Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yin Xiao and Version 3 by Camila Xu.
Porous nanomaterials (PNMs) are nanomaterials with a porous structure and high surface ratio, which are widely used in the fields of biomedical engineering such as bone regeneration, drug delivery, cell trace, and regulation of cell differentiation.
- nanomaterials
- autophagy
- osteogenesis
- immune microenvironment
- bone
1. Introduction
Bone is a metabolically active tissue that maintains physiological function and homeostasis through a continuous remodeling process that consists of bone resorption and formation [1]. For large bone defects caused by tumor, trauma, inflammation, or infection, it is necessary to implant materials to promote new bone regeneration and restore bone function. From the view of materials, healthy bone is a complex natural material composed of organic nanomaterials (collagen, nanofibers) and inorganic nanomaterials (nano-hydroxyapatite) with a multi-level structure from the micro-nanometer to the macro-level [2]. This multilayered structure is also linked to responding to stimuli/injury and activating regeneration [2]. Therefore, nanomaterials have a broad prospect for the development of functional bone regenerative materials. In recent years, nanomaterials have attracted increasing interest in bone regeneration studies, and many reviews have reported on the application of nanomaterials for bone regeneration. For instance, as early as 2009, Webster, T.J., reviewed the prospects for nanomaterials in bone, cartilage, blood vessel, nerve, and bladder tissue engineering [3]. Srinivasan, D.K., elucidated the use of nanoparticles as a drug delivery system to improve bone regeneration [4]. Liu, C., summarized the development of nanomaterials that can promote bone regeneration in a mimicked bone-healing model (e.g., compositional, nanocrystal formation, structural, and growth factor-related mimicking) [2]. Meanwhile, the mechanisms by which nanomaterials regulate cell behavior have been widely investigated [5]. Studies on the interactions between nanomaterials and cells have shown that cell phagocytosis and clearance of nanomaterials, cell function maintenance, cell differentiation, and stress response are strictly regulated by autophagy [6].
Autophagy is the response of cells to stress. It is an evolutionarily conserved process with multiple roles. Primarily, it maintains intracellular homeostasis by degrading and circulating metabolites within cells, providing energy and nutrients, eliminating cytotoxic substances such as damaged proteins and organelles [7]. Interestingly, in the skeletal system, autophagy activated by specific nanomaterials contributes to osteogenic differentiation [7][8][7,8]. At the same time, other studies have shown that the toxic effects of nanomaterials may also be associated with autophagy [9], which leads to bone loss [10] and osteolysis [11][12][11,12]. During bone remodeling, autophagy plays a vital role in the differentiation of osteoclasts and osteoblasts via the mediating immune regulation [13]. These results suggest that autophagy plays a bi-directionally regulatory role in the process of promoting or inhibiting osteogenesis; therefore, targeting autophagy is of great significance for the design of bone regenerative nanomaterials. Hence, targeting autophagy may be a practical approach for promoting bone regeneration.
PNMs are nanomaterials with a porous structure and high surface ratio, which are widely used in the fields of biomedical engineering [14][15][16][17][18][14,15,16,17,18] such as bone regeneration [18][19][20][21][18,19,20,21], drug delivery [22][23][24][22,23,24], cell trace, and regulation of cell differentiation. Since 2014, it has been reported that mesoporous bioactive glass nanomaterials can promote osteogenic differentiation through activation of autophagy [25]. Targeting autophagy has become a new research focus in the application of PNMs for bone regeneration. In the current review, the main application of PNMS in bone regeneration is summarized. Then, the crucial regulatory role of autophagy in bone regeneration is briefly introduced. The regulatory roles of PNMs, including mesoporous silica nanoparticles (MSNs) [20][26][27][20,26,27], mesoporous hydroxyapatite nanoparticles (HAP) [28][29][30][28,29,30], alumina nanoparticles (Al2O3) [31][32][31,32], mesoporous bioactive glass nanoparticles (MBGNs) [33][34][35][36][33,34,35,36], mesoporous ceria (MCeO2) [37][38][37,38], and metallic oxides [39][40][39,40] in bone regeneration via targeting autophagy, are reviewed and discussed.
