There are reported types of hearing loss includes noise-induced, genetic, sudden, ototoxicity, and age-related hearing loss. The application of exosomes for prevention or treatment can be classified according to the type of hearing loss, suggesting that they may help restore abnormalities in chemical and biological mechanism. The fundamental recovery of inner hair cells by exosome is difficult, because both noise-induced and sudden hearing loss occur due to physical stimulation of hair cell death.
- mesenchymal stem cell
- exosome
- auditory
- stem cell
- hearing loss
1. Strategies for Exosome Treatment
To allow overexpression of useful candidate factors with usually low expression levels in exosomes, a transporter capable of transferring the factors to MVB is required; furthermore, a technology for developing differentiated exosomes, in which the expressed stem cell exosomes can directly target specific receptor cells is being developed [1][2]. Biocompatibility of these exosomes can be demonstrated by mimicking molecular biological signaling mechanisms [3], and through the discovery of additional biomarkers, stem cell-derived exosomes can be applied not only for differentiation, antioxidation, and immunity, but also to regenerative medicine [1][2]. The fundamental recovery of inner hair cells by exosome is difficult, because both noise-induced and sudden hearing loss occur due to physical stimulation of hair cell death. In addition, the application of exosomes for prevention and treatment for hearing restoration is difficult because genetic hearing loss is caused by developmental disorders in the embryonic period.
Numerous researchers have reported applied studies using exosomes extracted from cultured media [4][5][6]. From a biological point of view, most studies have attempted to repair damaged tissues by exosomes treatment, which can be obtained from cell cultures and biofluids (blood, urine, etc.) [7][8][9]. In addition, the extracted exosomes can decrease inflammatory molecules in the infected cells, thereby increasing cell viability [10][11].
Various exosome isolation methods have been proposed to introduce exosomes directly into the cells or injured tissues (Figure 1b(i)).
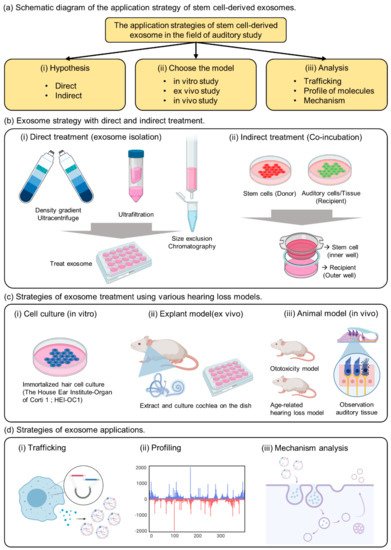
Figure 1. The summary of strategies for exosome application in the auditory field. (a) Schematic diagram of the application strategy of stem cell-derived exosomes. (b) Exosome strategy with direct and indirect treatment. (c) Strategies of exosome treatment using various hearing loss models. (d) Strategies of exosome applications.
The most common method includes fractionation of the sample with an ultracentrifuge and purification using a sucrose or iodixanol gradient [5][9][12]. Several studies have reported ultrafiltration methods to remove the impurities other than exosomes, to enrich the exosomes [4][7][11]. In addition, to maintain the function of exosomes after isolation, the exosomes must be resuspended in phosphate-buffered saline for use in cell or animal experiments. Exosomes obtained from cell culture fluids or biological samples have low stability and must be stored frozen at -80 °C or used immediately.
As previously reported, exosomes are vesicles composed of lipid membranes that are 100 to 200 nm in size; therefore, they have the property of being able to penetrate small-sized membranes [13][1][14]. Known to play an important role in cell-to-cell communication, useful factors can be divided into donor and recipient cells, and can be used to confirm delivery to the recipient cells [15][16][17]. As a strategy to confirm the effect of indirect exosomes, we propose a co-culture method (Figure 1b(ii)). This method can be used to determine the indirect effects of exosomes while delivering useful elements to the interior of the recipient cell.
