Glucosinolates are amino acid-derived plant-specialized metabolites that are largely found within the members of the family Brassicaceae, which includes vegetables such as broccoli, cabbage, and mustard, as well as the model plant Arabidopsis thaliana (thale cress). The aliphatic glucosinolates are derived from methionine, alanine, leucine, isoleucine, or valine; aromatic glucosinolates are built from phenylalanine or tyrosine; and the indole glucosinolates originate with tryptophan. Each of class of glucosinolate shares a core chemical structure consisting of a β-D-glucosyl residue linked to a (Z)-N-hydroximinosulfate ester through a sulfur and a variable amino acid-derived R group. To date, more than 130 glucosinolate molecules, of which Arabidopsis contains 40 mainly derived from methionine and tryptophan, have been described.
- glucosinolate
- glucosinolate biosynthesis
- secondary metabolism
- Brassicaceae
- amino acid-derived metabolite
- plant metabolite
1. Introduction to the Glucosinolates
Glucosinolates are amino acid-derived plant-specialized metabolites that are largely found within the members of the family Brassicaceae, which includes vegetables such as broccoli, cabbage, and mustard, as well as the model plant
(thale cress) [1]. They have been reported in 14 other families from the order Capparales, as well as in the family Euphorbiaceae from the genus
, which is unrelated to other glucosinolate-containing families [2]. Glucosinolates can be classified according to their precursor amino acids. The aliphatic glucosinolates are derived from methionine, alanine, leucine, isoleucine, or valine; aromatic glucosinolates are built from phenylalanine or tyrosine; and the indole glucosinolates originate with tryptophan. Each of class of glucosinolate shares a core chemical structure consisting of a β-D-glucosyl residue linked to a (Z)-N-hydroximinosulfate ester through a sulfur and a variable amino acid-derived R group (
) [1]. To date, more than 130 glucosinolate molecules, of which
contains 40 mainly derived from methionine and tryptophan, have been described [3].
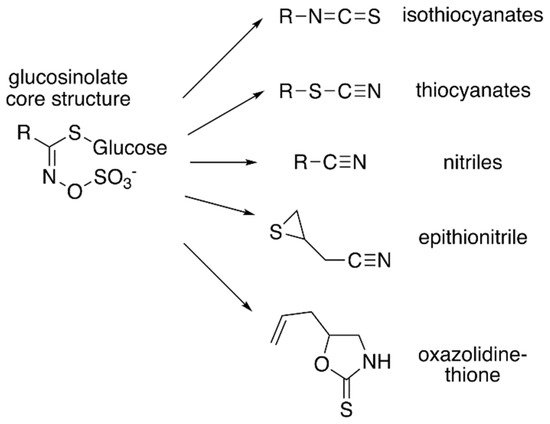
Core glucosinolate structure and hydrolysis product diversity. The core chemical structure of glucosinolates (
) consists of a β–D–glucosyl residue linked via a sulfur to a (Z)–N–hydroximinosulfate ester with and a variable amino acid-derived R group. Modifications of the aliphatic, aromatic, or indole R-group leads to the chemical diversity of this class of specialized metabolites. Hydrolysis of various glucosinolates leads to an array of bioactive molecules (
), including isothiocyanates, thiocyanates, nitriles, epithionitrile, and oxazolidine-thione.
Although the biological activities of all glucosinolates are yet to be elucidated, glucosinolates are believed to serve as responses to a wide range of external and/or environmental stimuli [4][5]. Much of glucosinolate function is related to their hydrolysis products (
), which accumulate in response to plant tissue damage. The various hydrolysis products are generated by a thioglucoside glucohydrolase known as myrosinase, which hydrolyzes the glucose moiety of the core glucosinolate structure [6][7]. The resulting products are glucose and an unstable aglycone compound that can rearrange to form isothiocyanates, nitriles, or other hydrolysis products, based on the starting glucosinolate structure type (
). The hydrolysis activity appears to be hindered by the physical separation of glucosinolates and myrosinase in intact plant tissue [1]. Glucosinolate hydrolysis products are involved in communicating a range of information pertaining to plant defense against insects, bacteria, and fungi. Some hydrolysis products, such as isothiocyanates, can be hydrolyzed further by the phenylalanine ammonia lyase to generate toxic compounds that can be injurious to certain pathogens [4]. Hence, some studies have proposed that glucosinolates may have a more direct role in plant defense.
