Thiophene derivatives provide useful intermediaries in various areas of science and industry, with a wide range of applications, and therapeutic properties. Thiophene derivatives attract both great academic interest, and interest from the agrochemical, pharmaceutical, and dye industries, as well. As to their biological and pharmacological applications, thiophene derivatives possess remarkable properties as antipsychotic, antianxiety, antifungal, antimicrobial, antioxidant, anticancer, and anti-inflammatory agents. The present work provides an update on the role of thiophene-based derivatives in inflammation processes.
- inflammation
- thiophene
- molecular docking
- cyclooxygenase
- lipoxygenase
- anti-inflammatory drugs
1. Introduction
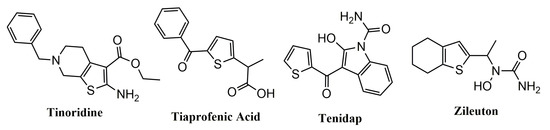
2. The Role of Thiophene Derivatives in Inflammation
2.1. Thiophene-Based Compounds Inhibitors of COX and/or LOX Enzymes
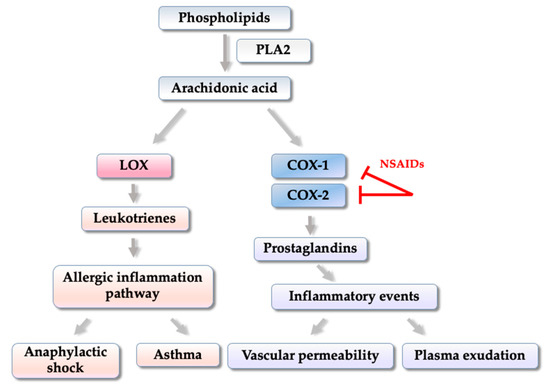
2.2. Thiophene Derivatives That Modulate Gene Expression and/or Inflammatory Cytokines
2.3. Thiophene Derivatives with In Vivo Anti-Inflammatory Activity in Classic Models of Inflammation
2.4. In Silico Studies Involving Thiophene-Based Compounds with Anti-Inflammatory Properties
The search for new bioactive compounds is a long process, which takes several years, and which gradually becomes expensive, costing tens of millions of dollars before reaching the goal of having a new chemical entity capable to be used in therapy. Aiming to reduce these costs, reducing the number of molecules that need to be synthesized, reducing the number of compounds that effectively need to be tested in the various in vitro, ex vivo, and in vivo assays, several computational methods, also known as in silico methods, or CADD (Computer-Aided Drug Design) studies were developed and have been constantly improved in order to reduce cost and times of the drug design and discovery process, and increase the chances of success. In silico methods are increasingly being used in both industry and in universities. They involve understanding molecular interactions from both a qualitative and quantitative point of view-based in mathematical tools. These methods generate and manipulate three-dimensional (3D) molecular structures, calculate descriptors and dependent molecular properties (pharmacokinetic (ADME) and toxicity properties, among others), model constructions, and employ other computational drug research tools. Analysis of the molecular structure of a given system allows relevant information to be extracted, as well as predicting the potential of the bioactive compound [40][41][42]. In silico methods are subdivided into two major general types of CADD approaches: Structure-Based Drug Design (SBDD) and Ligand-Based Drug Design (LBDD). SBDD methods are used to help investigators to predict, with precision and efficiency, in three dimensions, at a molecular level, the position (affinity) of small molecules (drug candidates or ligand) to molecular targets, usually proteins (enzymes) and nucleic acids (DNA and RNA). Where molecular docking is one of the most widespread and most used tool. In addition, LBDD approaches have been used when only the structure of the ligands are known, or when the data on the biological activities of those ligands were known. LBDD allows determinate the correlations between chemicals structures and the physicochemical properties of ligands and their biological activities. The three main categories of LBDD are: (a) pharmacophore models, which make it possible to identify the essential characteristics of the ligands for maintenance of biological activity; (b) Quantitative Structure–Activity Relationships (QSAR), which results in quantitative activity data based on the physicochemical properties of the ligands; and (c) similarity searching, which helps to predict the biological activity and the physicochemical properties of ligands based on existing data from other ligands with similar chemical structures [40][41][42]. In this context, Sagaama and Issaoui [43] performed a theoretical study involving molecular geometry, vibrational, pharmaceutical (1H and 13C Nuclear Magnetic Resonance (NMR) and UV-vis spectrum), and electronic properties (TD-DFT (time-dependent density-functional theory), HOMO-LUMO transitions (highest occupied molecular orbital and lowest unoccupied molecular orbital)), Hirshfeld surfaces, and molecular docking, using as a prototype 1-benzothiophene-2-carboxylic acid (2BT) (22) (Figure 5). Molecular docking was carried out using the iGEMDOCK program and Discovery studio software, against several targets, including: Human Immunodeficiency Virus type 1 (HIV) (PDB id: 1DLO), Bat SARS-like coronavirus (6LU7) (PDB id: 6LU7), and the inflammatory targets COX-2 (PDB id: 3LN1) and 5-LOX (PDB id: 3V92). The binding energy for COX-2 and 5-LOX, were respectively −81.44 and −72.48 kcal/mol.