The important point of this strategy is the composition of membranes and pore size when choosing the trans-well. If the pore size is 3 µm above the cell size, it would be impossible to move several cells at the same time; therefore, one should select a pore size below 0.4 µm [15][16]. When donor cells are attached to the insertion well, the recipient cells are cultured in the outer well, and when the insertion well is cultured together after 24 h, communication between the cells through exosomes is activated in the medium. Therefore, choosing co-culture as a method of confirming indirect effects without extracting exosomes from donor cells can be an important strategy to elucidate evidence for exosome transport.
2. Strategies of Exosome Therapy Using Various Hearing Loss Models
There are not many known auditory cells, but House Ear Institute-Organ of Corti 1 (HEI-OC1) has been mainly reported in in vitro experiments (Figure 1c(i)) Although it is possible to analyze the properties of a specific drug according to the purpose of the experiment, caution must be exercised because the physiological properties of these cells are different from those of conventional primary cells or cancer cells. Although ion channels located on the surface of auditory cells can be analyzed, hearing cannot be determined [18][19]. Therefore, we suggest that HEI-OC1 may be a useful model for investigating biological responses related to auditory cells, including auditory sensory cells, but we believe that a careful approach is needed when evaluating drug effects.
Although many researchers have attempted to study hearing in vitro, analytical techniques have been developed only for short-term biological analysis [20][21]. Ex vivo models include extraction of the animal’s auditory organ, the cochlea, and culturing it in a medium similar to that of in vitro experiments.
The human cochlea is difficult to obtain, but the use of mouse cochlea has been reported in previous studies [22]. In a previous study, we conducted experiments to confirm the viability of cochlear hair cells and to evaluate the activity of the special markers Myo7a and phalloidin [23]. The technique of extracting explants can be observed with immunofluorescence, and changes in the surface morphology of the tissue can be observed with transmission electron microscopy. Therefore, cochlear explants are a good tool to quickly screen for toxicity rates based on drug concentration and can be used prior to in vivo testing.
The most important aspect of auditory clinical studies is that animal testing is indispensable, and it is necessary to confirm the phenotype by measuring auditory brainstem response (ABR) to reliably prove hearing loss The type of animal model can represent an age-related or ototoxic hearing loss model [10][24][25][26]. Physiological analysis of auditory cells is possible through in vitro experiments (Figure 1c(iii)), but it is difficult to find a treatment for hearing loss or clearly analyze the efficacy of the drug. Demonstrating the phenotype of toxic substances in vitro and in vivo, we can confirm that hearing loss is a systemic action of the drug in animals.
To observe the anatomical phenotype of hearing loss, the morphology can be observed either through immune fluorescence staining (Figure 2a) or electron microscopy (Figure 2b). The observation of changes in hair cells in the organ of Cotri is evidence of direct damage to the auditory organ, and the Myo7a staining, a representative hair cell marker, may prove a physical phenomenon of hearing abnormalities. In addition, in chemical hearing, it can be confirmed that there is an abnormality in electrochemical transmission by comparing the dB reduction trend through ABR measurement (Figure 2c).
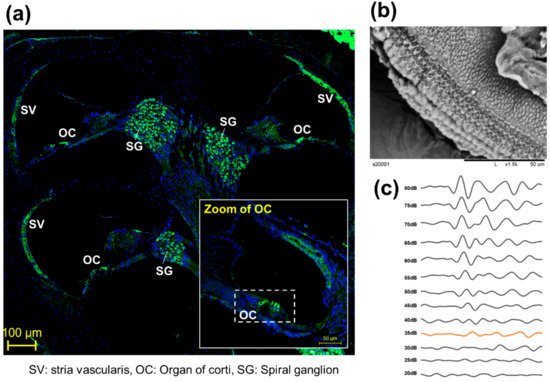
Figure 2. Representative anatomical analysis strategy for in vivo experiments. (a) Analysis of whole auditory organ by immune fluorescence staining using Myosin 7a (Myo7a). (Myo7a: green, DAPI: blue), (b) Observation of organ of corti (OC) by transmission electron microscopy [8], (c) Comparison of hearing kinetics depending on 80 db to 20 dB on 8000 kHz.