Interest in glucosinolates has long been present in human society, mainly due to the distinct taste and flavors of certain Brassicaceae vegetables (cabbage, cauliflower, broccoli) and condiments (mustard, horseradish, wasabi) that are present in our diet [8][9]. Glucosinolates have gained significance in an agricultural sense with the increased importance of rapeseeds/canola (
,
, and
) as oil crops worldwide [1]. To increase plant use efficiency, plant breeders have reduced the levels of glucosinolates in Brassica oil crops to allow the seedcake (i.e., the protein-rich residue left after seed processing) to be used as a nutritional supplement in animal feed [10]. This is done to avoid the anti-nutritive effects that glucosinolates can have on animals, as one of the major glucosinolates in canola hydrolyzes to an oxazolidine-2-thione, which causes goiter and has other negative effects in cattle [11]. Glucosinolate-rich plants can also be used as biofumigation agents. For example, post-harvest plant material can be incorporated into soils, where the glucosinolate-containing material can suppress pathogen, nematode, and/or weed growth [12][13][14]. Breeders have also tried to modify glucosinolate levels in rapeseed foliage to address damage from fungal and insect pests [15][16]. For human health, glucosinolates are potentially useful because of their reported cancer-preventative action in animal models. For example, 4-methylsulfinylbutyl glucosinolate, which is found in broccoli, hydrolyzes to the isothiocyanate sulforaphane, a molecule that blocks the cell cycle and promotes apoptosis to fight tumor growth [8][17][18][19][20]. Sulforaphane can also slow effects of
-caused gastritis and stomach cancer [21]. Overall, glucosinolate engineering in plants and production platforms such as bacteria offer useful tools for the application of these natural products in plant defense, agriculture, human health, and animal nutrition.
2. Glucosinolate Biosynthesis
Biosynthesis of glucosinolates requires the integration of multiple building blocks to generate their shared chemical structure. For the aliphatic glucosinolates, methionine provides the starting point of their biosynthesis through an iterative series of reactions that elongate the R-group, i.e., aliphatic chain elongation (
). In the second stage of aliphatic glucosinolate synthesis (
), the methionine-derived elongation products then undergo a series of modifications, including addition of the sugar and sulfate groups, to yield the core glucosinolate structure. A similar set of reactions for the aromatic and indole glucosinolates builds directly from the initial amino acids. Once the core glucosinolate structure is assembled, the third stage of biosynthesis consists of various secondary modifications to the R-group generate the diverse array of described glucosinolates [22][23][24][25][26][27].
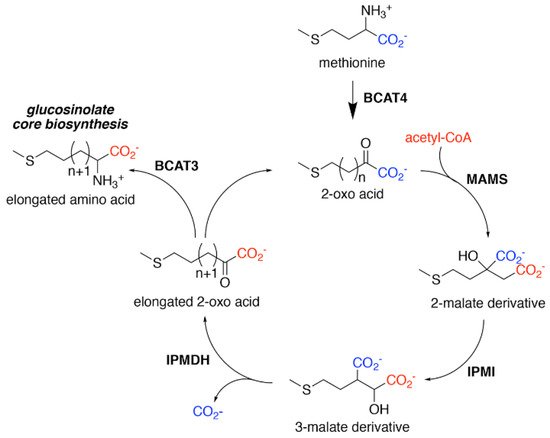
Aliphatic Glucosinolate Chain Elongation. Conversion of methionine to its corresponding 2-oxo acid (4–(methylsulfanyl)–2–oxobutanoate) by branched-chain amino transferase 4 (BCAT4) provides the starting point for the chain elongation cycle. A series of reactions catalyzed by methylthioalkylmalate synthase (MAMS), isopropylmalate isomerase (IPMI), and isopropylmalate dehydrogenase (IPMDH) result in elongation of the aliphatic chain by a single methylene group. The elongated 2–oxo can undergo transamination to the corresponding amino acid by BCAT3 or reenter the chain elongation cycle.