2. PNMs for Bone Regeneration
The type, structure, and morphology of nanomaterials have been continuously improved to achieve better bone regeneration. In particular, PNMs with porous structures have aroused the interest of researchers. Compared with nanomaterials without pores, porous materials have higher porosity and higher specific surface area. The high porosity facilitates the design for an excellent drug delivery carrier, and the high specific surface area makes it easily modifiable with bioactive molecules. The application of PNMs in bone regeneration is mainly focused on the following four aspects (Figure 1).
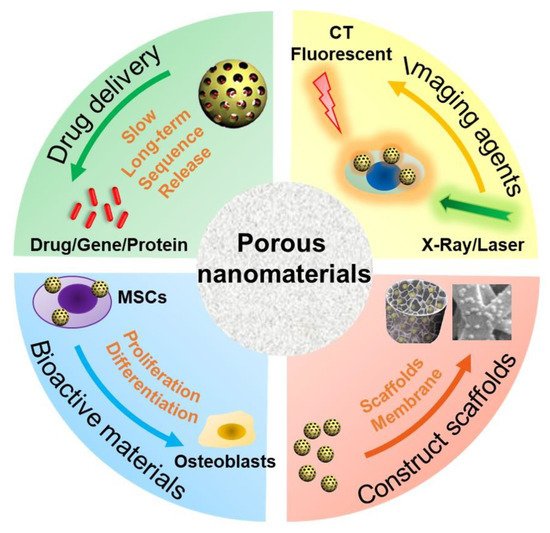
Figure 1. Schematic diagram of PNM applications in bone regeneration. MSCs: mesenchymal stem cells; CT: computer tomography.
(1) PNMs can be designed as efficient nanocarriers to promote bone regeneration through (controlled) delivery of beneficial factors such as small molecule compounds, genes, and proteins. A variety of microporous (<2 nm), mesoporous (2~50 nm), and macropore nanomaterials (>50 nm) are designed as drug delivery carriers [41]. In particular, due to the high porosity and specific surface area, PNMs are widely used to deliver drugs for bone regeneration such as mesoporous silica nanoparticles [42][43][44][42,43,44] and mesoporous hydroxyapatite nanoparticles [29][30][45][29,30,45]. The hollow and mesoporous structure of nanomaterials can enhance drug loading efficiency [46]. For example, Hae-Won Kim [36] used hollow porous nanoparticles to deliver small genetic molecules to silence the target gene thereby, in turn, stimulating osteogenic differentiation. In another study, mesoporous silica nanoparticles have been combined with hydroxyapatite to generate a composite coating for implant surface modification, which served as a drug-delivery tool to suppress osteoclastogenesis for improving bone regeneration and osteointegration [27]. These nanocarriers are often combined with other materials, such as polymers [47], hydrogels [48], and metal materials [49], to promote bone regrowth;
(2) PNMs can be used as an imaging contrast agent to trace the cells and monitor real-time tissue regeneration. Stem cell-based therapy is a promising approach in regenerative medicine [50]. However, the distribution and migration of stem cells after transplantation cannot be effectively monitored in vivo. To achieve this goal, an indirect or direct cell tracker is preferred [51]. Current approaches, such as indirect fluorescent reporter gene labeling, are challenging to obtain deep structure images in vivo with limited detection methods; furthermore, transgenic cells are difficult to use in clinical treatment due to the regulatory issues [52]. On the other hand, nanomaterials can be used to directly label cells and can be simultaneously detected with a variety of imaging methods including magnetic resonance (MRI) [53], computed tomography (CT) [54], and photoacoustic imaging (PI) [55][56][55,56]. Some products are already commercialized for this purpose as reviewed by Wang et al. [57]. PNMs have performed well in cell imaging studies. For instance, mesoporous silica shows excellent potential in stem cell tracking. The synthesized PEGylated gold/silica nanoparticle can simultaneously be detected by MRI, CT, and fluorescence imaging (FI) [58]. Jokerst, J.V., developed exosome-like silica nanoparticles, serving as a novel ultrasound contrast agent for stem cell imaging [59]. Gadolinium3+-doped mesoporous silica nanoparticles also served as a potential magnetic resonance tracer for monitoring the migration of stem cells in vivo [60];