3. Final Remarks
This work demonstrates the importance of thiophene-based compounds as privileged structures in drug design and in discovery of novel anti-inflammatory agents. The vast majority of their planned and synthesized derivatives present anti-inflammatory activity superior to the reference NSAIDs as was shown in in vitro, and in silico, and in vivo assays.
References
- Yeung, Y.T.; Aziz, F.; Guerrero-Castilla, A.; Arguelles, S. Signaling Pathways in Inflammation and Anti-inflammatory Therapies. Curr. Pharm. Des. 2018, 24, 1449–1484.
- Mack, M. Inflammation and fibrosis. Matrix Biol. 2018, 68–69, 106–121.
- Muszynska, B.; Grzywacz-Kisielewskaa, A.; Kala, K.; Gdula-Argasinska, J. Anti-inflammatory properties of edible mushrooms: A review. Food Chem. 2018, 243, 373–381.
- De Oliveira Pedrosa Rolim, M.; De Almeida, A.R.; Da Rocha Pitta, M.G.; De Melo Rêgo, M.J.B.; Quintans-Júnior, L.J.; De Souza Siqueira Quintans, J.; Heimfarth, L.; Scotti, L.; Scotti, M.T.; Da Cruz, R.M.D.; et al. Design, synthesis and pharmacological evaluation of CVIB, a codrug of carvacrol and ibuprofen as a novel anti-inflammatory agent. Int. Immunopharmacol. 2019, 76, 105856.
- Lahsasni, S.; Al-Hemyari, D.A.M.; Ghabbour, H.A.; Mabkhoot, Y.N.; Aleanizy, F.S.; Alothman, A.A.; Almarhoon, Z.M. Synthesis, Characterization, and Antibacterial and Anti-Inflammatory Activities of New Pyrimidine and Thiophene Derivatives. J. Chem. 2018, 2018, 8536063.
- Grondman, I.; Pirvu, A.; Riza, A.; Ioana, M.; Netea, M.G. Biomarkers of inflammation and the etiology of sepsis. Biochem. Soc. Trans. 2020, 48, 1–14.
- Von Vietinghoff, S.; Koltsova, E.K. Inflammation in atherosclerosis: A key role for cytokines. Cytokine 2019, 122, 154819.
- Barreto Mota, F.V.; Saraiva de Araújo Neta, M.; De Souza Franco, E.; Gomes Alves Bastos, I.V.; Cardoso Correia da Araújo, L.; Cabral da Silva, S.; Bezerra de Oliveira, T.; Souza, E.K.; Moraes de Almeida, V.; Matos Ximenes, R.; et al. Evaluation of anti-inflammatory activity and molecular docking study of new azabicyclic isooxazoline acylhydrazone derivatives. MedChemComm 2019, 10, 1916–1925.
- Yao, C.; Narumiya, S. Prostaglandin-cytokine crosstalk in chronic inflammation. Br. J. Pharmacol. 2019, 176, 337–354.
- Roe, K. An inflammation classification system using cytokine parameters. Scand. J. Immunol. 2021, 93, e12970.
- Sharma, S.; Kumar, D.; Singh, G.; Monga, V.; Kumar, B. Recent advancements in the development of heterocyclic anti-inflammatory agents. Eur. J. Med. Chem. 2020, 200, 112438.
- Shah, R.; Verma, P.K. Therapeutic importance of synthetic thiophene. Chem. Cent. J. 2018, 12, 137.
- Keri, R.S.; Chand, K.; Budagumpi, S.; Balappa Somappa, S.; Patil, S.A.; Nagaraja, B.M. An overview of benzo[b]thiophene-based medicinal chemistry. Eur. J. Med. Chem. 2017, 138, 1002–1033.