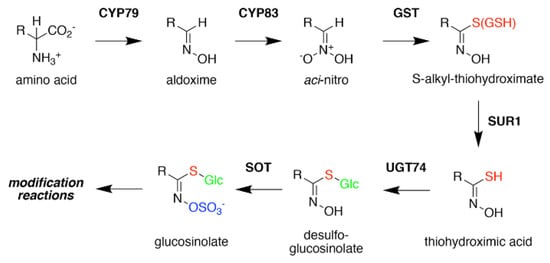
Glucosinolate Core Biosynthesis. A variety of amino acids (i.e., variable R-group), including elongated aliphatic methionine-derived molecules (see
), can be converted to aldoximes by members of the CYP79 cytochrome P450 family to begin construction of the core glucosinolate scaffold (see
, left). Members of the CYP83 family generate the unstable aci–nitro compounds believed to be the substrate of glutathione-S-transferases that introduce the shared sulfur atom. The SUR1 cysteine-sulfur lyase converts the S-alkyl-thiohydroximate to thiohydroximic acid, which is then modified with a glucosyl-residue by UDP-glucosyltransferase (UGT) family 74 enzymes. The final step of the pathway involves sulfation by PAPS-dependent sulfotransferases (SOT). Subsequent modification reactions of the core structure yield the molecular diversity of glucosinolates.
For aliphatic glucosinolate synthesis, the chain-elongation process begins with deamination of methionine to the corresponding 2-oxo acid, which is catalyzed by branched-chain amino acid aminotransferase 4 (BCAT4) (
) [28][29]. Although BCAT4 is localized in the cytosol, the rest of the enzymes involved in the elongation pathway are localized in the chloroplast [30][31][32][33][34]. This indicates the need for import of 2-oxo acids into the chloroplast, a function that has been potentially assigned to a chloroplast-localized bile acid transporter BAT5 [35]. The next reactions in the sequence require a set of three enzymes—methylthioalkylmalate synthase (MAMS), isopropylmalate isomerase (IPMI), and isopropylmalate dehydrogenase (IPMDH) (
). These enzymes form an iterative cluster of reactions that elongate the aliphatic chain of the 2-oxo acid. MAMS catalyzes the condensation of the 2-oxo acid and acetyl-CoA to form a 2-malate derivative [30][31][36][37][38]. Isomerization of the 2-malate derivative to a 3-malate derivative is catalyzed by IPMI [33][39]. The final step in the elongation cycle is performed by IPMDH, which oxidatively decarboxylates the 3-malate derivative to a 2-oxo acid, which elongates the original 2-oxo acid by a methylene group [34][40][41]. After each cycle, the elongated 2-oxo acid can either proceed through another round of chain elongation or be transaminated by BCAT3 to yield homomethionine (or its elongated derivatives), which enters the core glucosinolate assembly pathway [42][43] (
).
Formation of the glucosinolate core structure takes place in the cytosol and involves a set of reactions shared by aliphatic, aromatic, and indole glucosinolates (
). Conversion of elongated methionine-derived amino acids, along with phenylalanine, tyrosine, and tryptophan, into aldoximes is performed by a set of cytochrome P450s of the CYP79 family. CYP79F1 catalyzes the reaction with all elongated methionine derivatives, while CYP79F2 only converts long-chain methionine derivatives; CYP79B3 catalyzes the reaction with tryptophan; and CYP79A2 uses phenylalanine [44][45][46][47][48][49]. The aldoximes are oxidized to either nitrile oxides or aci-nitro compounds by CYP83 cytochrome P450s. CYP83B1 oxidizes both the tryptophan and phenylalanine-derived acetaldoximes, while CYP83A1 converts various aliphatic aldoximes [50][51][52][53][54].
The resulting cytochrome P450 products are conjugated to the sulfur donor glutathione by glutathione-S-transferases to produce S-alkyl-thiohydroximates, which serve as substrates for the carbon-sulfur lyase SUR1 [55]. This is the first enzymatic transformation in the core glucosinolate biosynthesis pathway that links their assembly to enzymes of sulfur metabolism [7][56]. The thiohydroximates generated by SUR1 undergo S-glycosylation catalyzed by glucosyltransferases of the UGT74 family to form desulfoglucosinolates. UGT74C1 glucosylates the methionine-derived molecules and UGT74B1 modified the aromatic amino acid-derived compounds [57][58].