(3) PNMs have been used to fabricate or modify tissue-engineering scaffolds. The primary goal of tissue-engineered scaffolds is to develop an implant to replace the original bone tissue while supporting the regeneration process [61]. As mentioned above, natural bone has a micro-nanometer to a macroscopic hierarchical structure; thus, the tissue-engineered scaffolds must be designed in a three-dimensional structure with a highly porous feature, forming an interconnected pore network to mimic the structure of natural bone [62]. To achieve this goal, nanomaterials have been used to develop tissue-engineering scaffolds to improve the bone formation properties, such as cell growth, nutrient transport, new bone growth, and angiogenesis [52]. The physicochemical stability and mechanical properties of collagen hydrogel were improved by modification of aminated mesoporous bioactive glass in order to be better applied in tissue engineering stem cell culture [33]. Titanium dioxide nanotubes (TiO2 NTs) are a type of classic PNMs with a diameter in the range of 30–100 nm, which have been widely used to construct and modify scaffolds to enhance cell attachment [63] and osseointegration [64][65][64,65]. N.K. summarized the potential applications of TNTs in implants [66]. Compared with the untreated titanium, TiO2 NT-modified titanium enhanced the deposition of type I collagen when implanted into the porcine frontal skull. In addition, bone implants with TiO2 NT modification have good contact with bone and will not be damaged due to the fact of simple stress [67]. In a tibial bone defect model of rabbits, the TiO2 NT-modified implants induced a nine-fold increase in the bone binding rate compared to the non-modified implants [68]. In vitro and in vivo studies have shown that titanium dioxide nanotubes can increase the deposition of calcium and phosphorus and enhance the expression of osteogenesis genes such as alkaline phosphatase (ALP), osterix (Osx), and collagen-I (COL-I) [69];
(4) PNMs have been regarded as an active substance to directly modulate cell behavior (cell adhesion and differentiation). Nanomaterials, themselves, are excellent enhancers of new bone formation. It is known that gold nanoparticle size and shape can influence the osteogenesis of mesenchymal stem cells [70]. In addition, porous materials, such as mesoporous bioactive glass nanoparticles [33][34][35][36][71][33,34,35,36,71], mesoporous hydroxyapatite [72], and mesoporous ceria (MCeO2) [37][38][37,38], have been found to directly promote osteoblast differentiation. For instance, cerium oxide nanoparticles-modified bioglass could enhance bone regeneration by activating the extracellular signal-regulated kinase (ERK) signaling pathway [38]. In another study, ceria nanocrystals, decorated with mesoporous silica nanoparticles, have been found to facilitate tissue regeneration via inducing reactive oxygen species-scavenging (therefore, avoiding tissue damage and inflammation), suggesting its potential in bone regeneration [73] Mesoporous Ce-doped bioactive glass nanoparticles could improve osteogenesis via Ce-induced anti-oxidation and anti-inflammation [74]. Similarly, nanoceria encapsulated within mesoporous silica nanoparticles (Ce@MSNs) have been found to facilitate bone regeneration in osteoporosis and that nanoceria could induce anti-oxidation and facilitate osteogenesis, ref. [75] suggesting that ceria could be considered a critical component in bone regenerative PNMs design. Moreover, ionic-doped PNMs can promote osteogenic differentiation and facilitate angiogenesis [76] and regulate immunity [77].
