Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by ALİ CENK ÖZAY and Version 2 by Catherine Yang.
Myo-inositol (myo-Ins) and D-chiro-inositol (D-chiro-Ins) are natural compounds involved in many biological pathways. Since the discovery of their involvement in endocrine signal transduction, myo-Ins and D-chiro-Ins supplementation has contributed to clinical approaches in ameliorating many gynecological and endocrinological diseases.
- myo-Inositol
- D-chiro-Inositol
- epimerase
- steroidogenesis
- testosterone
- neural tube defects
- polycystic ovary syndrome
- reproduction
- assisted reproduction techniques
1. Introduction: An Overview on Inositols
1.1. Inositol Discovery and Biology
Inositols caught the interest of clinicians, especially endocrinologists and gynecologists only in the past twenty years, although their story goes back a long way. It began in 1850 when the German physician and chemist, Johann Joseph Scherer, isolated a hexahydroxycyclohexane from muscle cells and named this molecule “Inositol” from the combination of the Greek terms [ìς (is, in-, “sinew, fiber”), -ose (indicating a carbohydrate), -ite (“ester”), -ol (“an alcohol”)] and also because of its sweet taste [1]. Only many years later, Maquenne established the inositol cyclohexanol structure, purifying it from leaves [2], while a century later the elegant work of Posternak described the configuration of the main inositol isomer in eukaryotic tissues: myo-inositol (myo-Ins) [3]. The structure of this hexahydroxycyclohexane allows the formation of nine different isomers: cis-, epi, allo-, myo-, neo-, scyllo-, L-chiro-, D-chiro- and muco- inositol (Figure 1) [4].
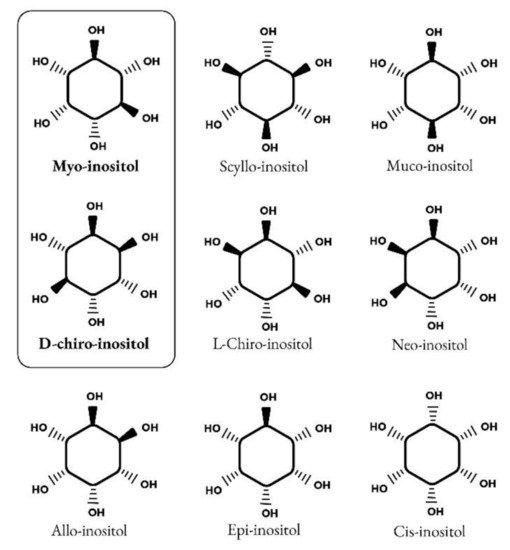
Figure 1.
Structure of nine isomers of inositol. Myo-inositol and D-chiro-inositol are the most common isomers of inositol.
Myo-Ins is an organic osmolyte that regulates cellular responses to hypertonic environments. Although myo-Ins absorption can occur by a diffusion process when it is highly concentrated, inositols uptake by cells is primarily carried out by a complex system of transporters, which mediate an active transport. Na+-coupled transport is exerted by sodium/myo-inositol transporter-1 (SMIT1) and sodium/myo-inositol transporter-2 (SMIT2), and H+-coupled transport is exerted by H+/myo-inositol transporter (HMIT) [5][6][7][5,6,7]. Those Myo-Ins transporters have been found in several tissues including kidney, brain, liver, pancreas, placenta, heart and skeletal muscle [8]. In particular, the expression of SMIT1 is upregulated by extracellular hypertonicity via transcriptional mechanisms, resulting in increased uptake of myo-Ins into cells. This prevents an increase in the concentration of inorganic ions without perturbing the activity of macromolecules [9].
Inositol is an important component of structural lipids, namely phosphatidyl-inositol (PI) and its various phosphates, including phosphatidyl-inositol phosphate (PIP) lipids [10]. Myo-Ins is basically incorporated into eukaryotic cell membranes as phosphatidyl-myo-inositol, the precursor of inositol triphosphate (InsP3), which acts as second messenger in the transduction of several endocrine signals, including FSH, TSH and insulin.
In humans a large amount of myo-Ins (about 1 g/day) is provided by dietary intake, with cereals, legumes, oil seeds and nuts representing the main sources [11], but a significant proportion of daily requirements is still synthesized endogenously (about 4 g/day), with kidneys being the major contributors.
