Posterior capsule opacification (PCO) is the most common complication arising from the corrective surgery used to treat cataract patients. PCO arises when lens epithelial cells (LEC) residing in the capsular bag post-surgery undergo hyper-proliferation and transdifferentiation into myofibroblasts, migrating from the posterior capsule over the visual axis of the newly implanted intraocular lens (IOL). The developmental pathways underlying PCO are yet to be fully understood and the current literature is contradictory regarding the impact of the recognised risk factors of PCO. The aim of this review is firstly to collate the known biochemical pathways that lead to PCO development, providing an up-to-date chronological overview from surgery to established PCO formation. Secondly, the risk factors of PCO are evaluated, focussing on the impact of IOLs’ properties. Finally, the latest experimental model designs used in PCO research are discussed to demonstrate the ongoing development of clinical PCO models, the efficacy of newly developed IOL technology, and potential therapeutic interventions. This review will contribute to current PCO literature by presenting an updated overview of the known developmental pathways of PCO, an evaluation of the impact of the risk factors underlying its development, and the latest experimental models used to investigate PCO. Furthermore, the review should provide developmental routes for research into the investigation of potential therapeutic interventions and improvements in IOL design in the aid of preventing PCO for new and existing patients.
- posterior capsule opacification
- pathophysiology
- wound-healing response
- experimental models
- lens epithelial cells
- intraocular lenses
1. Introduction
1.1. Pathophysiology of Posterior Capsule Opacification
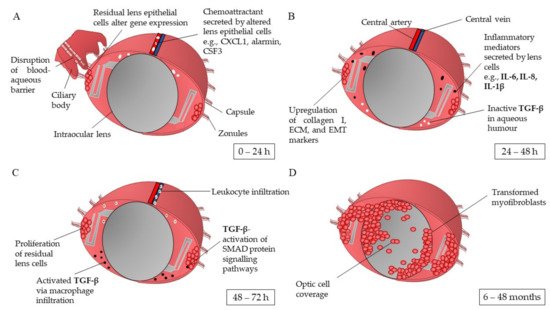
1.2. Risk Factors for Developing Posterior Capsule Opacification
1.3. Therapeutic Interventions for Posterior Capsule Opacification
1.4. Capsular Devices to Prevent Posterior Capsule Opacification Development
2. Experimental Models to Investigate Posterior Capsule Opacification
2.1. In Vitro Models
2.2. In Vivo Models
In vivo models can be exploited in many aspects of PCO research. Such applications include the investigation of intraocular lenses, underlying biomolecular mechanisms, and the efficacy of surgical interventions, and the testing of therapeutic inhibitors of developmental pathways [17][67][68][69][70]. Animal donors include murine, rabbit, and porcine species. However, caution is required when comparing and extrapolating pathological responses between animals and humans due to differences in species biology [1][17]. Furthermore, each animal donor type has its own limitations.2.3. Ex Vivo Models
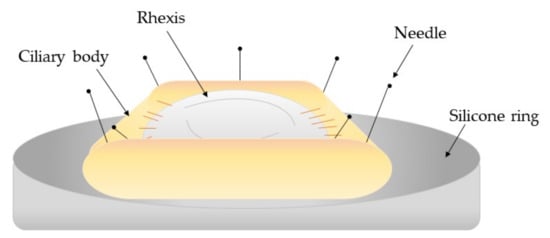
2.4. Clinical Studies
3. Conclusions
References
- Wormstone, I.M.; Wang, L.; Liu, C.S.C. Posterior capsule opacification. Exp. Eye Res. 2009, 88, 257–269.
- Nibourg, M.L.; Gelens, E.; Kuijwe, R.; Hooymans, J.M.M.; Kooten Gvan, T.; Koopmans, A.S. Prevention of posterior capsular opacification. Exp. Eye Res. 2015, 136, 100–115.
- Goshe, J.; Awh, C.; Houser, K. Posterior Capsule Opacification. Am. J. Ophthalmol. 2020, 1. Available online: (accessed on 13 September 2020).
- Sinha, R.; Shekhar, H.; Sharma, N.; Titiyal, J.S.; Vajpayee, R.B. Posterior capsular opacification: A review. Indian J. Ophthalmol. 2013, 61, 371–376.