Sulfation of the desulfoglucosinolates to yield the final glucosinoate molecule is mediated by a set of sulfotransferases (SOTs) that use 3′-phosphoadenosine-5′-phosphosulfate (PAPS) as a sulfate donor [59][60][61][62][63]. This is the second step in the assembly of glucosinolates that depends on sulfur metabolism, as the activity of the adenosine-5′-phosphosulfate kinase is essential for PAPS formation and generation of a wide array of sulfonated metabolites and that PAPS synthesis responds to sulfur nutrient levels in plants [64][65][66][67][68][69][70][71]. Like many of the enzyme families involved in glucosinolate biosynthesis, plants encode multiple SOTs with certain members of the SOT family, such as SOT16, SOT17, and SOT18 in
, catalyzing the modification of desulfoglucosinolates [60][61][62]. Recent biochemical and X-ray crystallographic studies of Arabidopsis SOT18 identified the active site responsible for the reaction chemistry and key residues required for binding of PAPS; these features are conserved across SOT from plants and other organisms, including humans [72]. Interestingly, the molecular basis of substrate preference of the SOT in glucosinolate biosynthesis remains elusive. For example, comparison of the residues in the SOT active site between Arabidopsis SOT16, which prefers indole desulfoglucosinolates and the methionine-derived desulfoglucosinolate metabolizing SOT17 and SOT18, suggested that the basis of substrate selectivity involves residues beyond the ligand binding site and may be dictated by multiple conformationally flexible loops near the active site [72]. This is a biochemical problem not limited to the plant SOT, but is relevant for SOT families in other organisms, including humans.
Although the various biosynthetic steps in the aliphatic chain-elongation process and the assembly of the core glucosinolate scaffold have been determined, the molecular details for the evolution of these specialized processes and the biochemical selectivity for glucosinolate biosynthesis remain to be determined. Bioengineering efforts for the application of glucosinolates in plant defense, agriculture, human health, and animal nutrition require the knowledge of not only how glucosinolates are synthesized, but also what the specific enzymatic mechanisms and structures of enzymes involved in the glucosinolate biosynthetic pathway are.
References
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333.
- Rodman, J.; Karol, K.; Price, R.; Sytsma, K. Molecules, morphology, and Dahlgren's expanded order Capparales. Syst. Bot. 1996, 21, 289–307.
- Sønderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of glucosinolates- gene discovery and beyond. Trends Plant Sci. 2010, 15, 283–290.
- Singh, A. Glucosinolates and plant defense. In Glucosinolates- Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2017; pp. 237–246.
- Nguyen, V.P.T.; Stewart, J.; Lopez, M.; Ioannou, I.; Allais, F. Glucosinolates: Natural occurrence, biosynthesis, accessibility, isolation, structures, and biological activities. Molecules 2020, 25, 4537.
- Burmeister, W.P.; Cottaz, S.; Driguez, H.; Iori, R.; Palmieri, S.; Henrissat, B. The crystal structures of Sinapis alba myrosinase and a covalent glycosyl-enzyme intermediate provide insights into the substrate recognition and active-site machinery of an S-glycosidase. Structure 1997, 5, 663–675.
- Ravilious, G.E.; Jez, J.M. Structural biology of plant sulfur metabolism: From assimilation to biosynthesis. Nat. Prod. Rep. 2012, 29, 1138–1152.
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51.
- Engel, E.; Baty, C.; Le Corre, D.; Souchon, I.; Martin, N. Flavor-active compounds potentially implicated in cooked cauliflower acceptance. J. Agric. Food Chem. 2002, 50, 6459–6467.
- Nour-Eldin, H.H.; Madsen, S.R.; Engelen, S.; Jørgensen, M.E.; Olsen, C.E.; Andersen, J.S.; Seynnaeve, D.; Verhoye, T.; Fulawka, R.; Denolf, P.; et al. Reduction of antinutritional glucosinolates in Brassica oilseeds by mutation of genes encoding transporters. Nat. Biotechnol. 2017, 35, 377–382.