Endogenously, myo-Ins is synthetized from glucose-6-phosphate (G6P), which is isomerized to inositol-3-phosphate (InsP3) by D-3-myo-inositol-phosphate synthase enzyme (inositol synthase, Ino1, or MIPS1) [12]. Then, through inositol monophosphatase-1 (IMPA-1 or IMPase), InsP3 is dephosphorylated into free myo-Ins [13]. Free myo-Ins could also be obtained through dephosphorylation of inositol-1,4,5-trisphosphate (InsP3) and inositol-1,4-bisphosphate (InsP2).
When the endogenous production of myo-Ins is insufficient to meet the human biological needs, an adequate intake of inositol either through specific food and/or supplements becomes necessary [14] (Figure 2).
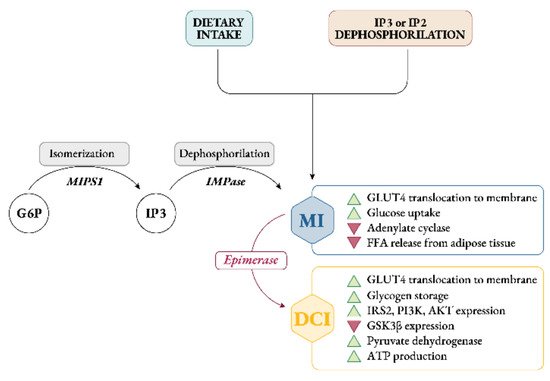
Figure 2. Synthesis, sources and role of myo-inositol and D-chiro-inositol in insulin signaling pathway. Abbreviations: G6P, glucose-6-phosphate; MIPS1, myo-inositol-phosphate synthase; IMPase, inositol monophosphatase; IP3, inositol-trisphosphate; IP2, inositol-biphosphate; MI, myo-inositol; DCI, D-chiro-inositol; GLUT4, glucose transporter type 4; FFA, free fatty acids; IRS2, insulin receptor type 2; PI3K, phosphoinositide 3-kinase; GSK3β, glycogen synthase kinase 3β.
Intracellularly, myo-Ins promotes GLUT4 translocation to the plasma membrane to enhance glucose uptake, inhibits adenylate cyclase and reduces free fatty acid release from adipose tissue [15][16][15,16]. This could explain why tissues with high glucose utilization, like brain, heart and ovary contain a large amount of myo-Ins compared with other tissues [17].
Under insulin stimulation, tissue-specific epimerase enzymes convert myo-Ins into its stereoisomer D-chiro-inositol (D-chiro-Ins) [18]. This is a unidirectional reaction which allows each organ and tissue to benefit from a specific and proper balance between myo-Ins and D-chiro-Ins content, ensuring the correct metabolic functions and consequent physiological status.
D-chiro-Ins stimulates glycogen synthase, and its levels are relatively increased in those tissues involved in glycogen storage such as liver or skeletal muscle [16]. Moreover, D-chiro-Ins increases mRNA and protein expression of IRS2, PI3K and AKT, upregulating the level of the P-AKT protein, and downregulating the level of the GSK3β protein [19] (Figure 2); all of which are key players in insulin and other hormone signal transduction.
By these activities D-chiro-Ins reduces the amount of cytosolic glucose, creating a glucose gradient that facilitates additional uptake of glucose through the mobilization of GLUT4 transporters, which are expressed in intracellular vesicles that are subsequently translocated to the cell membrane [20]. Moreover, D-chiro-Ins is involved in stimulating pyruvate dehydrogenase, which induces glycolysis and the Kreb’s cycle, resulting in the production of adenosine triphosphate (ATP) [21][22][21,22].
Through all these mechanisms myo-Ins and D-chiro-Ins may exert their insulin-sensitizing effect thereby decreasing insulin requirements, which are consequently reflected by lower circulating insulin concentrations [23].
Independently, Nestler and coworkers confirmed the insulin-mimetic action of a glycan containing D-chiro-Ins (called INS-2) on human ovarian thecal cells [24][25][27,28]. Nestler et al. observed that both insulin and D-chiro-Ins stimulated the biosynthesis of testosterone which was blocked by an antibody directed against this glycan (Figure 3).
