Environmental microplastics are gaining interest due to their ubiquity and the threat they pose to environmental and human health. Critical studies have revealed the abundance of microplastics in nature, while others have tested the impacts of these small plastics on organismal health in the laboratory. Yet, there is often a mismatch between these two areas of research, resulting in major discrepancies and an inability to interpret certain findings. Here, we focus on several main lines of inquiry. First, even though the majority of environmental microplastics are plastic microfibers from textiles, laboratory studies still largely use spherical microbeads. There are also inconsistencies between the measurements of microplastics in the environment as compared to the concentrations that tend to be used in experimental studies. Likewise, the period of exposure occurring in experimental studies and in the environment are vastly different. Lastly, although experimental studies often focus on a particular subset of toxic chemicals present on microplastics, textile microfibers carry other dyes and chemicals that are understudied. They also cause types of physical damage not associated with microspheres.
- microplastics
- microfibers
- aquatic effects
- environmental relevancy
1. Introduction
Ever since the discovery of microplastics (MPs) in the ocean, there have been large numbers of laboratory studies on their effects. Many studies have been conducted on aquatic organisms, many of which consume these pollutants, and some of which are eaten by humans. Other experimental studies have been on mammalian surrogates (rats or mice) or tissue cultures to provide evidence for potential impacts of these ubiquitous pollutants on humans. For both types of studies, questions have arisen about the lack of realism. For example, Burns and Boxall (2018) noted that the levels detected in the environment are orders of magnitude lower than those used in studies of effects on biochemistry, reproduction, growth, feeding, and inflammation in aquatic organisms [1]. Similarly, Bucci et al. (2020) who performed a meta-analysis of the literature, concluded that there was a mismatch between field studies and the laboratory studies, which tend to use different plastic shapes (fibers being the most common in the field; spheres in the lab), virgin MPs in the lab as compared to aged MPs with biofilm in the field, and different sizes of MPs [2]. Fibers have been found to be the most common MP type in aquatic ecosystems [3]. These fibers come predominantly from washing of synthetic textiles, but also from cigarette butts [4]. The degree of effect detected is a response to concentration, shape, particle size, and polymer type. Comparing concentrations used in most lab exposures to the concentrations and sizes of MPs in the environment, Bucci et al. (2020) noted that 17% of the concentrations used in lab studies have been seen in the environment, and that 80% of the particle sizes used in experimental studies are below the sizes found in most environmental sampling [2].
Kogel et al. (2020) in a general review found that toxicity of plastic particles depends on particle size, concentration, particle condition, exposure time, polymer type, and shape [5]. Other factors, relating to the experimental subject included species, sex, developmental stage, and food availability. Effects that have been found in experimental studies were on energy metabolism, feeding, growth, activity, metabolism, physiological stress, pathology, immune system, intestinal damage, development, hormonal regulation, and cell death. Photosynthetic disruption was reported in phytoplankton.
2. Shape (Spheres vs. Fibers)
2.1. Aquatic Effects
As Ward et al. (2019) point out, microspheres are available to be purchased with uniform size and shape, with added fluorescence or dyes allowing them to be visualized in tissues [6]. Therefore, they are useful for studies of ingestion and egestion in many species. However useful they are, they are not representative of MPs in the environment, and are therefore not realistic (Figure 1). Despite not being so easily obtained, other researchers have found ways to study effects of microfibers, which are the predominant shape of MPs in the water, air, and soil. They can be acquired from ropes, lint in dryers, textile samples, microfilaments used to make synthetic textiles, and other methods.
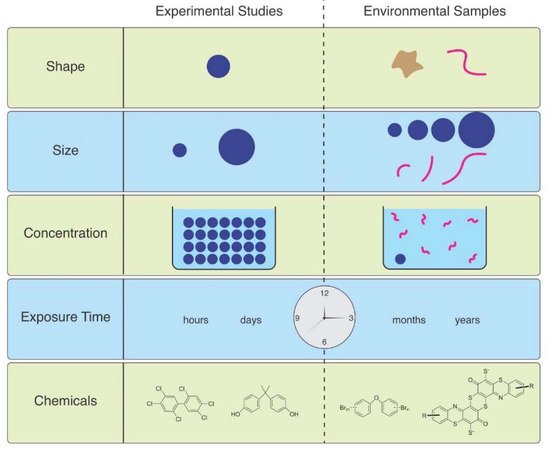
Figure 1. The mismatch between MPs used in laboratory studies and those in the environment.