- Griffiths, D.W.; Birch, A.N.E.; Hillman, J.R. Antinutritional compounds in the Brasi analysis, biosynthesis, chemistry and dietary effects. J. Hort. Sci. Biotech. 1998, 73, 1–18.
- Brown, P.D.; Morra, M.J. Glucosinolate-containing plant tissues as bioherbicides. J. Agric. Food Chem. 1995, 43, 3070–3074.
- Vaugh, S.F.; Palmquist, D.E.; Duval, S.M.; Berhow, M.A. Herbicidal activity of glucosinolate-containing seedmeals. Weed Sci. 2006, 54, 743–748.
- Zasada, I.A.; Ferris, H. Nematode suppression with brassicaceous amendments: Application based upon glucosinolate profiles. Soil Biol. Biochem. 2004, 36, 1017–1024.
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2009, 54, 57–83.
- Mithen, R. Leaf glucosinolate profiles and their relationship to pest and disease resistance in oilseed rape. Euphytica 1992, 64, 71–83.
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403.
- Thornalley, P.J. Isothiocyanates: Mechanism of cancer chemopreventive action. Anticancer Drugs 2002, 13, 331–338.
- Keum, Y.S.; Jeong, W.S.; Kong, A.N. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutat. Res. 2004, 555, 191–202.
- Hayes, J.D.; Kelleher, M.O.; Eggleston, I.M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur. J. Nutr. 2008, 47, 73–88.
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzopyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615.
- Ibdah, M.; Chen, Y.T.; Wilkerson, C.G.; Pichersky, E. An aldehyde oxidase in developing seeds of Arabidopsis converts benzaldehyde to benzoic acid. Plant Physiol. 2009, 150, 416–423.
- Li, J.; Hansen, B.G.; Ober, J.A.; Kliebenstein, D.J.; Halkier, B.A. Subclade of flavin-monooxygenases involved in aliphatic glucosinolate biosynthesis. Plant Physiol. 2008, 148, 1721–1733.
- Hansen, B.G.; Kliebenstein, D.J.; Halkier, B.A. Identification of a flavin-monooxygenase as the S-oxygenating enzyme in aliphatic glucosinolate biosynthesis in Arabidopsis. Plant J. 2007, 50, 902–910.
- Kliebenstein, D.J.; D’Auria, J.C.; Behere, A.S.; Kim, J.H.; Gunderson, K.L.; Breen, J.N.; Lee, G.; Gershenzon, J.; Last, R.L.; Jander, G. Characterization of seed-specific benzoyloxyglucosinolate mutations in Arabidopsis thaliana. Plant J. 2007, 51, 1062–1076.
- Brown, P.D.; Tokuhisa, J.G.; Reichelt, M.; Gershenzon, J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 2003, 62, 471–481.
- Kliebenstein, D.J.; Lambrix, V.M.; Reichelt, M.; Gershenzon, J.; Mitchell-Olds, T. Gene duplication in the diversification of secondary metabolism: Tandem 2-oxoglutarate-dependent dioxygenases control glucosinolate biosynthesis in Arabidopsis. Plant Cell 2001, 13, 681–693.
- Diebold, R.; Schuster, J.; Däschner, K.; Binder, S. The branched-chain amino acid transaminase gene family in Arabidopsis encodes plastid and mitochondrial proteins. Plant Physiol. 2002, 129, 540–550.
- Schuster, J.; Knill, T.; Reichelt, M.; Gershenzon, J.; Binder, S. Branched-chain aminotransferase4 is part of the chain elongation pathway in the biosynthesis of methionine-derived glucosinolates in Arabidopsis. Plant Cell 2006, 18, 2664–2679.
- Falk, K.L.; Vogel, C.; Textor, S.; Bartram, S.; Hick, A.; Pickett, J.A.; Gershenzon, J. Glucosinolate biosynthesis: Demonstration and characterization of the condensing enzyme of the chain elongation cycle in Eruca sativa. Phytochemistry 2004, 65, 1073–1084.