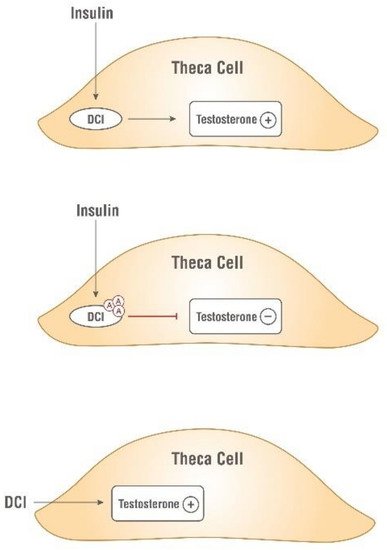
Figure 3. Insulin-mimetic action of D-chiro-Ins (DCI). Both insulin and D-chiro-Ins stimulate testosterone production by theca cell. Antibodies, Ⓐ, against this glycan block testosterone production.
These findings raised the interest of the scientific community in inositols’ insulin-mimetic properties, and the investigation of their usefulness in clinical practice increased concomitantly.
1.2. Inositols and Human Reproduction
It soon became clear that besides glucose metabolism, inositols are deeply involved in the physiology of female and male reproduction. In women, inositol, specifically myo-Ins, acts as an FSH second messenger (Figure 4) and is involved in FSH-mediated pathways that regulate proliferation and maturation of granulosa cells. Based on this role, myo-Ins modulates the FSH-mediated anti-Mullerian hormone (AMH) production, playing a pivotal role in determining oocyte maturation and transport in the oviduct as well as ensuring the good quality of embryos [26][29].
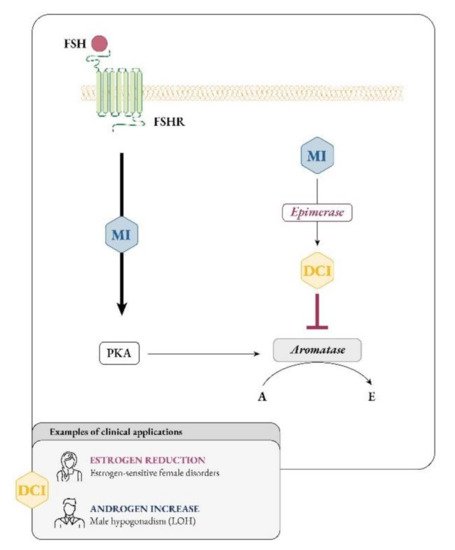
Figure 4. Myo-inositol and D-chiro-inositol affect aromatase activity in an opposite manner. Abbreviations: FSH, follicular stimulating hormone; FSHR, FSH receptor; MI, myo-inositol; DCI, D-chiro-inositol; PKA, protein kinase A; A, androgen; E, estrogen; LOH, late onset hypogonadism.
Ovaries, as well as other organs and tissues, are characterized by a specific ratio of myo-Ins to D-chiro-Ins, which ensures their healthy state and proper functionality (Table 1). In agreement with its functions, it can be guessed that the concentration of myo-Ins in a healthy female reproductive tract should be higher than that of D-chiro-Ins, supporting its important role in the ovaries. On the other hand, high D-chiro-Ins levels can negatively impact on the quality of oocytes and blastocysts [27][30], therefore its abundance needs to be tightly regulated.
Table 1. Myo-Ins (MI) to D-chiro-Ins (DCI) ratios in different tissues, both in physiological and insulin resistance conditions. Data were retrieved and adapted from the references [18][27][28][18,30,32].
| Physiological Conditions | Insulin Resistance Conditions | |||
|---|---|---|---|---|
| MI (%) | DCI (%) | MI (%) | DCI (%) | |
| Fat | 65 | 35 | 98 | 2 |
| Liver | 70 | 30 | 99.3 | 0.7 |
| Muscle | 74 | 26 | 98.1 | 1.9 |
| Blood | 97 | 3 | 99.6 | 0.4 |
| Kidney | 98 | 2 | 98.2 | 1.8 |
| Intestine | 98 | 2 | 98.2 | 1.8 |
| Spleen | 98.8 | 1.2 | 99 | 1 |
| Heart | 99.5 | 0.5 | 99.3 | 0.7 |
| Brain | 99.5 | 0.5 | 99.2 | 0.8 |
| Follicular fluid | 99.02 | 0.98 | 15 | 85 |
| Ovary (Theca) | 95.24 | 4.76 | 16.67 | 83.33 |
The impact of inositols on reproductive physiology must consider another inositol effect, namely, their influence on steroidogenesis, though this remains a poorly explored pathway. Indeed, both myo-Ins and D-chiro-Ins deeply affect the androgenic and estrogenic pools, likely in opposite directions.