- Ma, B.; Yang, L.; Jing, R.; Liu, J.; Quan, Y.; Hui, Q.; Li, J.; Qin, L.; Pei, C. Effects of Interleukin-6 on posterior capsular opacification. Exp. Eye Res. 2018, 172, 94–103.
- Meacock, W.R.; Spalton, D.J.; Stanford, M.R. Role of cytokines in the pathogenesis of posterior capsule opacification. Br. J. Ophthalmol. 2000, 84, 332–336.
- Awasthi, N.; Guo, S.; Wagner, B.J. Posterior capsular opacification: A problem reduced but not yet eradicated. JAMA Ophthalmol. 2009, 127, 555–562.
- Trent, N. Global intraocular Lens market 2019: Size, share, demand, trends, growth and 2022 forecasts explored in latest research. Wise Guy Rep. 2018, 1–2.
- Vasavada, A. Cataract and surgery for cataract. Br. Med. J. 2006, 333, 128–132.
- Laser Eye Surgery Hub. Cataract Statistics & Resources. 2018, p. 1. Available online: (accessed on 9 November 2020).
- Ursell, P.G.; Dhariwal, M.; Majirska, K.; Ender, F.; Klason-Ray, S.; Venerus, A.; Miglio, C.; Bouchet, C. Three-year incidence of Nd:YAG capsulotomy and posterior capsule opacification and its relationship to monofocal acrylic IOL biomaterial: A UK real world evidence study. Eye 2018, 32, 1579–1589.
- Sadoughi, M.; Einollahi, B.; Roshandel, D.; Sarimohammadli, M.; Feizi, S. Visual and refractive outcomes of phacoemulsification with implantation of accommodating versus standard monofocal intraocular lenses. J. Ophthalmic Vis. Res. 2015, 10, 370–374.
- Jaffe, N.S. History of cataract surgery. Ophthalmology. 1996, 103, 5–16.
- Raj, M.S.; Vasavada, A.R.; Johar, S.R.V.; Vasavadam, A.V.; Vasavada, A.V. Post-operative capsular opacification: A review. Int. J. Biomed. Sci. 2007, 3, 237–250.
- Davidson, M.G.; Morgan, D.K.; McGahan, M.C. Effect of surgical technique on in vitro posterior capsule opacification. J. Cataract Refract. Surg. 2000, 26, 1550–1554.
- Jiang, J.; Shihan, M.H.; Wang, Y.; Duncan, M.K. Lens epithelial cells initiate an inflammatory response following cataract surgery. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4986–4997.
- Wormstone, I.M.; Eldred, J.A. Experimental models for posterior capsule opacification research. Exp. Eye Res. 2016, 142, 2–12.
- Mohammadpour, M.; Jafarinasab, M.R.; Javadi, M.A. Outcomes of acute postoperative inflammation after cataract surgery. Eur. J. Ophthalmol. 2007, 17, 20–28.
- Perez-Vives, C. Biomaterial influence on intraocular lens performance: An overview. J. Ophthalmol. 2018, 15, 2687385.
- Rouillard, A.; Gundersen, G.; Fernandez, N.; Wang, Z.; Monteiro, C.; McDermott, M.; Ma’ayan, A. The harmonizome: A collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database 2016, 2016, baw100.
- Lewis, A.C. Interleukin-6 in the pathogenesis of posterior capsule opacification and the potential role for interleukin-6 inhibition in the future of cataract surgery. Med. Hypotheses 2013, 80, 466–474.
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27.
- Nishi, O.; Nishi, K.; Ohmoto, Y. Synthesis of interleukin-1, interleukin-6, and basic fibroblast growth factor by human cataract lens epithelial cells. J. Cataract Refract. Surg. 1996, 22, 852–858.
- Basu, A.; Krady, J.K.; Levison, S.W. Interleukin-1: A master regulator of neuroinflammation. J. Neurosci. Res. 2004, 78, 151–156.
- Ferrick, M.R.; Thurau, S.R.; Oppenheim, M.H.; Herbort, C.P.; Ni, M.; Zachariae, C.O.C.; Matsushima, K.; Chan, C.C. Ocular inflammation stimulated by intravitreal interleukin-8 and interleukin-1. Investig. Ophthalmol. Vis. Sci. 1991, 32, 1534–1539.