Microfiber (MF) uptake by Asian clams (Corbicula fluminea) varied by polymer type and size. The uptake of fibers was greater in those exposed to greater concentrations than lower concentrations, and clams were more likely to take up polyester fibers of smaller size [7]. Horn et al. (2019) used polypropylene rope as a source of microfibers, the concentrations of which were based on concentrations found in the beach from where the experimental subjects, the mole crab, Emerita analoga, were collected [8]. Adult crabs exposed to these microfibers had increased mortality and impaired embryo development. Larval midges, Chironomus riparius, were exposed to PET MFs, and effects including survival, time till emergence, growth, head capsule length, and general stress response, were examined in 28-day sediment chronic toxicity tests [9]. They used artificial sediments spiked with MFs at concentrations of 500, 5000, and 50,000 particles/kg sediment dry weight. The lowest concentration was comparable to 500 particles/kg sediment dry weight in Lake Ontario, Canada and 4900 particles/kg sediment dry weight as in the Rhine River. Larvae ingested the microfibers which were later found in the adults. However, no significant effects were seen on time until emergence, head capsule lengths, weight, or HSP 70 (stress response) compared to control organisms. Alnajar et al. (2021) conducted a seven-day exposure of the mussel Mytilus galloprovinciallis to MFs at 56–180 mg L−1 (far higher than environmental levels) and observed a reduction in mean clearance rate, abnormality in gills and digestive gland, and an increase in DNA damage [10]. They felt that these effects were due to a combination of the fibrous material itself and chemicals mobilized from the polymers into seawater or the digestive tract, the latter being consistent with an increase in trace elements (e.g., zinc) in the exposure medium with increasing lint concentration. Lobster (Homarus americanus) larvae were exposed to 0, 1, 10 and 25 MF mL−1 [11]. Only the highest concentration decreased early larval survival, and the timing or rate of molting was not altered. All larval and post-larval stages accumulated MFs under the carapace, and ingestion increased with larval stage and with MF concentration; oxygen consumption rates were reduced in later larval stages exposed to high concentrations.
Some studies have compared responses to and effects of fibers vs. spheres. Ward et al. (2019) indicated that mussels reject most of the fibers and spheres they ingest, but a much smaller percentage of spheres were rejected than polyester fibers (ca. 65–260 μm long × 16 μm wide) [6]. The amphipod Hyalella azteca had slower egestion of fibers than microspheres, but eventually both showed complete egestion [12]. MFs had greater toxicity than microbeads however, possibly because of slower gut passage. In a 28-day feeding experiment, Blarer and Burkhart-Holm (2016) studied effects of fibers and spheres on the feeding rate, assimilation efficiency and change in wet weight of the amphipod Gammarus fossarum [13]. While both types were ingested and egested, only the fibers impaired the health of the animals. Lower concentrations of MF had more severe effects on the amphipod G. fossarum than higher concentrations of MP particles. Frydkjær et al. (2017) reported that elevated concentrations of PE particles decreased the mobility of Daphnia magna, while irregular shaped fragments (10–75 µm) had greater effects than beads (10–106 µm) [14]. D. magna egested regular-shaped PE faster than irregular ones, indicating that “spiky” particles are retained longer and have more severe effects. Polyethylene terephthalate granular particles (p-PET, approximately 150 μm diam) and fibers (f-PET, approximately 3–5 mm L and 20 μm diam) were compared for effects on development of zebrafish (Danio rerio) embryos and their joint effects with cadmium [15]. Both types of MP accelerated blood flow and heart rate and inhibited hatching. Both forms decreased the toxicity of Cd. The detoxification effect of f-PET was greater than that of p-PET. Mendrik et al. (2021) found that MP fibers, but not spheres, reduced photosynthesis of algal symbionts of Acropora sp. corals, with a 41% decrease in photochemical efficiency after 12 days [16]. Grass shrimp (Palaemonetes pugio) were exposed to either sediment, polyethylene spheres, polypropylene fragments, tire fragments, polyester fibers, or clean-water for 96 h at a nominal concentration of 50,000 particles/L before a bacterial challenge with V. campbellii [17]. Mortality was not observed in any of the exposures, and survival following the bacterial challenge was similar among shrimp exposed to particle-free water, sediment, polypropylene fragments, polyethylene spheres, tire fragments, and polyester fibers. The grass shrimp cleared most of the ingested particles and all of the ventilated particles within 48 h.