3. Strategies of Exosome Applications
EV studies reported over the past decade have demonstrated that EVs retain their activity and carry several types of macromolecules as they are transferred from parent cells to recipient cells [13][27][28]. Previous studies have confirmed that stem cells with embedded nanoparticles can increase exosome production [29]. This demonstrates the possibility of using tube particles to transport specialized proteins to nanoparticles that could influence exosome development. In addition, a previous study reported direct engineering of a fluorescent substance into exosomes to confirm trafficking in human cells (Figure 1d(i))
Therefore, understanding the pathways leading to exosome development and internalization within target cells is an important goal in the current field of EV research. In addition, studies that uncover the unique biomolecules of exosomes are related to disease states and treatments; therefore, the discovery of new EV biomarkers is also a potential research topic.
Next, the presence of DNA molecules in EVs has been widely reported [30][31]. Mitochondria and genomic DNA were found in EVs derived from double-stranded DNA, and those isolated from cell culture media and biological fluids (human and mouse) were found inside EVs (Figure 1d(ii)).
Finally, there is increasing interest in miRNA studies, which are known to be the main causes of gene and protein expression, and there are reports that the miRNA profile of the released exosomes cannot be randomly generated under certain immune response conditions. Proteins inherent in EVs include a complex set of proteins derived from parent cells (cytoplasmic, nuclear, mitochondrial, and membrane-bound proteins). Several proteomic studies have determined EV activity by defining unique EV properties associated with parent cells. Vesicle-specific proteins are often used as markers for specific heat shock proteins, tetraspanins (CD9, CD63, and CD81), major histocompatibility complex molecules, and proteins in the endosomal sorting complex required for transport (ESCRT) complex (Alix and TSG101)
Exosome viability is regulated by the MVB, which redirects endocytosis proteins to their destinations (such as cells or organs) (Figure 1d(iii)). Substances are internalized by endocytosis on the cell surface and transported through early and late endosomes. They are surrounded by lipids that form a plasma membrane, which protects the contents of the vesicle and promotes its migration to the target cells.
However, clinically, the study of exosome endocytosis is of great importance [32][33]. The response at the receptor carries a risk of toxicity or side effects of exosomes; however, to date, this has not been confirmed when using exosomes in auditory cells [34][35][36]. In the case of cancer cell-derived exosomes, a factor that promotes the proliferation of cancer cells can metastasize [17]. Therefore, we believe that when using exosomes to be inserted into the auditory organs, we should test for toxicity and analyze which substances in the surrounding cells are stimulated.
References
- Jeppesen, D.; Fenix, A.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18.
- Colombo, M.; Moita, C.F.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013, 126, 5553–5565.
- Park, D.J.; Seo, Y.J. Engineering of Extracellular Vesicles Based on Payload Changes for Tissue Regeneration. Tissue Eng. Regen. Med. 2021, 1–13.
- Lu, K.; Li, H.-Y.; Yang, K.; Wu, J.-L.; Cai, X.-W.; Zhou, Y.; Li, C.-Q. Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: In-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 1–11.
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.M.; Sadik, M.; Alaarg, A.; Smith, C.I.E.; Lehtiö, J.; El Andaloussi, S.; et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016, 6, 22519.
- Guan, S.; Yu, H.; Yan, G.; Gao, M.; Sun, W.; Zhang, X. Characterization of Urinary Exosomes Purified with Size Exclusion Chromatography and Ultracentrifugation. J. Proteome Res. 2020, 19, 2217–2225.
- He, L.; Zhu, D.; Wang, J.; Wu, X. A highly efficient method for isolating urinary exosomes. Int. J. Mol. Med. 2018, 43, 83–90.