- Xiong, W.; Frasch, S.C.; Thomas, S.M.; Bratton, D.L.; Henson, P.M. Induction of TGF-β1 synthesis by macrophages in response to apoptotic cells requires activation of the scavenger receptor CD36. PLoS ONE 2013, 8, e72772.
- Wallentin, N.; Wickström, K.; Lundberg, C. Effect of cataract surgery on aqueous TGF-β and lens epithelial cell proliferation. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1410–1418.
- Walton, K.L.; Johnson, K.E.; Harrison, C.A. Targeting TGF-β mediated SMAD signaling for the prevention of fibrosis. Front. Pharmacol. 2017, 8, 461.
- Taiyab, A.; Holms, J.; West-Mays, J.A. β-Catenin/Smad3 interaction regulates transforming growth factor-β-induced epithelial to mesenchymal transition in the lens. Int. J. Mol. Sci. 2019, 20, 2078.
- Saika, S. TGFbeta pathobiology in the eye. Lab. Investig. 2006, 86, 106–115.
- Weizman Institute of Science. S100A9 gene S100 calcium binding protein A9. Hum. Gene Database 2020. Available online: (accessed on 9 November 2020).
- Nishi, O.; Nishi, K.; Ohmoto, Y. Effect of interleukin 1 receptor antagonist on the blood-aqueous barrier after intraocular lens implantation. Br. J. Ophthalmol. 1994, 78, 917–920.
- Wilson, S.E.; Esposito, A. Focus on molecules: Interleukin-1: A master regulator of the corneal response to injury. Exp. Eye Res. 2009, 89, 124–125.
- Ooi, K.G.J.; Galatowicz, G.; Calder, V.L.; Lightman, S.L. Cytokines and chemokines in uveitis—Is there a correlation with clinical phenotype? Clin. Med. Res. 2006, 4, 294–309.
- Kubo, E.; Shibata, S.; Shibata, T.; Kiyokawa, E.; Sasaki, H.; Singh, D.P. FGF2 antagonizes aberrant TGFβ regulation of tropomyosin: Role for posterior capsule opacity. J. Cell Mol. Med. 2017, 21, 916–928.
- Kubo, E.; Shibata, T.; Singh, P.D.; Sasaki, H. Roles of TGFB and FGF signals in the lens: Tropomyosin regulation for posterior capsule opacification. Int. J. Mol. Sci. 2018, 19, 3093.
- Tandon, A.; Tovey, J.C.K.; Sharma, A.; Gupta, R.; Mohan, R.R. Role of transforming growth factor beta in corneal function, biology and pathology. Curr. Mol. Med. 2012, 10, 565–578.
- Nishi, O.; Nishi, K.; Wada, K.; Ohmoto, Y. Expression of transforming growth factor (TGF)-α, TGF-β2 and interleukin 8 messenger RNA in postsurgical and cultured lens epithelial cells obtained from patients with senile cataracts. Graefe’s Arch. Clin. Exp. Ophthalmol. 1999, 237, 806–811.
- Yamashita, H. Functions of the transforming growth factor-β superfamily in eyes. J. Jpn. Ophthalmol. Soc. 1997, 101, 927–947.
- Pei, C.; Ma, B.; Kang, Q.-Y.; Qin, L.; Cui, L.-J. Effects of transforming growth factor beta-2 and connective tissue growth factor on induction of epithelial mesenchymal transition and extracellular matrix synthesis in human lens epithelial cells. Int. J. Ophthalmol. 2013, 6, 752–757.
- Cho, H.J.; Baek, K.E.; Saika, S.; Jeong, M.J.; Yoo, J. Snail is required for transforming growth factor-beta-induced epithelial-mesenchymal transition by activating PI3 kinase/Akt signal pathway. Biochem. Biophys. Res. Commun. 2007, 353, 337–343.
- Lois, N.; Taylor, J.; McKinnon, A.D.; Smith, G.C.; Van’t Hof, R.; Forrester, J.V. Effect of TGF-β2 and anti-TGF-β2 antibody in a new in vivo rodent model of posterior capsule opacification. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4260–4266.