2.2. Human Effects
In humans, relevant MP shapes will depend on exposure source (i.e., air, water, food), as well as how particles degrade over time in the environment and in the body. One of the earliest signs of MPs in human tissues came in 1998, when plastic microfibers were identified in biopsies of human lung tissue [18]. This study indicated two things: humans are exposed to MPs from the environment, and these MPs become embedded in our tissue. This study also importantly brought to our attention the relevance of plastic microfibers, as compared to more commonly studied spherical MPs. A more recent study built upon this work, showing that a combination of plastic particles and fibers were present in 13 of 20 human lung tissue samples examined [19].
Although inhaling airborne MPs is a major route of exposure, MPs also enter through ingestion and can become embedded in other organs. In a screening of 11 human colon samples, 96.1% of embedded particles were plastic filaments or fibers [20]. Furthermore, as was widely publicized, MPs were recently identified in human placentas, with a total of 12 irregularly shaped MP fragments being found in 4 placenta samples [21].
Interestingly, in a characterization of excreted MPs in human stool samples, mostly fragments and films were identified [22]. As few plastic microfibers were found in human stool samples and fibers are a major class of environmental MPs, one can infer that plastic microfibers that enter our bodies are becoming embedded in our tissues. This conclusion is in line with the aforementioned data.
Through experimental studies, it is known that ingested MP spheres (5 µm) hamper metabolism and gut microbial function. Furthermore, these spheres accumulate in the gut [23]. Spherical MPs have also been used in new in vitro models and organoid cultures of the human intestines. Similar to mammalian models, these systems show varied effects, ranging from no significant cytotoxicity to high levels of cytotoxicity and a strong immune response [24]. The advantage of these in vitro approaches is that they have the potential to more closely resemble how humans might respond to MP exposure. However, the lack of available evidence surrounding how both mammalian models and in vitro systems respond to plastic microfibers as compared to spherical MPs leaves much to be desired.
A more recent studied began to explore this question, studying how MPs of varied shape relates to cytotoxicity in human cell lines. They found that MPs with rigid, sharp, or irregular edges appeared to be more harmful than those with smooth, round edges [25]. Future studies should find which shapes of MPs are most prevalent in various tissues, and then test the impacts of MPs with those same shapes in mammalian models or in vitro cultures for the corresponding cell types.
3. Size
3.1. Aquatic Effects
Particle size is a critical factor in uptake and egestion. For small organisms such as plankton, particles larger than their mouth opening cannot be ingested. However, using only particles small enough to be eaten is also unrealistic, because it maximizes organism exposure [26]. One might expect that after ingestion, smaller particles would be better able to penetrate through tissues and cause greater toxicity, but that is not always the case. Roch et al. (2021) found that rainbow trout evacuated 50% of particles in 12 h for 42.7 µm particles and 4 h for 1086 µm particles (which is less than the time for evacuating food) [27]. In contrast, the differences between sizes for evacuation by common carp were smaller: 7 h for 42.7 µm particles and 4.6 h for 1086 µm particles. They concluded that it is likely that large particles in rainbow trout must be actively transported out of the stomach, since they had shorter evacuation times than food, while in carp, evacuation rates of all particle sizes were in the same range as food, suggesting a passive process.
In Daphnia, accumulation of fragments was higher than that of beads, and inhibition of feeding and growth depended on the size of MP fragments [28]. Survival of D. magna exposed to small- and large-sized fragments (17 vs. 34 μm) was significantly lower than that of daphnids exposed to MP beads. Small fragments significantly reduced feeding, and body retention time in the digestive system.