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031.
- Palviainen, M.; Saari, H.; Kärkkäinen, O.; Pekkinen, J.; Auriola, S.; Yliperttula, M.; Puhka, M.; Hanhineva, K.; Siljander, P.R.-M. Metabolic signature of extracellular vesicles depends on the cell culture conditions. J. Extracell. Vesicles 2019, 8, 1596669.
- Paciello, F.; Di Pino, A.; Rolesi, R.; Troiani, D.; Paludetti, G.; Grassi, C.; Fetoni, A.R. Anti-oxidant and anti-inflammatory effects of caffeic acid: In vivo evidences in a model of noise-induced hearing loss. Food Chem. Toxicol. 2020, 143, 111555.
- Choi, J.S.; Cho, W.L.; Choi, Y.J.; Kim, J.D.; Park, H.-A.; Kim, S.Y.; Park, J.H.; Jo, D.-G.; Cho, Y.W. Functional recovery in photo-damaged human dermal fibroblasts by human adipose-derived stem cell extracellular vesicles. J. Extracell. Vesicles 2019, 8, 1565885.
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977.
- Li, M.; Li, S.; Du, C.; Zhang, Y.; Li, Y.; Chu, L.; Han, X.; Galons, H.; Zhang, Y.; Sun, H.; et al. Exosomes from different cells: Characteristics, modifications, and therapeutic applications. Eur. J. Med. Chem. 2020, 207, 112784.
- Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Chiaradia, E.; Urbanelli, L.; Emiliani, C. Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular. Int. J. Mol. Sci. 2020, 21, 2576.
- Danzer, K.M.; Kranich, L.R.; Ruf, W.P.; Cagsal-Getkin, O.; Winslow, A.R.; Zhu, L.; Vanderburg, C.R.; McLean, P.J. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol. Neurodegener. 2012, 7, 42.
- Zhou, X.; Brown, B.A.; Siegel, A.P.; El Masry, M.S.; Zeng, X.; Song, W.; Das, A.; Khandelwal, P.; Clark, A.; Singh, K.; et al. Exosome-Mediated Crosstalk between Keratinocytes and Macrophages in Cutaneous Wound Healing. ACS Nano 2020, 14, 12732–12748.
- Kulkarni, B.; Gondaliya, P.; Kirave, P.; Rawal, R.; Jain, A.; Garg, R.; Kalia, K. Exosome-mediated delivery of miR-30a sensitize cisplatin-resistant variant of oral squamous carcinoma cells via modulating Beclin1 and Bcl2. Oncotarget 2020, 11, 1832–1845.
- Guo, X.; Bai, X.; Li, L.; Li, J.; Wang, H. Forskolin protects against cisplatin-induced ototoxicity by inhibiting apoptosis and ROS production. Biomed. Pharmacother. 2018, 99, 530–536.
- Kalinec, G.; Thein, P.; Park, C.; Kalinec, F. HEI-OC1 cells as a model for investigating drug cytotoxicity. Hear. Res. 2016, 335, 105–117.
- Landegger, L.D.; Dilwali, S.; Stankovic, K.M. Neonatal Murine Cochlear Explant Technique as an In Vitro Screening Tool in Hearing Research. J. Vis. Exp. 2017, 124, e55704.
- Ogier, J.M.; Burt, R.A.; Drury, H.R.; Lim, R.; Nayagam, B.A. Organotypic Culture of Neonatal Murine Inner Ear Explants. Front. Cell. Neurosci. 2019, 13, 170.
- Park, J.-E.; Lee, S.H.; Park, D.J.; Seo, Y.J.; Kim, S.K. In vitro Time-lapse Live-Cell Imaging to Explore Cell Migration toward the Organ of Corti. J. Vis. Exp. 2020, 166, e61947.
