennan et al., 2014 research article [41].
Additionally, recombinant attenuated Salmonella vaccines (RASV) have been used as vectors to deliver vaccine antigens for various other bacterial pathogens. RASV expressing pneumococcal surface protein A (PspA) elicited an IgG response with a predominant rPspA specific IgG1 (Th2-type) and Salmonella LPS and OMP specific IgG2a (Th1-type) responses [43]. Similarly, oral immunization of mice with RASV vectors expressing genes from seven different serotypes of CPS from S. pneumoniae elicited a Th1-type immune response and subsequent in vitro clearance of the S. pneumoniae expressing the corresponding CPS serotype [44]. Immunization with RASV vectors expressing Salmonella and M. tuberculosis effector protein SopE resulted in similar observations [45]. Furthermore, RASVs with regulated delayed attenuation have seen significantly higher anti-PspA responses (i.e., IgG, IL-4, and INF-γ), compared to the controls [46]. These vaccines express wild-type phenotypes at immunization and are attenuated after colonization of the host tissues, by inactivating the virulence genes [46]. Thus, RASVs not only facilitate the induction of an immune response against the vaccine antigen from other bacteria but also induce protective responses against Salmonella -specific antigens [47][48][49].
Protein-based subunit vaccines are composed of multiple antigenic epitopes and can have broad coverage. A subunit approach is currently in use for multivalent pneumococcal and meningococcal conjugate vaccines. In another strategy to develop broad coverage, cross-protective vaccines can use highly conserved protein antigens produced through recombinant technology [50][51][52]. Proper antigens can be selected using bioinformatics analysis of whole-genome sequences and reverse vaccinology [53]. Examples of highly conserved Salmonella protein antigens include flagellin and porins (i.e., OmpC, F, and D). Both recombinant and purified protein vaccine candidates have been tested against conserved protein epitopes [2]. Subunit vaccines can induce both T-cell responses and antibody production; a balanced Th1-Th2 approach is vital to proper clearance of Salmonella . One of the downsides of subunit vaccines developed through recombinant technology is that it is difficult to maintain and preserve the proper conformation of proteins, especially when there are several membrane-spanning domains. This can result in the induction of a poor antibody response. One approach is to purify proteins from whole Salmonella cells rather than relying on recombinant proteins [52].
As glycoconjugate vaccines primarily rely on the production of antibodies specific for surface carbohydrate moieties, it may not be sufficient to efficiently deal with the growing iNTS disease. The development of new live-attenuated, protein-based, or Generalized Modules for Membrane Antigens (GMMA)-based vaccines will serve to provide broader protection by activating Salmonella -specific T-cell responses [41][54][55][56]. Vaccine development has been hindered due to a lack of understanding regarding specific antigenic epitopes of Salmonella and preservation of proper protein confirmations. GMMA technology (i.e., outer membrane blebs) is used to deliver outer membrane proteins and surface polysaccharides in the correct orientation and conformation to induce protective immunity. In addition, the membrane components of GMMAs have adjuvant activity by co-delivering multiple pathogen-associated molecular patterns (PAMPs) along with the target antigens [2]. In our hands, immunization of mice with GMMAs isolated from colanic acid overproducing strains was sufficient to significantly reduce Salmonella colonization [23]. It is possible that the development of a GMMA-CPS-based vaccine against NTS can be explored in the future.
5. Looking Ahead
The primary goal of this review was to provide a perspective of the current state of NTS vaccine development and to encourage vaccine candidate testing and clinical development. Despite the type of vaccine developed, in terms of delivery, time and the associated cost of administration, it will be advantageous to develop vaccines that require only one or two doses to mount an optimal immune response, compared to the required three doses of the current Ty21 vaccine [53]. Although the current vaccine pipeline is looking weak for NTS, there are candidates not listed in official databases, therefore the prospects are stronger than they appear. In our opinion, there needs to be a greater commitment of scientific expertise and availability of resources to accelerate vaccine candidates, especially taking S. Typhi as a proof of principle for vaccine development. Less expensive conjugate methods and technological advancements should be considered to facilitate vaccine accessibility in low-income countries. Subunit vaccines, especially those using glycoconjugate technologies, are costly to produce and since most vaccines are to be used in low-income countries, affordability remains a key factor in vaccine development. To balance the cost-effectiveness, it has been proposed that multivalent vaccines composed of 5 to 6 antigens could provide cross-protection against the majority of the iNTS serovars [127]. Furthermore, a better understanding of the regional burden of NTS/iNTS infections will enhance informed policy decisions.