In the marine medaka (Oryzias melastigma), body weight, adipocyte size and liver lipid contents were significantly increased in fish exposed to large (200 μm) PS-MPs, while fish exposed to smaller (2 and 10 μm) MPs had liver injury, specifically fibrosis and inflammation [29]. Since the larger particles did not enter the circulatory system, their impacts on intestinal microbes were investigated. Gut microbial diversity and composition were altered in fish exposed to PS-MPs, particularly the larger particles. However, when goldfish (Carassius auratus) were exposed to two sizes (0.25 and 8 μm) of polystyrene MPs at different environmentally relevant concentrations, enzyme changes and histological lesions were more severe in those that were exposed to the smaller sized MPs [30].
Bour et al., 2018 exposed the benthic bivalves Ennucula tenuis and Abra nitida to polyethylene MPs at 1, 10, and 25 mg/kg of sediment for four weeks [31]. Three sizes (4–6; 20–25 and 125–500 um) were used. No effects were seen on survival, condition index or burrowing behavior. However, A. nitida showed a significant decrease of protein in those exposed to the largest particles, at all concentrations. No changes were seen in protein, carbohydrate or lipid in E. tenuis, but total energy decreased in a dose-related manner in ones exposed to the largest size particles.
In a rare (and welcome) paper comparing effects of polyethylene terephthalate MFs of different lengths (50 and 100 μm), mussels (Mytilus galloprovincialis) were exposed to environmental (0.5 μg/L) and high (100 mg/L) MF concentrations for four days [32]. Short MFs accumulated in the lower intestinal organs, but long MFs were only observed in the upper intestinal organs. Both sized MFs affected necrosis, DNA damage, reactive oxygen species, nitric oxide, and acetylcholinesterase. Fiber length-dependent effects occurred at environmental concentrations for DNA damage (long MFs) and AChE activity (short MFs).
3.2. Human Effects
Due to the availability of various sized MP spheres, there is more known about how particle size impacts mammalian models and human cells. In human lung tissue samples, MP particles were found to be smaller than 5.5 µm, whereas plastic microfibers were found to range from 8.12 to 16.8 µm [19]. In human colon samples, MPs were surprisingly larger, with an average size of 1.1 mm and a range of 0.8 to 1.6 mm [20]. This is particularly relevant because it suggests that different tissues might be exposed to sizes of MPs that vary greatly. Perhaps those that are airborne and inhaled are generally smaller than those ingested. These considerations should be incorporated into future studies.
Keeping this point in mind, human colon cell lines (Caco-2 cells) were exposed to 0.1 µm and 5 µm MP spheres. Although both sizes disrupted mitochondrial membrane potential, 5 µm particles displayed a stronger effect. However, both sizes inhibited ATP-binding cassette transporter activity, but in this context, 0.1 µm MPs were more disruptive [33].
The size-dependent impacts of MPs were found in another study in mice. Pregnant mice were exposed to either 0.5 µm or 5.0 µm polystyrene MP. Their offspring were examined for metabolic disorders, and it was found that those coming from mothers exposed to 5.0 µm MPs had more severe metabolic defects, including altered metabolites and hepatic gene expression [34]. Similarly, in a different study larger MPs (10 µm) were shown to more strongly damage testis tissue architecture, and decrease viability than smaller MPs (0.5 µm), although all three size were able to enter cells in vitro [35]. Interestingly, in a human vascular endothelial cell line (HUVECs), size-dependent impacts of MPs were also observed. However, in this case, smaller MPs (0.5 µm) were shown to have a greater impact on cell viability in an autophagy-dependent manner, as compared to larger MPs (5.0 µm) [36].
Furthermore, a study where mice were fed with MPs found that smaller MPs (5 µm) were more likely to translocate to the gut and kidney after ingestion. Larger MPs (20 µm) accumulated in the liver [37].
As these selected references show, there is a clear size dependent impact of MPs. Yet, in a recent report, no effects were observed at any MP size tested—1 µm, 4 µm, 10 µm—despite all three having some level of cellular uptake [38]. The discrepancies between these results, and between the sizes of MPs observed in various tissues compared to those used in experimental studies requires further investigation. Furthermore, there are a dearth of studies investigating how plastic microfibers of various sizes interact with cells and tissues in mammalian models and human cells. This is critical, as it appears that fibers are one of the more common classes of MP that become embedded in tissues.