Larner and Nestler mostly focused on D-chiro-Ins, concluding that this specific inositol influences steroidogenesis stimulating the ovarian production of androgens by thecal cells. Recently, Sacchi and coworkers proposed a second mechanism by which D-chiro-Ins could influence steroidogenesis, namely, by modulating the expression of aromatase enzyme and directly downregulating the synthesis of estrogens [29][31]. Considering that the healthy physiological status of tissues depends on a proper ratio of inositol concentrations, it is likely that an alteration of myo-Ins to D-chiro-Ins ratio may explain the imbalance in sex hormones observed in some pathological conditions, such as polycystic ovarian syndrome (PCOS), or secondary to pharmacological treatments, malabsorption or competition with glucose in food and beverages.
Overall, considering the growing interest in the clinical use of inositols, and based on the new findings on D-chiro-Ins activity, the present position paper aims to gather the most established evidence on the use of inositols in clinical practice as well as to introduce some novel therapeutic approaches, to allow clinicians to tailor inositol therapy for use in other medical areas, hitherto unexplored.
2. Emerging Roles for Inositol Treatments
2.1. The Importance of Myo- to D-chiro-Inositol Ratio in Steroidogenesis
As research progresses, it is becoming clearer that, besides constituting the intracellular second messengers of insulin signaling, inositols also function as endocrine modulators, influencing steroidogenesis. In 1998 Nestler first observed that D-chiro-Ins increased testosterone levels in theca cells from women with PCOS, even though the mechanism underlying this effect remained unknown [25][28]. Recently, new experiments suggested that D-chiro-Ins directly regulates the gene expression of enzymes involved in steroidogenesis in human granulosa cells, dose-dependently reducing the expression of both aromatase and cytochrome P450 side-chain cleavage genes [29][31]. Therefore, D-chiro-Ins modulates estrogen levels without completely blocking their biosynthesis.
On the other hand, evidence on the effect of myo-Ins is still needed and we speculate that this isomer may affect aromatase activity in an opposite manner from D-chiro-Ins. In support to this idea, myo-Ins is involved in modulating FSH signaling, and FSH stimulates aromatase synthesis, a fundamental step for conversion of androgens to estrogens and for follicle maturation [30][126]. FSH downregulation and the subsequent decrease in aromatase synthetized by granulosa cells constitute a hallmark of PCOS [31][127]. Thus, while D-chiro-Ins inhibits aromatase, myo-Ins seems to enhance aromatase synthesis in granulosa cells [32][33][128,129]. Moreover, myo-Ins could modulate ovarian steroidogenesis by rearranging cytoskeletal proteins [34][130].
In this regard, higher myo-Ins/D-chiro-Ins ratios should increase aromatase activity in granulosa, fostering estrogen biosynthesis, while lower myo-Ins/D-chiro-Ins ratios promote androgen production in thecal cells [35][131].
As a matter of fact, under normal homeostatic conditions, ovarian myo-Ins/D-chiro-Ins ratio ranges from 70:1 to 100:1, while in PCOS women this ratio decreased pathologically in favor of D-chiro-Ins [21][36][21,40]. The increased D-chiro-Ins concentration promotes androgen synthesis, while myo-Ins depletion worsens the energy state of the oocytes, impairing FSH signaling and oocyte quality [27][30].