An NTS vaccine would be both beneficial as a vaccine for travelers in developed countries, and as an early childhood vaccine in endemic regions. Although an NTS vaccine would require no new implementation provided the vaccine is introduced to childhood vaccination schedules, vaccination in high-disease-burden regions may require additional infrastructure for storage. One of the biggest hurdles is that commercial attractiveness for NTS vaccines remains low, given the demand remains relatively low in middle- and high-income countries. Thus, it should be noted that the limited candidates in the pipeline are a reflection of a lack of interest and resources to drive clinical testing, rather than a reflection of the feasibility of developing an NTS vaccine.
References
- Hurley, D.; McCusker, M.P.; Fanning, S.; Martins, M. Salmonella-host interactions—Modulation of the host innate immune system. Front. Immunol. 2014, 5, 481.
- WHO. Product Development for Vaccines Advisory Committee (PD-VAC) Meeting. 2015. Available online: http://www.who.int/immunization/research/meetings_workshops/pdvac/en/ (accessed on April 2021).
- Pathogen Safety Data Sheet: Infectious Substances—Salmonella Enterica Spp. Available online: http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/salmonella-ent-eng.php (accessed on April 2021).
- FoodNet Canada Annual Report 2016. Available online: https://www.canada.ca/en/public-health/services/surveillance/foodnet-canada/publications/foodnet-canada-annual-report-2016.html (accessed on April 2021).
- De Jong, H.K.; Parry, C.M.; van der Poll, T.; Wiersinga, W.J. Host-pathogen interaction in invasive salmonellosis. PLoS Pathog. 2012, 8, e1002933.
- Salmonella. Available online: https://www.cdc.gov/salmonella/index.html (accessed on April 2021).
- Salmonella Outbreak Investigations: Timeline for Reporting Cases. Available online: http://www.cdc.gov/salmonella/reportingtimeline.html (accessed on April 2021).
- Antibiotic Resistance Threats in the United States. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjiqq6U0sDwAhVH_54KHbTICiUQFjAOegQIEhAD&url=https%3A%2F%2Fwww.cdc.gov%2Fdrugresistance%2Fpdf%2Fthreats-report%2F2019-ar-threats-report-508.pdf&usg=AOvVaw1709sEktvf-ovR38riUreg (accessed on April 2021).
- Brisabois, A.; Cazin, I.; Breuil, J.; Collatz, E. Surveillance of antibiotic resistance in Salmonella. Eurosurveillance 1997, 2, 181.
- Salmonella Heidelberg Ceftiofur-Related Resistance in Human and Retail Chicken Isolates. Available online: http://www.phac-aspc.gc.ca/cipars-picra/heidelberg/heidelberg-eng.php (accessed on April 2021).
- Owens, T.W.; Taylor, R.J.; Pahil, K.S.; Bertani, B.R.; Ruiz, N.; Kruse, A.C.; Kahne, D. Structrual basis of unidirectional export of lipopolysaccharide to the cell surface. Nature 2019, 567, 550–553.
- Li, Y.; Orlando, B.J.; Liao, M. Structural basis of lipopolysaccharide extraction by the LptB2FGC complex. Nature 2019, 567, 486–490.
- Whitfield, C. Biosynthesis and assembly of capsular polysaccharides in escherichia coli. Annu. Rev. Biochem. 2006, 75, 39–68.
- Whitfield, C.; Amor, P.A.; Koplin, R. Modulation of the surface architecture of gram-negative bacteria by the action of surface polymer: Lipid a-core ligase and by determinants of polymer chain length. Mol. Microbiol. 1997, 23, 629–638.
- Whitfield, C.; Roberts, I.S. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol. Microbiol. 1999, 31, 1307–1319.