References
- Burns, E.E.; Boxall, A.B.A. Microplastics in the aquatic environment: Evidence for or against adverse impacts and major knowledge gaps. Environ. Toxicol. Chem. 2018, 37, 2776–2796.
- Bucci, K.; Tulio, M.; Rochman, C.M. What is known and unknown about the effects of plastic pollution: A meta-analysis and systematic review. Ecol. Appl. 2020, 30, e02044.
- Gago, J.; Carretero, O.; Filgueiras, A.V.; Viñas, L. Synthetic microfibers in the marine environment: A review on their occurrence in seawater and sediments. Mar. Pollut. Bull. 2018, 127, 365–376.
- Belzagui, F.; Buscio, V.; Gutiérrez-Bouzán, C.; Vilaseca, M. Cigarette butts as a microfiber source with a microplastic level of concern. Sci. Total Environ. 2021, 762, 144165.
- Kögel, T.; Bjorøy, Ø.; Toto, B.; Bienfait, A.; Sanden, M. Micro- and nanoplastic toxicity on aquatic life: Determining factors. Sci. Total Environ. 2020, 709, 136050.
- Ward, J.E.; Rosa, M.; Shumway, S.E. Capture, ingestion, and egestion of microplastics by suspension-feeding bivalves: A 40-year history. Anthr. Coasts 2019, 2, 39–49.
- Li, L.; Su, L.; Cai, H.; Rochman, C.M.; Li, Q.; Kolandhasamy, P.; Peng, J.; Shi, H. The uptake of microfibers by freshwater Asian clams (Corbicula fluminea) varies based upon physicochemical properties. Chemosphere 2019, 221, 107–114.
- Horn, D.A.; Granek, E.F.; Steele, C.L. Effects of environmentally relevant concentrations of microplastic fibers on Pacific mole crab (Emerita analoga) mortality and reproduction. Limnol. Oceanogr. Lett. 2020, 5, 74–83.
- Setyorini, L.; Michler-Kozma, D.; Sures, B.; Gabel, F. Transfer and effects of PET microfibers in Chironomus riparius. Sci. Total Environ. 2021, 757, 143735.
- Alnajar, N.; Jha, A.N.; Turner, A. Impacts of microplastic fibres on the marine mussel, Mytilus galloprovinciallis. Chemosphere 2021, 262, 128290.
- Woods, M.N.; Hong, T.J.; Baughman, D.; Andrews, G.; Fields, D.M.; Matrai, P.A. Accumulation and effects of microplastic fibers in American lobster larvae (Homarus americanus). Mar. Pollut. Bull. 2020, 157, 111280.
- Au, S.; TF, B.; Bridges, W.; Klaine, S. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ. Toxicol. Chem. 2015, 34, 2564–2572.
- Blarer, P.; Burkhardt-Holm, P. Microplastics affect assimilation efficiency in the freshwater amphipod Gammarus fossarum. Environ. Sci. Pollut. Res. 2016, 23, 23522–23532.
- Frydkjær, C.; Iversen, N.; Roslev, P. Ingestion and egestion of microplastics by the cladoceran daphnia magna: Effects of regular and irregular shaped plastic and sorbed phenanthrene. Bull. Environ. Contam. Toxicol. 2017, 99, 655–661.
- Cheng, H.; Feng, Y.; Duan, Z.; Duan, X.; Zhao, S.; Wang, Y.; Gong, Z.; Wang, L. Toxicities of microplastic fibers and granules on the development of zebrafish embryos and their combined effects with cadmium. Chemosphere 2021, 269, 128677.
- Mendrik, F.M.; Henry, T.B.; Burdett, H.; Hackney, C.R.; Waller, C.; Parsons, D.R.; Hennige, S.J. Species-specific impact of microplastics on coral physiology. Environ. Pollut. 2021, 269, 116238.
- Leads, R.R.; Burnett, K.G.; Weinstein, J.E. The effect of microplastic ingestion on survival of the grass shrimp Palaemonetes pugio (Holthuis, 1949) challenged with Vibrio campbellii. Environ. Toxicol. Chem. 2019, 38, 2233–2242.