- Textor, S.; de Kraker, J.W.; Hause, B.; Gershenzon, J.; Tokuhisa, J.G. MAM3 catalyzes the formation of all aliphatic glucosinolate chain lengths in Arabidopsis. Plant Physiol. 2007, 144, 60–71.
- Sawada, Y.; Kuwahara, A.; Nagano, M.; Narisawa, T.; Sakata, A.; Saito, K.; Hirai, M.Y. Omics-based approaches to methionine side chain elongation in Arabidopsis: Characterization of the genes encoding methylthioalkylmalate isomerase and methylthioalkylmalate dehydrogenase. Plant Cell. Physiol. 2009, 50, 1181–1190.
- Knill, T.; Reichelt, M.; Paetz, C.; Gershenzon, J.; Binder, S. Arabidopsis thaliana encodes a bacterial-type heterodimeric isopropylmalate isomerase involved in both Leu biosynthesis and the Met chain elongation pathway of glucosinolate formation. Plant Mol. Biol. 2009, 71, 227–239.
- He, Y.; Mawhinney, T.P.; Preuss, M.L.; Schroeder, A.C.; Chen, B.; Abraham, L.; Jez, J.M.; Chen, S. A redox-active isopropylmalate dehydrogenase functions in the biosynthesis of glucosinolates and leucine in Arabidopsis. Plant J. 2009, 60, 679–690.
- Gigolashvili, T.; Yatusevich, R.; Rollwitz, I.; Humphry, M.; Gershenzon, J.; Flügge, U.I. The plastidic bile acid transporter 5 is required for the biosynthesis of methionine-derived glucosinolates in Arabidopsis thaliana. Plant Cell 2009, 21, 1813–1829.
- Textor, S.; Bartram, S.; Kroymann, J.; Falk, K.L.; Hick, A.; Pickett, J.A.; Gershenzon, J. Biosynthesis of methionine-derived glucosinolates in Arabidopsis thaliana: Recombinant expression and characterization of methylthioalkylmalate synthase, the condensing enzyme of the chain-elongation cycle. Planta 2004, 218, 1026–1035.
- de Kraker, J.W.; Gershenzon, J. From amino acid to glucosinolate biosynthesis: Protein sequence changes in the evolution of methylthioalkylmalate synthase in Arabidopsis. Plant Cell 2011, 23, 38–45.
- Kumar, R.; Lee, S.G.; Augustine, R.; Reichelt, M.; Vassão, D.G.; Palavalli, M.H.; Allen, A.; Gershenzon, J.; Jez, J.M.; Bisht, N.C. Molecular basis of the evolution of methylthioalkylmalate synthase and the diversity of methionine-derived glucosinolates. Plant Cell 2019, 31, 1633–1647.
- He, Y.; Chen, B.; Pang, Q.; Strul, J.M.; Chen, S. Functional specification of Arabidopsis isopropylmalate isomerases in glucosinolate and leucine biosynthesis. Plant Cell. Physiol. 2010, 51, 1480–1487.
- He, Y.; Galant, A.; Pang, Q.; Strul, J.M.; Balogun, S.F.; Jez, J.M.; Chen, S. Structural and functional evolution of isopropylmalate dehydrogenases in the leucine and glucosinolate pathways of Arabidopsis thaliana. J. Biol. Chem. 2011, 286, 28794–28801.
- He, Y.; Chen, L.; Zhou, Y.; Mawhinney, T.P.; Chen, B.; Kang, B.H.; Hauser, B.A.; Chen, S. Functional characterization of Arabidopsis thaliana isopropylmalate dehydrogenases reveals their important roles in gametophyte development. New Phytol. 2011, 189, 160–175.
- Knill, T.; Schuster, J.; Reichelt, M.; Gershenzon, J.; Binder, S. Arabidopsis branched-chain aminotransferase 3 functions in both amino acid and glucosinolate biosynthesis. Plant Physiol. 2008, 146, 1028–1039.
- Lächler, K.; Imhof, J.; Reichelt, M.; Gershenzon, J.; Binder, S. The cytosolic branched-chain aminotransferases of Arabidopsis thaliana influence methionine supply, salvage and glucosinolate metabolism. Plant Mol. Biol. 2015, 88, 119–131.