- Liston, S.D.; Ovchinnikova, O.G.; Whitfield, C. Unique lipid anchor attaches vi antigen capsule to the surface of Salmonella enterica serovar Typhi. Proc. Natl. Acad. Sci. USA 2016, 113, 6719–6724.
- Wangdi, T.; Lee, C.-Y.; Spees, A.M.; Yu, C.; Kingsbury, D.D.; Winter, S.E.; Hastey, C.J.; Wilson, R.P.; Heinrich, V.; Baumler, A.J. The vi capsular polysaccharide enables salmonella enterica serovar typhi to evade microbe-guided neutrophil chemotaxis. PLoS Pathog. 2014, 10, e1004306.
- Wilson, R.P.; Winter, S.E.; Spees, A.M.; Winter, M.G.; Nishimori, J.H.; Sanchez, J.F.; Nuccio, S.-P.; Crawford, R.W.; Tukel, C.; Baumler, A.J. The vi capsular polysaccharide prevents complement receptor 3-mediated clearance of Salmonella enterica serotype typhi. Infect. Immun. 2011, 79, 830–837.
- Raffatellu, M.; Chessa, D.; Wilson, R.P.; Tukel, C.; Akcelik, M.; Baumler, A.J. Capsule-mediated immune evasion: A new hypothesis explaining aspects of typhoid fever pathogenesis. Infect. Immun. 2006, 74, 19–27.
- Hirose, K.; Ezaki, T.; Miyake, M.; Li, T.; Khan, A.Q.; Kawamura, Y.; Yokoyama, H.; Takami, T. Survival of vi-capsulated and vi-deleted salmonella typhi strains in cultured macrophage expressing different levels of CD14 antigen. FEMS Microbiol. Lett. 1997, 147, 259–265.
- Gibson, D.L.; White, A.P.; Snyder, S.D.; Martin, S.; Heiss, C.; Azadi, P.; Surette, M.; Kay, W.W. Salmonella produces an o-antigen capsule regulated by agfd and important for environmental persistence. J. Bacteriol. 2006, 188, 7722–7730.
- Quintero, E.; Weiner, R.M. Evidence for the adhesive function of the exopolysaccharide of hyphomonas strain MHS-3 in its attachment to surfaces. Appl. Environ. Microb. 1995, 61, 1897–1903.
- Sokaribo, A.S.; Perera, S.R.; Sereggela, Z.; Krochak, R.; Balezantis, L.R.; Xing, X.; Lam, S.; Deck, W.; Attah-Poku, S.; Abbott, D.W.; et al. A GMMA-CPS-based vaccine for non-typhoidal salmonella. Vaccines 2021, 9, 165.
- Wilder-Smith, A. Meningococcal vaccines: A neglected topic in travel medicine? Expert Rev. Vaccines 2009, 8, 1343–1350.
- Sood, R.K.; Fattom, A. Capsular polysaccharide-protein conjugate vaccines and intravenous immunoglobulins. Expert Opin. Investig. Drugs. 1998, 7, 333–347.
- State Exemption Levels Low, National Vaccination Rates High. Available online: https://www.cdc.gov/media/releases/2015/p0827-vaccination-rates.html (accessed on April 2021).
- Makela, P.H.; Kayhty, H. Evolution of conjugate vaccines. Expert Rev. Vaccines 2002, 1, 399–410.
- Ramsay, M.E.; McVernon, J.; Andrews, N.J.; Heath, P.T.; Slack, M.P. Estimating haemophilus influenzae type b vaccine effectiveness in England and wales by use of the screening method. J. Infect. Dis. 2003, 188, 481–485.
- Heath, P.T.; McVernon, J. The UK hib vaccine experience. Arch. Dis. Child. 2002, 86, 396–399.
- Ramsay, M.E.; Andrews, N.J.; Trotter, C.L.; Kaczmarski, E.B.; Miller, E. Herd immunity form meningococcal serogroup C conjugate vaccination in England: Database analysis. BMJ 2003, 326, 365–366.
- Robbins, J.B.; Chu, C.; Schneerson, R. Hypothesis for vaccine development: Protective immunity to enteric diseases caused by nontyphoidal salmonella and shigellae may be conferred by serum igG antibodies to the O-specific polysaccharide of their lipopolysaccharides. Clin. Infect. Dis. 1992, 15, 346–361.