- Pauly, J.L.; Stegmeier, S.J.; Allaart, H.A.; Cheney, R.T.; Zhang, P.J.; Mayer, A.G.; Streck, R.J. Inhaled cellulosic and plastic fibers found in human lung tissue. Cancer Epidemiol. Prev. Biomarkers 1998, 7, 419–428.
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; Dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124.
- Ibrahim, Y.S.; Anuar, S.T.; Azmi, A.A.; Khalik, W.M.A.W.M.; Lehata, S.; Hamzah, S.R.; Ismail, D.; Ma, Z.F.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of microplastics in human colectomy specimens. JGH Open 2021, 5, 116–121.
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274.
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of various microplastics in human stool: A prospective case series. Ann. Intern. Med. 2019, 171, 453–457.
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317.
- Fournier, E.; Etienne-Mesmin, L.; Grootaert, C.; Jelsbak, L.; Syberg, K.; Blanquet-Diot, S.; Mercier-Bonin, M. Microplastics in the human digestive environment: A focus on the potential and challenges facing in vitro gut model development. J. Hazard. Mater. 2021, 415, 125632.
- Choi, D.; Hwang, J.; Bang, J.; Han, S.; Kim, T.; Oh, Y.; Hwang, Y.; Choi, J.; Hong, J. In vitro toxicity from a physical perspective of polyethylene microplastics based on statistical curvature change analysis. Sci. Total Environ. 2021, 752, 142242.
- Bour, A.; Hamann Sandgaard, M.; Syberg, K.; Palmqvist, A.; Carney Almroth, B. Comprehending the complexity of microplastic organismal exposures and effects, to improve testing frameworks. J. Hazard. Mater. 2021, 415, 125652.
- Roch, S.; Ros, A.F.H.; Friedrich, C.; Brinker, A. Microplastic evacuation in fish is particle size-dependent. Freshw. Biol. 2021, 66, 926–935.
- An, D.; Na, J.; Song, J.; Jung, J. Size-dependent chronic toxicity of fragmented polyethylene microplastics to Daphnia magna. Chemosphere 2021, 271, 129591.
- Zhang, X.; Wen, K.; Ding, D.; Liu, J.; Lei, Z.; Chen, X.; Ye, G.; Zhang, J.; Shen, H.; Yan, C.; et al. Size-dependent adverse effects of microplastics on intestinal microbiota and metabolic homeostasis in the marine medaka (Oryzias melastigma). Environ. Int. 2021, 151, 106452.
- Abarghouei, S.; Hedayati, A.; Raeisi, M.; Hadavand, B.S.; Rezaei, H.; Abed-Elmdoust, A. Size-dependent effects of microplastic on uptake, immune system, related gene expression and histopathology of goldfish (Carassius auratus). Chemosphere 2021, 276, 129977.
- Bour, A.; Haarr, A.; Keiter, S.; Hylland, K. Environmentally relevant microplastic exposure affects sediment-dwelling bivalves. Environ. Pollut. 2018, 236, 652–660.
- Choi, J.; Kim, K.; Hong, S.; Park, K.; Park, J. Impact of polyethylene terephthalate microfiber length on cellular responses in the Mediterranean mussel Mytilus galloprovincialis. Mar. Environ. Res. 2021, 168, 105320.
- Wu, B.; Wu, X.; Liu, S.; Wang, Z.; Chen, L. Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2 cells. Chemosphere 2019, 221, 333–341.
- Luo, T.; Zhang, Y.; Wang, C.; Wang, X.; Zhou, J.; Shen, M.; Zhao, Y.; Fu, Z.; Jin, Y. Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ. Pollut. 2019, 255, 113122.
- Jin, H.; Ma, T.; Sha, X.; Liu, Z.; Zhou, Y.; Meng, X.; Chen, Y.; Han, X.; Ding, J. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 2021, 401, 123430.
- Lee, H.-S.; Amarakoon, D.; Wei, C.; Choi, K.Y.; Smolensky, D.; Lee, S.-H. Adverse effect of polystyrene microplastics (PS-MPs) on tube formation and viability of human umbilical vein endothelial cells. Food Chem. Toxicol. 2021, 154, 112356.
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 1–10.
- Stock, V.; Böhmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.; et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch. Toxicol. 2019, 93, 1817–1833.