- Park, D.J.; Park, J.-E.; Lee, S.H.; Eliceiri, B.P.; Choi, J.S.; Kim, S.K.; Seo, Y.J. Protective Effect of Msc-Derived Exosomes Against Cisplatin-Induced Apoptosis via Heat Shock Protein 70 in Auditory Explant Model. Available online: (accessed on 4 March 2021).
- Lee, C.H.; Park, S.-S.; Lee, D.-H.; Lee, S.M.; Kim, M.Y.; Choi, B.Y.; Kim, S.Y. Tauroursodeoxycholic acid attenuates cisplatin-induced hearing loss in rats. Neurosci. Lett. 2020, 722, 134838.
- Park, D.J.; Ha, S.; Choi, J.S.; Lee, S.H.; Park, J.-E.; Seo, Y.J. Induced Short-Term Hearing Loss due to Stimulation of Age-Related Factors by Intermittent Hypoxia, High-Fat Diet, and Galactose Injection. Int. J. Mol. Sci. 2020, 21, 7068.
- Bowl, M.; Dawson, S.J. The Mouse as a Model for Age-Related Hearing Loss—A Mini-Review. Gerontology 2014, 61, 149–157.
- Joshi, B.S.; De Beer, M.A.; Giepmans, B.N.G.; Zuhorn, I.S. Endocytosis of Extracellular Vesicles and Release of Their Cargo from Endosomes. ACS Nano 2020, 14, 4444–4455.
- Shi, X.; Cheng, Q.; Hou, T.; Han, M.; Smbatyan, G.; Lang, J.; Epstein, A.L.; Lenz, H.-J.; Zhang, Y. Genetically Engineered Cell-Derived Nanoparticles for Targeted Breast Cancer Immunotherapy. Mol. Ther. 2020, 28, 536–547.
- Park, D.J.; Yun, W.S.; Kim, W.C.; Park, J.-E.; Lee, S.H.; Ha, S.; Choi, J.S.; Key, J.; Seo, Y.J. Improvement of stem cell-derived exosome release efficiency by surface-modified nanoparticles. J. Nanobiotechnology 2020, 18, 1–17.
- Savina, A.; Furlán, M.; Vidal, M.; Colombo, M.I.; Gamel-Didelon, K.; Kunz, L.; Föhr, K.J.; Gratzl, M.; Mayerhofer, A. Exosome Release Is Regulated by a Calcium-dependent Mechanism in K562 Cells. J. Biol. Chem. 2003, 278, 20083–20090.
- Lin, Y.; Anderson, J.D.; Rahnama, L.M.A.; Gu, S.V.; Knowlton, A.A. Exosomes in disease and regeneration: Biological functions, diagnostics, and beneficial effects. Am. J. Physiol. Circ. Physiol. 2020, 319, H1162–H1180.
- Garcia-Romero, N.; Esteban-Rubio, S.; Rackov, G.; Carrión-Navarro, J.; Belda-Iniesta, C.; Ayuso-Sacido, A. Extracellular vesicles compartment in liquid biopsies: Clinical application. Mol. Asp. Med. 2018, 60, 27–37.
- Liu, C.; Su, C. Design strategies and application progress of therapeutic exosomes. Theranostics 2019, 9, 1015–1028.
- Müller, U. Exosome-mediated protection of auditory hair cells from ototoxic insults. J. Clin. Investig. 2020, 130, 2206–2208.
- Breglio, A.M.; May, L.A.; Barzik, M.; Welsh, N.C.; Francis, S.P.; Costain, T.Q.; Wang, L.; Anderson, D.E.; Petralia, R.S.; Wang, Y.-X.; et al. Exosomes mediate sensory hair cell protection in the inner ear. J. Clin. Investig. 2020, 130, 2657–2672.
- Jiang, P.; Zhang, S.; Cheng, C.; Gao, S.; Tang, M.; Lu, L.; Yang, G.; Chai, R. The Roles of Exosomes in Visual and Auditory Systems. Front. Bioeng. Biotechnol. 2020, 8, 525.