These results were confirmed in a clinical trial by Nordio et al. [37][44]. In this study fifty-six patients with PCOS were supplemented with different myo-Ins/D-chiro-Ins ratios (namely 0:1; 1:3.5; 2.5:1; 5:1; 20:1; 40:1 and 80:1) with the aim to restore ovulatory function, as evidenced by progesterone assay, and ameliorate abnormalities in metabolic parameters, like FSH, LH, SHBG, E2, free testosterone, HOMA index and basal and postprandial insulin. The authors observed that the 40:1 combination produced the most significant improvements, followed by the 20:1 and 80:1, while the other ratios showed less relevant outcomes. Despite these promising results, of course, future studies are mandatory to shed light on the molecular aspects of ovarian inositol activity and to investigate the beneficial effects of an ideal myo-Ins/D-chiro-Ins formulation in larger cohorts of patients, possibly with different PCOS phenotypes.
2.2. Myo-Inositol Support to Assisted Reproduction Techniques (ART)
The correlation between the levels of myo-Ins and D-chiro-Ins in FF and the blastocyst quality only received a clearer elucidation with the study of Ravanos and coworkers [27][30]. They observed that blastocysts showing good quality were associated with a higher myo-Ins to D-chiro-Ins ratio in FF, compared to those rated as poor-quality that exhibited a large amount of D-chiro-Ins. This striking result suggests that the ratio between myo-Ins and D-chiro-Ins may be a valuable biomarker for blastocyst quality, especially in forecasting achievement of pregnancy.
From this evidence, the use of myo-Ins to support ART in PCOS women has figured prominently in scientific literature, since PCOS patients often experience infertility. In a retrospective study Wdowiak demonstrated myo-Ins effectiveness on PCOS women undergoing intracytoplasmic sperm injection (ICSI), concluding that 2 g myo-Ins plus 200 µg folic acid twice a day increased embryo development dynamics and accelerated blastocyst stage reaching time [38][134].
Further demonstrations of myo-Ins efficacy came from two other studies: the first is an IVF clinical trial on 133 PCOS women [39][135], in which the authors reported that supplementation with 1 g myo-Ins and 400 µg folic acid significantly augmented the number of mature oocytes compared to the control group, treated with folic acid and cyanocobalamin. In the second prospective, controlled, randomized trial by Özay and colleagues, the clinicians provided a supplement of 4 g myo-Ins and 400 µg folic acid to 98 infertile PCOS patients undergoing controlled ovarian hyperstimulation with rFSH and intrauterine insemination [40][136]. They observed that the myo-Ins treated group displayed a significant decrease in total rFSH dose required and cycle duration, as well as a higher pregnancy rate, when compared with controls.
Overall, based on these findings, it could be interesting to make some pharmaco-economic considerations on the possible reduced costs related to assisted reproduction techniques, with myo-Ins supplementation and less gonadotropin use, before or during these procedures.
2.3. D-chiro-Inositol Dual Effects (Reduction in Estrogens in Estrogen-Sensitive Female Disorders; Increase in Androgens in Male Hypogonadism)
The discovery that D-chiro-Ins modulates the expression of the aromatase enzyme, influencing steroidogenesis and consequently the androgen/estrogen balance [29][31], led to the consideration that D-chiro-Ins supplementation may be a valid approach for male and female clinical conditions that would benefit from androgen increase and/or estrogen decrease [32][41][128,140] (Figure 4).
Interestingly, the study by Monastra et al. [42][141] showed that supplementation with 1 g/day D-chiro-Ins for 1 month to male volunteers with altered glycemia and/or hormonal status reduced serum estrone and estradiol levels (−85.0% and −14.4% respectively) and increased testosterone and dehydroepiandrosterone (+23.4% and +13.8% respectively). Besides normalizing the hormonal balance, the treatment with D-chiro-Ins decreased glycemia, insulinemia and HOMA index as well.
These results seem to confirm that D-chiro-Ins functions as an aromatase down-modulator, clearly opening new perspectives of research and therapeutic applications with this inositol.
Within the male population a potential target for D-chiro-Ins administration might be elderly men suffering from late-onset hypogonadism (LOH), who present with an impaired production of adequate levels of testosterone by testis and sperm cells, resulting in androgen deficiency [43][44][142,143]. Decrease in sexual activity, loss of body hair, subfertility and erectile dysfunction represent the main symptoms that these patients experience, followed by an inescapable decline in their quality of life.
Testosterone replacement therapy (TRT) is widely used for the treatment of LOH, although there is still an extensive debate whether it should be administered to men with this condition [43][142].