- Rodrigues, L.P.; Schneerson, R.; Robbins, J.B. Immunity to hemophilus influenzae type b. i. The isolation, and some physicochemical, serologic and biologic properties of the capsular polysaccharide of hemophilus influenzae type b. J. Immunol. 1971, 107, 1071–1080.
- Smith, D.H.; Peter, G.; Ingram, D.L.; Harding, A.L.; Anderson, P. Responses of children immunized with the capsular polysaccharide of hemophilus influenzae type B. Pediatrics 1973, 52, 637–644.
- Dagan, R.; Melamed, R.; Muallem, M.; Piglansky, L.; Greenberg, D.; Abramson, O.; Mendelman, P.M.; Bohidar, N.; Yagupsky, P. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J. Infect. Dis. 1996, 174, 1271–1278.
- Snape, M.D.; Kelly, D.F.; Salt, P.; Green, S.; Snowden, C.; Diggle, L.; Borkowski, A.; Yu, L.-M.; Moxon, R.; Pollard, A.J. Serogroup C meningococcal glycoconjugate vaccine in adolescents: Persistence of bactericidal antibodies and kinetics of the immune reponse to a booster vaccine more than 3 years after immunization. Clin. Infect. Dis. 2006, 43, 1387–1394.
- Snape, M.D.; Kelley, D.F.; Lewis, S.; Banner, C.; Kibwana, L.; Moore, C.E.; Diggle, L.; John, T.; Yu, L.-M.; Borrow, R.; et al. Seroprotection against serogroup C meningococcal disease in adolescents in the United Kingdom: Observational study. BMJ 2008, 336, 1487–1491.
- Pollard, A.J.; Perrett, K.P.; Beverley, P.C. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat. Rev. Immunol. 2009, 9, 213–220.
- Parke, J.C.; Schneerson, R.; Relmer, C.; Black, C.; Welfare, S.; Bryla, D.; Levi, L.; Pavliakova, D.; Cramton, T.; Schulz, D.; et al. Clinical and immunologic reponses to haemophilus influenzae type b-tetanus toxoid conjugate vaccine in infants injected at 3,5,7 and 18 months of age. J. Pediatr. 1991, 118, 184–190.
- Claesson, B.A.; Trolfors, B.; Lagergard, T.; Taranger, J.; Bryla, D.; Otterman, G.; Cramton, T.; Yang, Y.; Reimer, C.B.; Robbins, J.B.; et al. Clinical and immunologic responses to capsular polysaccharide of haemophilus influenzae type b alone or conjugated to tetanus toxoid in 18- to 23-month-old children. J. Pediatr. 1988, 112, 695–702.
- Adams, W.G.; Deaver, K.A.; Cochi, S.L.; Plikaytis, B.D.; Zell, E.R.; Broome, C.V.; Wenger, J.D. Decline of childhood haemophilus influenzae type b (Hib) disease in the Hib vaccine era. JAMA—J. Am. Med. Assoc. 1993, 269, 221–226.
- MacLennan, C.A.; Martin, L.B.; Micoli, F. Vaccines against invasive salmonella disease. Hum. Vaccines Immunother. 2014, 10, 1478–1493.
- Svenson, S.B.; Lindberg, A.A. Artificial Salmonella vaccines: Salmonella typhimurium O-antigen-specific oligosaccharide-protein conjugates elicit protective antibodies in rabbits and mice. Infect. Immun. 1981, 32, 490–496.
- Kang, H.Y.; Srinivasan, J.; Curtiss, R., III. Immune responses to recombinant pneumococal PspA antigen delivered by live attenuated Salmonella enterica serovar typhimurium vaccine. Infect. Immun. 2002, 70, 1739–1749.
- Su, H.; Liu, Q.; Bian, X.; Wang, S.; Curtiss, R., 3rd; Kong, Q. Synthesis and delivery of streptococcus pneumoniae capsular polysaccharides by recombinant attenuated Salmonella vaccines. Proc. Natl. Acad. Sci. USA 2021, 118, e2013350118.