3. Autophagy Modulation and Bone Reconstruction
There are three main types of autophagy, namely, macroautophagy, microautophagy, and partner-mediated autophagy [78]. This review focuses on macroautophagy (hereinafter referred to as autophagy), a degradation process during which cellular wastes, such as damaged macromolecules and organelles, are accumulated at lysosomes by autophagy vesicles and removed [78]. Autophagy begins with cytoplasmic organelle isolation in bi-membranous vesicles called autophagosomes, which then fuse with lysosomes to form autophagosomes to degrade its contents by lysosomal hydrolases, such as damaged organelles, intracellular pathogens, glycogens, lipids, and nucleotides proteins, which then turned into a nutrient source for maintaining cellular activity [79]. In this progress, the cytosolic form of microtubule-associated protein 1A/1B-light chain 3 (LC3-I) is converted to form LC3-phosphatidylethanolamine conjugate (LC3-II), which is attached to the autophagosome membrane and then degraded [80]. The transition from LC3-I to LC3-II is considered one of the hallmarks of autophagy. Autophagy plays a quality control role in cell homeostasis [81].
Autophagy is one of the main mechanisms promoting cell survival, which is activated under stress conditions such as nutrient deprivation, oxidative stress, hypoxia, and infection [82]. For example, autophagy promotes the circulation of cellular components, thus providing energy for starving cells [83]. On the other hand, autophagy functions on clearing dysfunctional/damaged proteins and organelles [84]. For example, autophagy mediates the clearance of damaged mitochondria, also known as mitochondrial autophagy, inhibits the accumulation of reactive oxygen species (ROS), thereby protecting cells from oxidative stress and apoptosis [85]. These functions are thought to be essential in bone cell differentiation and immune cell polarization; thus, autophagy is believed to play a central role in bone regeneration (Figure 2).
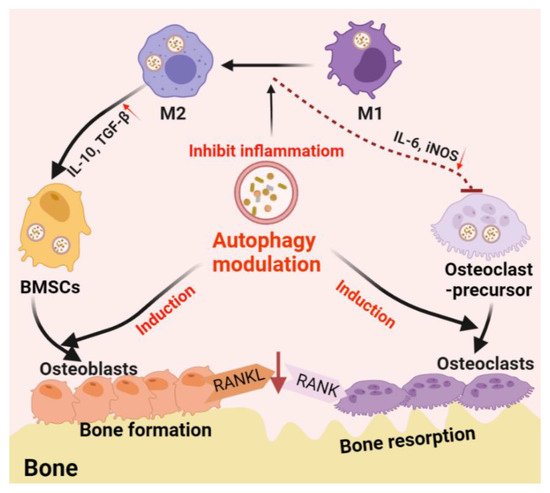
Figure 2. Schematic diagram of autophagy-derived regulation on the differentiation/function of osteoclast/osteoblast and osteoimmunology. On the one hand, autophagy can facilitate the differentiation/function of both osteoclasts and osteoblasts; on the other hand, autophagy induces the phenotype switch from M1 (inflammatory phenotype, which facilitating osteoclastogenesis by producing IL-6 and iNOS) to M2 (tissue-regenerative phenotype, which facilitating osteogenesis by producing IL-10 and TGF-β) in the macrophage population, thereby generating an immune microenvironment favoring bone formation. Furthermore, autophagy induction on osteoblasts can reduce osteoblast-originated RANKL production, hence, reducing osteoclastogenesis by inhibiting the RANKL–RANK signaling pathway.