- Mikkelsen, M.D.; Hansen, C.H.; Wittstock, U.; Halkier, B.A. Cytochrome P450 CYP79B2 from Arabidopsis catalyzes the conversion of tryptophan to indole-3-acetaldoxime, a precursor of indole glucosinolates and indole-3-acetic acid. J. Biol. Chem. 2000, 275, 33712–33717.
- Wittstock, U.; Halkier, B.A. Cytochrome P450 CYP79A2 from Arabidopsis thaliana L. catalyzes the conversion of L-phenylalanine to phenylacetaldoxime in the biosynthesis of benzylglucosinolate. J. Biol. Chem. 2000, 275, 14659–14666.
- Hull, A.K.; Vij, R.; Celenza, J.L. Arabidopsis cytochrome P450s that catalyze the first step of tryptophan-dependent indole-3-acetic acid biosynthesis. Proc. Natl. Acad. Sci. USA 2000, 97, 2379–2384.
- Hansen, C.H.; Wittstock, U.; Olsen, C.E.; Hick, A.J.; Pickett, J.A.; Halkier, B.A. Cytochrome P450 CYP79F1 from Arabidopsis catalyzes the conversion of dihomomethionine and trihomomethionine to the corresponding aldoximes in the biosynthesis of aliphatic glucosinolates. J. Biol. Chem. 2001, 276, 11078–11085.
- Chen, S.; Glawischnig, E.; Jørgensen, K.; Naur, P.; Jørgensen, B.; Olsen, C.E.; Hansen, C.H.; Rasmussen, H.; Pickett, J.A.; Halkier, B.A. CYP79F1 and CYP79F2 have distinct functions in the biosynthesis of aliphatic glucosinolates in Arabidopsis. Plant J. 2003, 33, 923–937.
- Glawischnig, E. The role of cytochrome P450 enzymes in the biosynthesis of camalexin. Biochem. Soc. Trans. 2006, 34, 1206–1208.
- Bak, S.; Tax, F.E.; Feldmann, K.A.; Galbraith, D.W.; Feyereisen, R. CYP83B1, a cytochrome P450 at the metabolic branch point in auxin and indole glucosinolate biosynthesis in Arabidopsis. Plant Cell 2001, 13, 101–111.
- Bak, S.; Feyereisen, R. The involvement of two P450 enzymes, CYP83B1 and CYP83A1, in auxin homeostasis and glucosinolate biosynthesis. Plant Physiol. 2001, 127, 108–118.
- Hansen, C.H.; Du, L.; Naur, P.; Olsen, C.E.; Axelsen, K.B.; Hick, A.J.; Pickett, J.A.; Halkier, B.A. CYP83B1 is the oxime-metabolizing enzyme in the glucosinolate pathway in Arabidopsis. J. Biol. Chem. 2001, 276, 24790–24796.
- Hemm, M.R.; Ruegger, M.O.; Chapple, C. The Arabidopsis ref2 mutant is defective in the gene encoding CYP83A1 and shows both phenylpropanoid and glucosinolate phenotypes. Plant Cell 2003, 15, 179–194.
- Naur, P.; Petersen, B.L.; Mikkelsen, M.D.; Bak, S.; Rasmussen, H.; Olsen, C.E.; Halkier, B.A. CYP83A1 and CYP83B1, two nonredundant cytochrome P450 enzymes metabolizing oximes in the biosynthesis of glucosinolates in Arabidopsis. Plant Physiol. 2003, 133, 63–72.
- Mikkelsen, M.D.; Naur, P.; Halkier, B.A. Arabidopsis mutants in the C-S lyase of glucosinolate biosynthesis establish a critical role for indole-3-acetaldoxime in auxin homeostasis. Plant J. 2004, 37, 770–777.
- Jez, J.M. Structural biology of plant sulfur metabolism: From sulfate to glutathione. J. Exp. Bot. 2019, 70, 4089–4103.
- Grubb, C.D.; Zipp, B.J.; Ludwig-Müller, J.; Masuno, M.N.; Molinski, T.F.; Abel, S. Arabidopsis glucosyltransferase UGT74B1 functions in glucosinolate biosynthesis and auxin homeostasis. Plant J. 2004, 40, 893–908.