- Juarez-Rodriguez, M.D.; Arteaga-Cortes, L.T.; Kader, R.; Curtiss, R., 3rd. Live attenuated Salmonella vaccines against mycobacterium tuberculosis with antigen delivery via the type III secretion system. Infect. Immun. 2012, 80, 798–814.
- Li, Y.; Wang, S.; Scarpellini, G.; Gunn, B.; Xin, W.; Wanda, S.-Y.; Roland, K.L.; Curtis, R., 3rd. Evaluation of new generation of Salmonella enterica serovar typhimurium vaccines with regulated delayed attenuation to induce immune responses against PspA. Proc. Natl. Acad. Sci. USA 2009, 106, 593–598.
- Wang, S.; Kong, Q.; Curtis, R., 3rd. New technologies in developing recombinant attenuated Salmonella vaccine vectors. Microb. Pathog. 2013, 58, 17–28.
- Wang, S.; Li, Y.; Scarpellini, G.; Kong, W.; Shi, H.Y.; Baek, C.-H.; Gunn, B.; Wanda, S.-Y.; Roland, K.L.; Zhang, X.; et al. Salmonella vaccine vectors displaying delayed antigen synthesis in vivo to enhance immunogenicity. Infect. Immun. 2010, 78, 3969–3980.
- Kang, H.Y.; Curtis, R., 3rd. Immune responses dependent on antigen location in recombinant attenuated Salmonella typhimurium vaccines following oral immunization. FEMS Immunol. Med. Mic. 2003, 37, 99–104.
- Gil-Cruz, C.; Bobat, S.; Marshall, J.L.; Kingsley, R.A.; Ross, E.A.; Henderson, I.R.; Leyton, D.L.; Coughlan, R.E.; Khan, M.; Jensen, K.T.; et al. The porin ompd from nontyphoidal salmonella is a key target for a protective B1b cell antibody response. Proc. Natl. Acad. Sci. USA 2009, 106, 9803–9808.
- Secundino, I.; Lopez-Macias, C.; Cervantes-Barragan, L.; Gil-Cruz, C.; Rios-Sarabia, N.; Pastelin-Palacios, R.; Villasis-Keever, M.A.; Becker, I.; Puente, J.L.; Calva, E.; et al. Salmonella porins induce a sustained, lifelong specific bactericidal antibody memory response. Immunology 2005, 117, 59–70.
- Salazar-Gonzalez, R.M.; Maldonado-Bernal, C.; Ramirez-Cruz, N.E.; Rios-Sarabia, N.; Beltran-Nava, J.; Castanon-Gonzalez, J.; Castillo-Torres, N.; Palma-Aguirre, J.A.; Carrera-Camargo, M.; Lopez-Macias, C.; et al. Induction of cellular immune response and anti-Salmoneall enterica serovar typhi bactericidal antibodies in healthy volunteers by immunization with a vaccine candidate against typhoid fever. Immunol. Lett. 2004, 93, 115–122.
- Sette, A.; Rappuoli, R. Reverse vaccinology: Developing vaccines in the era of genomics. Immunity 2010, 33, 530–541.
- Fiorino, F.; Pettini, E.; Koeberling, O.; Ciabattini, A.; Pozzi, G.; Martin, L.B.; Medaglini, D. Long-term anti-bacterial immunity against systemic infection by salmonella enterica serovar typhimurium elicited by a gmma-based vaccine. Vaccines 2021, 9, 495.
- Micoli, F.; Bjarnarson, S.P.; Arcuri, M.; Pindi, A.A.A.; Magnusdottir, G.J.; Necchi, F.; Benedetto, R.D.; Carducci, M.; Schiavo, F.; Giannelli, C.; et al. Short vi-polysaccharide abroages t-independent immune response and hyporesponsiveness elicited by long Vi-CRM197 conjugate vaccine. Proc. Natl. Acad. Sci. USA 2020, 117, 24443–24449.
- Micoli, F.; Alfini, R.; Di Benedetto, R.; Necchi, F.; Schiavo, F.; Mancini, F.; Carducci, M.; Palmieri, E.; Balocchi, C.; Gasperini, G.; et al. GMMA is a versatile platform to design effective multivalent combination vaccines. Vaccines 2020, 8, 540.