Importantly, in 2015, the FDA issued a warning about potential cardiovascular risks resulting from TRT. In addition, another concern derives from the fact that exogenous testosterone can suppress the hypothalamic-pituitary-gonadal axis through negative feedback, and TRT may lead to secondary spermatogenic failure and subsequent infertility [45][144].
Since they normalize testosterone to estradiol ratio and improve sperm concentration, motility and morphology, aromatase inhibitors (AIs) and selective estrogen receptor modulators (SERMs) could represent off-label options to TRT, especially for obese individuals or those at high risk of TRT [46][145]. However, these medications have not yet been established as common clinical practice.
Based on these premises and due to its proven safety [47][146], D-chiro-Ins represents a valuable alternative approach for these patients.
As such, encouraging results have been obtained from a recent pilot study by Nordio and colleagues. The authors reported that, after 1 month of a daily supplementation with 1800 mg D-chiro-Ins, 10 patients showed significantly increased serum testosterone and androstenedione levels. Conversely, estradiol and estrone levels were reduced, thereby providing an important demonstration that D-chiro-Ins behaved as a molecule that could affect aromatase activity. Furthermore, the treatment with D-chiro-Ins positively impacted on insulin resistance and waist circumference, improving patients’ sexual performance and physical strength [48][147].
Clearly, further studies with larger cohorts of patients should be encouraged in order to confirm these exciting data but such promising results should not be overlooked.
Notably, estrogen deficiency predisposes males to increased adiposity and metabolic dysfunction. Paradoxically, however, obesity in men has been associated with hyperestrogenism. Moreover, excessive estradiol stimulation has been postulated to play an exacerbating role in the progression of obesity and metabolic dysregulation [49][148]. Obese men are often characterized by low circulating androgens but elevated levels of circulating estrone and 17β-estradiol [50][51][149,150]. The reasons for this co-occurrence of obesity with hyperestrogenemia in men are not well defined but may include polymorphism in the aromatase gene CYP19A [52][151]. It has been proposed that increased peripheral aromatization of testosterone in obese men may enhance central estradiol signaling, suppressing gonadotropin production and contributing to a sustained state of hypogonadotropic hypogonadism [53][152].
From these observations, it becomes clear that obese men would greatly benefit from D-chiro-Ins supplementation, since this inositol optimizes glucose metabolism, reduces plasma insulin levels, and concomitantly, by modulating aromatase activity, promotes androgen production.
Additionally, in women, decreasing estrogen levels represents an interesting therapeutic target, particularly in the clinical management of uterine leiomyomas, also called fibroids. Indeed, estrogens, as well as progesterone, play a pivotal role in promoting cell proliferation and growth of myomas, that represent the most frequent form of benign neoplasia affecting female reproductive organs [54][153]. Although leiomyomas are mostly asymptomatic and tend to resolve spontaneously after menopause [55][154], some women may experiment severe symptoms, such as pelvic pain, dysmenorrhea and bleeding.
Unfortunately, up-to-date specific and safe treatments for myomas are still lacking, especially after ulipristal acetate (UPA) was withdrawn from the market in September 2020 due to the rare but serious side effect of liver failure [56][155].
On the other hand, off-label pharmacological treatments including progestogens, androgens, estrogen receptors antagonists, selective progesterone receptor modulators (SPRM) and gonadotropin-releasing hormone agonists (GnRHa) have been proven effective in reducing tumor size and symptoms before surgery or, possibly, to completely avoid the surgical procedure.
Furthermore, it is noteworthy that neoplastic tissues express high levels of the aromatase enzyme compared with the lower expression observed in non-neoplastic (normal) tissues [57][156]. In accordance, several groups reported that aromatase is significantly overexpressed in myoma cells compared with the adjacent normal myometrium [58][59][157,158], leading to a high level of in situ estrogens that may contribute to the growth advantage of myomas through an intracrine/autocrine mechanism [60][159]. Therefore, lowering estrogen levels could be clearly considered a valid therapeutic approach for fibroid management [61][160]. Indeed, aromatase inhibitors, like Letrozole, proved to be effective in reducing myoma size and volume, ameliorating patient symptoms and improving quality of life [62][161].