- Gachon, C.M.; Langlois-Meurinne, M.; Henry, Y.; Saindrenan, P. Transcriptional co-regulation of secondary metabolism enzymes in Arabidopsis: Functional and evolutionary implications. Plant Mol. Biol. 2005, 58, 229–245.
- Klein, M.; Papenbrock, J. The multi-protein family of Arabidopsis sulphotransferases and their relatives in other plant species. J. Exp. Bot. 2004, 55, 1809–1820.
- Klein, M.; Reichelt, M.; Gershenzon, J.; Papenbrock, J. The three desulfoglucosinolate sulfotransferase proteins in Arabidopsis have different substrate specificities and are differentially expressed. FEBS J. 2006, 273, 122–136.
- Piotrowski, M.; Schemenewitz, A.; Lopukhina, A.; Müller, A.; Janowitz, T.; Weiler, E.W.; Oecking, C. Desulfoglucosinolate sulfotransferases from Arabidopsis thaliana catalyze the final step in the biosynthesis of the glucosinolate core structure. J. Biol. Chem. 2004, 279, 50717–50725.
- Klein, M.; Papenbrock, J. Kinetics and substrate specificities of desulfo-glucosinolate sulfotransferases in Arabidopsis thaliana. Physiol. Plant. 2009, 135, 140–149.
- Hirschmann, F.; Papenbrock, J. The fusion of genomes leads to more options: A comparative investigation on the desulfo-glucosinolate sulfotransferases of Brassica napus and homologous proteins of Arabidopsis thaliana. Plant Physiol. Biochem. 2015, 91, 10–19.
- Mugford, S.G.; Yoshimoto, N.; Reichelt, M.; Wirtz, M.; Hill, L.; Mugford, S.T.; Nakazato, Y.; Noji, M.; Takahashi, H.; Kramell, R.; et al. Disruption of adenosine-5'-phosphosulfate kinase in Arabidopsis reduces levels of sulfated secondary metabolites. Plant Cell 2009, 21, 910–927.
- Mugford, S.G.; Matthewman, C.A.; Hill, L.; Kopriva, S. Adenosine-5′-phosphosulfate kinase is essential for Arabidopsis viability. FEBS Lett. 2010, 584, 119–123.
- Yatusevich, R.; Mugford, S.G.; Matthewman, C.; Gigolashvili, T.; Frerigmann, H.; Delaney, S.; Koprivova, A.; Flügge, U.I.; Kopriva, S. Genes of primary sulfate assimilation are part of the glucosinolate biosynthetic network in Arabidopsis thaliana. Plant J. 2010, 62, 1–11.
- Mugford, S.G.; Lee, B.R.; Koprivova, A.; Matthewman, C.; Kopriva, S. Control of sulfur partitioning between primary and secondary metabolism. Plant J. 2011, 65, 96–105.
- Ravilious, G.E.; Nguyen, A.; Francois, J.A.; Jez, J.M. Structural basis and evolution of redox regulation in plant adenosine-5’-phosphosulfate kinase. Proc. Natl. Acad. Sci. USA 2012, 109, 309–314.
- Ravilious, G.E.; Jez, J.M. Nucleotide binding site communication in Arabidopsis thaliana adenosine 5′-phosphosulfate kinase. J. Biol. Chem. 2012, 287, 30385–30389.
- Ravilious, G.E.; Westfall, C.S.; Jez, J.M. Redox-linked gating of nucleotide binding by the N-terminal domain of adenosine 5'-phosphosulfate kinase. J. Biol. Chem. 2013, 288, 6107–6115.
- Jez, J.M.; Ravilious, G.E.; Herrmann, J. Structural insights on the regulation of the plant sulfation pathway. Chem.-Bio. Interact. 2016, 259, 31–38.
- Hirschmann, F.; Krause, F.; Baruch, P.; Chizhov, I.; Mueller, J.W.; Manstein, D.J.; Papenbrock, J.; Fedorov, R. Structural and biochemical studies of sulphotransferase 18 from Arabidopsis thaliana explain its substrate specificity and reaction mechanism. Sci. Rep. 2017, 7, 4160.