- Gotoh, N.; Perdue, N.R.; Matsushima, H.; Sage, E.H.; Yan, Q.; Clark, J.I. An in vitro model of posterior capsular opacity: SPARC and TGF-β2 minimize epithelial-to-mesenchymal transition in lens epithelium. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4679–4687.
- Amoozgar, B.; Fitzpatrick, S.D.; Sheardown, H. Effect of anti-TGF-β2 surface modification of polydimethylsiloxane on lens epithelial cell markers of posterior capsule opacification. J. Bioact. Compat. Polym. 2013, 28, 637–651.
- Kurosaka, D.; Kato, K.; Nagamoto, T. Presence of alpha smooth muscle actin in lens epithelial cells of aphakic rabbit eyes. Br. J. Ophthalmol. 1996, 80, 906–910.
- Wormstone, I.M.; Tamiya, S.; Anderson, I.; Duncan, G. TGF-β2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2301–2308.
- Wu, S.; Tong, N.; Pan, L.; Jiang, X.; Li, Y.; Guo, M.; Li, H. Retrospective analyses of potential risk factors for posterior capsule opacification after cataract surgery. J. Ophthalmol. 2018, 2018, 9089285.
- Spalton, D.J. Posterior capsular opacification after cataract surgery. Eye 1999, 13, 489–492.
- Elgohary, M.A.; Dowler, J.G. Incidence and risk factors of Nd:YAG capsulotomy after phacoemulsification in non-diabetic and diabetic patients. Clin. Exp. Ophthalmol. 2006, 34, 526–534.
- Burq, M.A.; Taqui, A.M. Frequency of retinal detachment and other complications after neodymium:YAG capsulotomy. J. Cataract Refract. Surg. 2008, 58, 550–552.
- Werner, L.; Mamalis, N.; Pandey, S.K.; Izak, A.M.; Nilson, C.D.; Davis, B.L.; Weight, C.; Apple, D.J. Posterior capsule opacification in rabbit eyes implanted with hydrophilic acrylic intraocular lenses with enhanced square edge. J. Cataract Refract. Surg. 2004, 30, 2403–2409.
- Mencucci, R.; Favuzza, E.; Boccalini, C.; Gicquel, J.J.; Raimondi, L. Square-edge intraocular lenses and epithelial lens cell proliferation: Implications on posterior capsule opacification in an in vitro model. BMC Ophthalmol. 2015, 15, 1–5.
- Nixon, D.R.; Woodcock, M.G. Pattern of posterior capsule opacification models 2 years postoperatively with 2 single-piece acrylic intraocular lenses. J. Cataract Refract. Surg. 2010, 36, 929–934.
- Shao, J.Z.; Qi, Y.; Du, S.S.; Du, W.W.; Li, F.Z.; Zhang, F.Y. In vitro inhibition of proliferation, migration and epithelial-mesenchymal transition of human lens epithelial cells by fasudil. Int. J. Ophthalmol. 2018, 11, 1253–1257.
- Kramer, G.D.; Werner, L.; Mamalis, N. Prevention of postoperative capsular bag opacification using intraocular lenses and endocapsular devices maintaining an open or expanded capsular bag. J. Cataract Refract. Surg. 2016, 42, 469–484.
- Hasanee, K.; Butler, M.; Ahmed, I.I.K. Capsular tension rings and related devices: Current concepts. Curr. Opin. Ophthalmol. 2006, 17, 31–41.
- Hara, T.; Hara, T.; Yamada, Y. “Equator ring” for maintenance of the completely circular contour of the capsular bag equator after cataract removal. Ophthalmic Surg. 1991, 22, 358–359.
- Hara, T.; Hara, T.; Narita, M. Long-term study of posterior capsular opacification prevention with endocapsular equator rings in humans. Arch Ophthalmol. 2011, 129, 855–863.
- Menapace, R.; Sacu, S.; Georgopoulos, M.; Findl, O.; Rainer, G.; Nishi, O. Efficacy and safety of capsular bending ring implantation to prevent posterior capsule opacification: Three-year results of a randomized clinical trial. J. Cataract Refract. Surg. 2008, 34, 1318–1328.