Since D-chiro-Ins affects aromatase expression, it can be a promising clinical option for managing uterine myomas. Montanino Oliva reported the case of two patients with leiomyomas associated with heavy menstrual bleeding, who sought pregnancy through ART [63][162]. These women were supplemented daily with a combination of Epigallocatechin gallate (EGCG), vitamin D and low dose of D-chiro-Ins for 3 months.
Several studies [64][65][66][163,164,165] had indeed demonstrated that natural compounds, like EGCG and vitamin D were effective in reducing myoma size and, in this contest, D-chiro-Ins may improve their efficacy in arresting myoma cell growth. Montanino Oliva observed a reduction in the fibroid volume of 73.8% and 68.4%. Moreover, a decrease in menstrual blood loss was recorded (−42.1% and −48.7%). Interestingly, 3 months after the end of the treatment, both patients underwent the ART procedure without the need for surgical intervention.
These results suggested a potentially high efficacy of this combination in reducing fibroid volume and menstrual bleeding, so that surgery could be avoided. D-chiro-Ins action on modulating aromatase activity might help to explain the important volume reduction reported in both cases, and it is tempting to speculate that vitamin D and EGCG exerted their anti-proliferative and proapoptotic effects, enhanced by the activity of D-chiro-Ins.
Certainly, these outstanding results need further confirmation on a larger sample of women.
Down-modulation in the expression of the aromatase enzyme by D-chiro-Ins, similar to the action of Letrozole, can be considered a valid approach to restore ovulation. However, because of its activity on aromatase and the subsequent androgen-rising effect, the baseline clinical condition of patients as well as the duration of supplementation should be carefully evaluated before starting therapy with D-chiro-Ins, in order to avoid a negative impact on the ovaries.
When hyperinsulinemic anovulatory women are supplemented with D-chiro-Ins, it restores ovulation, mainly functioning as an insulin-sensitizer by improving insulin signaling and decreasing the systemic hyperinsulinemia accordingly. Unfortunately, results on non-hyperinsulinemic patients are still lacking in the literature.
Based on this finding and considering that the examined cases were normo-insulinemic, it is unlikely that insulin regulation played a pivotal part in restoring ovulatory function. The authors rather concluded that in such specific cases D-chiro-Ins probably acted mainly on aromatase expression, impairing estrogen biosynthesis and contributing to FSH release.
Hopefully, controlled studies with an appropriate sample of patients will be able to confirm this preliminary observation.
2.4. D-chiro-Inositol Supplementation: All a Matter of Dose and Time of Administration with a Focus on PCOS
While scientific evidence indicates that myo-Ins and D-chiro-Ins can synergistically be integrated into the clinical management of PCOS, exerting therapeutic effects and representing a reliable alternative to conventional medications, the emerging role of D-chiro-Ins as an aromatase modulator and androgen-raising molecule clearly requires further reflection on its clinical use. In particular, when D-chiro-Ins is supplemented alone, the appropriate dosage and timing of treatment should be carefully evaluated and tailored to the different clinical pictures. Indeed, as previously discussed, following long-term or high-dose treatments, D-chiro-Ins could predominantly affect steroidogenesis increasing androgen levels and worsening patients’ clinical picture, especially in case of PCOS patients, already characterized by hyperandrogenism.
The disruption of the ovarian organization constituted the principal result in mice treated with 500 and 1000 mg/kg/day D-chiro-Ins, while they exhibited minimal serum levels of testosterone and the ovarian content of aromatase was similar in both negative and positive controls. The authors speculated that these treatments could block the normal ovarian hormonal pathways, likely by inhibiting the expression/activity of cytochrome P450scc that catalyzes the first step of the steroidogenic cascade. Overall, these findings should encourage physicians to identify the most appropriate intervention with D-chiro-Ins, keeping in mind its dual function as well as patients’ metabolic and hormonal baseline conditions.
Indeed, as reiterated, D-chiro-Ins can improve some clinical conditions, but at the same time it can also worsen others. Those findings prompted Gambioli et al. to recommend specific daily doses and timing of D-chiro-Ins administration for several different medical conditions [32][128]. Of course, these observations and speculations need to be supported and confirmed hopefully through randomized, placebo-controlled, double-blind studies with greater sample sizes.