3.1. Autophagy in the Differentiation/Function of Osteoclasts and Osteoblasts
Bone is a metabolically active tissue composed of a network of various types of cells through multiple factors. To maintain physiological bone metabolism, different types of cells (e.g., stromal cells and immune cells) need to continuously interact with each other to ensure osteoblast differentiation, functional mineralization, and osteoclast phagocytosis. The process requires close coordination between cellular organelles and regulators and consumes a large amount of biological energy [86]. Bone metabolic homeostasis is maintained by the balance between osteoblast-derived bone formation and osteoclast-derived bone absorption [87]. Recent studies have confirmed that autophagy is involved in the mineralization process of osteoblasts and the maintenance of bone homeostasis [86]. Autophagy plays an essential role in the differentiation and function of osteoblasts and osteoclasts during bone regeneration. During the receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclast differentiation, autophagy-associated protein ATG 5/7/12 expression and LC3-II /LC3-I ratio increase with the degradation of p62 [88]. ATG5/7/4B and LC3 have also been reported to play a decisive role in regulating the production of osteoclast wrinkle boundary and lysosome secretion, thus determining the function of osteoclasts in vitro and in vivo [89].
On the other hand, autophagy participates in the differentiation and mineralization of osteoblasts. Autophagosomes act as cargos transporting intracellular mineral crystal-like structures to facilitate extracellular mineralization [90]. Inhibition of autophagy can result in impaired mineralization in vitro and reduced bone mass and volume in vivo, which is followed by oxidative stress and the production of RANKL in general [91]. These results suggest the fundamental role of autophagy in osteoblast differentiation and mineralization, which acts as a mineralization carrier to protect osteoblasts from increased oxidative stress and, in addition, to reduce the production of RANKL, thereby inhibiting osteoclastogenesis during bone formation [91].
3.2. Autophagy-Associated Immunomodulation in Bone Remodeling
Not only directly involved in the differentiation and function of osteoblast and osteoclast, autophagy also regulates the immune system which, in turn, regulates bone regeneration through modulating the immune microenvironment [13]. Among the immune cells, macrophages play an important role in the innate immune system. Macrophages are divided into un-activated M0 macrophages, proinflammatory M1 phenotype, and anti-inflammatory M2 phenotype. M1 macrophages are usually activated by microbial lipopolysaccharide (LPS) or Th1 cell-derived IFN, which are considered to promote osteoclastogenesis [92][93][92,93]. M2 macrophages, which are usually activated by TH2 cell-derived IL-4 or IL-13, are considered to be the subtypes that inhibit osteoclast differentiation and promote bone regeneration [92][93][92,93]. Especially in biomaterial-associated bone regeneration, macrophage phenotype switch from M1 to M2 is considered as an essential strategy in material design/development [94].
Autophagy plays an immunosuppressive role in macrophage inflammatory response. Atg5- or Atg16L1-deficiency on macrophages was found to induce the conversion of M2 macrophages into M1-like phenotypes with enhanced secretion of proinflammatory cytokines [95][96][95,96]. Mice with macrophage-specific ATG5-knockout showed induced systemic inflammation [97]. Primary bone marrow-derived macrophages (BMDMs) obtained from this mice type showed abnormal polarization with increased M1 polarization and decreased M2 polarization, indicating that inhibition or deficiency of autophagy can upregulate inflammation in macrophages [97]. Further studies have found that autophagy facilitated the clearance of damaged mitochondria (mitochondrial autophagy, mitophagy). This process can effectively eliminate dysfunctional or damaged mitochondria, which can trigger inflammation and cause cell apoptosis or necrosis, thereby inhibiting inflammation and preventing unnecessary cell loss [98]. Autophagy plays a quality control role in inflammation regulation, and poor quality control can lead to inflammation and cell population death [98]. As previously mentioned, the inflammatory response of macrophages has been shown to induce osteoclast formation and bone loss. At the same time, the transformation of the proinflammatory M1 to anti-inflammatory M2 phenotype is thought to improve bone repair [99]. Therefore, this autophagy-mediated regulation of macrophage response is beneficial to bone regeneration. Nanomaterial-derived autophagy induction has been shown to potentially introduce M2 polarization to improve bone regrowth [100], which further suggests that autophagy may be a potential immunomodulatory target in regenerative medicine, particularly for the treatment of bone loss diseases such as osteoporosis [101], arthritis [102], and periapical lesions [103].