- Nishi, O.; Nishi, K.; Menapace, R.; Akura, J. Capsular bending ring to prevent posterior capsule opacification: 2 year follow-up. J. Cataract Refract. Surg. 2001, 27, 1359–1365.
- Slutzky, L.; Kleinmann, G. Further enhancement of intraocular open-capsule devices for prevention of posterior capsule opacification. Transl. Vis. Sci. Technol. 2018, 7, 21.
- Alon, R.; Assia, E.I.; Kleinmann, G. Prevention of posterior capsule opacification by an intracapsular open capsule device. Lens 2014, 55, 4005–4013.
- Chung, K.K.; Schumacher, J.F.; Sampson, E.M.; Burne, R.; Antonelli, P.J.; Brennan, A.B.; Reddy, S.T. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases 2007, 2, 89–94.
- VanSlyke, J.K.; Boswell, B.A.; Musil, L.S. Fibronectin regulates growth factor signaling and cell differentiation in primary lens cells. J. Cell Sci. 2018, 131, 22.
- Wertheimer, C.; Liegl, R.; Kernt, M.; Mayer, W.; Docheva, D.; Kampik, A.; Eibl-Lindner, K.H. EGF receptor inhibitor erlotinib as a potential pharmacological prophylaxis for posterior capsule opacification. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 1529–1540.
- Zukin, L.M.; Pedler, M.G.; Groman-Lupa, S.; Pantcheva, M.; Ammar, D.A.; Mark Petrash, J. Aldose reductase inhibition prevents development of posterior capsular opacification in an in vivo model of cataract surgery. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3591–3598.
- Han, Y.; Tang, J.; Xia, J.; Wang, R.; Qin, C.; Liu, S.; Zhao, X.; Chen, H.; Lin, Q. Anti-adhesive and antiproliferative synergistic surface modification of intraocular lens for reduced posterior capsular opacification. Int. J. Nanomed. 2019, 14, 9047–9061.
- Shihan, M.H.; Kanwar, M.; Wang, Y.; Jackson, E.E.; Faranda, A.P.; Duncan, M.K. Fibronectin has multifunctional roles in posterior capsular opacification (PCO). Matrix Biol. 2020, 90, 79–108.
- Gerhart, J.; Withers, C.; Gerhart, C.; Werner, L.; Mamalis, N.; Bravo-Nuevo, A.; Scheinfeld, V.; FitzGerald, P.; Getts, R.; George-Weinstein, M. Myo/Nog cells are present in the ciliary processes, on the zonule of Zinn and posterior capsule of the lens following cataract surgery. Exp. Eye Res. 2018, 171, 101–105.
- Sternberg, K.; Terwee, T.; Stachs, O.; Guthoff, R.; Lobler, M.; Schmitz, K.P. Drug-induced secondary cataract prevention: Experimental ex vivo and in vivo results with disulfiram, methotrexate and actinomycin. D. Ophthalmic Res. 2010, 44, 225–236.
- Wormstone, I.M. The human capsular bag model of posterior capsule opacification. Eye 2019, 34, 225–231.
- Kassumeh, S.A.; Wertheimer, C.M.; von Studnitz, A.; Hillenmayer, A.; Priglinger, C.; Wolf, A.; Mayer, W.J.; Teupser, D.; Holdt, L.M.; Priglinger, S.G.; et al. Poly(lactic-co-glycolic) Acid as a slow-release drug-carrying matrix for methotrexate coated onto intraocular lenses to conquer posterior capsule opacification. Curr. Eye Res. 2018, 43, 702–708.
- D’Antin, J.C.; Barraquer, R.I.; Tresserra, F.; Michael, R. Prevention of posterior capsule opacification through intracapsular hydrogen peroxide or distilled water treatment in human donor tissue. Sci. Rep. 2018, 8, 1–10.
- Eldred, J.A.; Zheng, J.; Chen, S.; Wormstone, I.M. An in vitro human lens capsular bag model adopting a graded culture regime to assess putative impact of iols on pco formation. Investig. Ophthalmol. Vis. Sci. 2019, 60, 113–122.
- Aronson, J.K. What is a clinical trial? Br. J. Clin. Pharmacol. 2004, 58, 1–3.