2.5. The Consequences of Inositol Deficiency Due to Pharmacological Treatments, Malabsorption or Competition with Dietary Glucose
2.6. The Consequences of Inositol Deficiency Due to Pharmacological Treatments, Malabsorption or Competition with Dietary Glucose
It should also be stressed that, when high doses of D-chiro-Ins or other sugar-like molecules are supplemented concomitantly with myo-Ins, they seem to negatively impact on its availability in the human body. As a matter of fact, Garzon et al. observed that D-chiro-Ins, sorbitol and maltodextrin, when provided together with myo-Ins, significantly decreased its absorption and plasma concentration, compared to when myo-Ins was supplemented alone [7]. As a possible explanation, the authors postulated a competitive action among these molecules at inositol transporters, mainly SMIT2. Indeed, this protein exhibits similar affinity for both inositol stereoisomers, as demonstrated by the Km values (120–150 µM for myo-Ins and 110–130 µM for D-chiro-Ins). However, considering that under physiological conditions the serum concentration of D-chiro-Ins is less than 100 nM, it is unlikely that it can interfere with myo-Ins absorption, since serum concentrations of myo-Ins typically range from 26.8 to 43.0 µM, which is significantly higher [67][68][185,186]. The same shall apply to the formulations in which myo-Ins and D-chiro-Ins are combined in a proper ratio, such as the 40:1 formula.
On the contrary, when D-chiro-Ins or other sugars from food are consumed at high dosage (≥1 g) and/or concomitantly with myo-Ins, a strong competition for SMIT2 transporters may occur, decreasing myo-Ins absorption in the gut and consequently altering plasma myo-Ins/D-chiro-Ins ratio that possibly accounts for pathological conditions.
In addition, it should not be underestimated that some pharmacological treatments, particularly antiepileptic drugs (AEDs), such as sodium valproate (VA), and mood stabilizers for bipolar disorder, such as lithium (Li+), can cause myo-Ins depletion. All of these medications share the “inositol-depletion hypothesis”, put forward to explain their therapeutic mechanism [69][187]. According to this theory, Li+ acts principally by inhibiting the monophosphatase (IMPase) and the inositol polyphosphatase (IPP), key enzymes in inositol synthesis, while VA inhibits the myoinositol-phosphate synthase (MIPS).
Although these drugs superficially appear beneficial to patients, since they are able to control seizures and mood disbalances, their chronic administration is not completely safe and may expose patients to side effects mostly associated with inositol depletion in peripheral tissues. Among these effects are hypothyroidism, weight gain, hyperinsulinemia, dyslipidemia, impairment of kidney function and adverse dermatological effects that are quite commonly reported [70][71][72][188,189,190]. Noteworthy, PCOS is one of the most serious side-effects reported by AEDs-treated women [72][73][74][75][190,191,192,193], who experience reduced estradiol and progesterone and increased testosterone, leading to hypogonadism, amenorrhea or oligomenorrhea, along with sexual dysfunction and infertility [76][194].
Importantly, even though these conditions may sometimes spontaneously resolve after a few weeks of treatment, or revert to baseline with drug discontinuation, they certainly worsen patients’ quality of life, weakening their compliance to therapeutic protocols.
Several scientific studies have documented myo-Ins supplementation as a safe and efficient approach in treating PCOS symptoms, improving hormonal profile, hyperandrogenism, menstrual cycle, oocyte quality and psychological disturbances [77][78][54,195]. Moreover, myo-Ins in combination with Selenium, has been reported to be effective in restoring euthyroidism in patients with subclinical hypothyroidism or autoimmune thyroiditis [79][196]. Therefore, supplementing with this inositol could be a valid option to counteract the peripherical side effects connected to bipolar disorder and epileptic treatment. Clearly, the dose of myo-Ins supplemented should be properly settled so as not to increase its levels in the brain and interfere with the central pharmacological therapeutic effect.
The possible ratio between myo-Ins and D-chiro-Ins may physiologically range from 10:1 to 100:1 and the ratio 80:1 in favor of myo-Ins is particularly encouraged [72][190].
From these premises, the concomitant supplementation with inositol to patients in therapy with Li+ or AEDs could represent an intriguing proposal. Interestingly, this combined treatment would safely reduce the peripheral side effects, ameliorating patients’ compliance and improving quality of life.