- Da Cruz, R.M.D.; Braga, R.M.; De Andrade, H.H.N.; Monteiro, Á.B.; Luna, I.S.; Cruz, R.M.D.; Scotti, M.T.; Mendonça-Junior, F.J.B.; De Almeida, R.N. RMD86, a thiophene derivative, promotes antinociceptive and antipyretic activities in mice. Heliyon 2020, 6, e05520.
- Bozorov, K.; Nie, L.F.; Zhao, J.; Aisa, H.A. 2-Aminothiophene scaffolds: Diverse biological and pharmacological attributes in medicinal chemistry. Eur. J. Med. Chem. 2017, 140, 465–493.
- Lisboa, T.; Silva, D.; Duarte, S.; Ferreira, R.; Andrade, C.; Lopes, A.L.; Ribeiro, J.; Farias, D.; Moura, R.; Reis, M.; et al. Toxicity and Antitumor Activity of a Thiophene-Acridine Hybrid. Molecules 2019, 25, 64.
- Madhavi, K.; Sree Ramya, G. Synthesis, antioxidant and anti-inflammatory activities of ethyl 2-(2-cyano-3-(substituted phenyl)acrylamido)-4,5-dimethylthiophene-3-carboxylates. Asian J. Pharm. Clin. Res. 2017, 10, 95–100.
- Kalariya, P.D.; Patel, P.N.; Kavya, P.; Sharma, M.; Garg, P.; Srinivas, R.; Talluri, M.V.N.K. Rapid structural characterization of in vivo and in vitro metabolites os tinoridine using UHPLC-QTOF-MS/MS and in silico toxicological screening of its metabolites. J. Mass Spectrom. 2015, 50, 1222–1233.
- Wu, Q.-Q.; Deng, W.; Xiao, Y.; Chen, J.-J.; Liu, C.; Wnag, J.; Guo, Y.; Duan, M.; Cai, Z.; Xie, S.; et al. The 5-Lipoxygenase Inhibitor Zileuton Protects Pressure Overload-Induced Cardiac Remodeling via Activating PPARα. Oxid. Med. Cell. Longev. 2019, 2019, 7536803.
- Li, M.; Yu, C.; Zeng, X. Comparative efficacy of traditional non-selective NSAIDs and selective cyclo-oxygenase-2 inhibitors in patients with acute gout: A systematic review and meta-analysis. BJM Open 2020, 10, e036748.
- Dona, I.; Salas, M.; Perkins, J.R.; Barrionuevo, E.; Gaeta, F.; Cornejo-Garcia, J.A.; Campo, P.; Torres, M.J. Hypersensitivity Reactions to Non-Steroidal Anti-Inflammatory Drugs. Curr. Pharm. Des. 2016, 45, 6784–6802.
- Bashir, S.; Elegunde, B.; Morgan, W.A. Inhibition of lipolysis: A novel explanation for the hypothermic actions of acetaminophen in non-febrile rodents. Biochem. Pharmacol. 2020, 172, 113774.
- Przybyla, G.W.; Szychowski, K.A.; Gmiński, J. Paracetamol—An old drug with new mechanisms of action. Clin. Exp. Pharmacol. Physiol. 2021, 48, 3–19.
- Fraenkel, L.; Buta, E.; Suter, L.; Dubreuil, M.; Levy, C.; Najem, C.; Brennan, M.; Corn, B.; Kerns, R.; Goulet, J. Nonsteroidal Anti-inflammatory Drugs vs. Cognitive Behavioral Therapy for Arthritis Pain: A Randomized Withdrawal Trial. JAMA Intern. Med. 2020, 180, 1194–1202.
- Tai, F.W.D.; McAlindon, M.E. NSAIDs and the small bowel. Curr. Opin. Gastroenterol. 2018, 34, 175–182.
- Prozzi, G.R.; Cañás, M.; Urtasun, M.A.; Buschiazzo, H.O.; Dorati, C.M.; Mordujovich-Buschiazzo, P. Cardiovascular risk of non-steroidal anti-inflammatory drugs. Medicina 2018, 78, 349–355.
- Foley, K.G.; Christian, A.; Peaker, J.; Marshall, C.; Spezi, E.; Kynaston, H.; Roberts, A. Cyclo-oxygenase-2 expression is associated with mean standardised uptake value on 18F-Fluorodeoxyglucose positron emission tomography in oesophageal adenocarcinoma. Br. J. Radiol. 2019, 1099, 1–5.
- Gunter, B.R.; Butler, K.A.; Wallace, R.L.; Smith, S.M.; Harirforoosh, S. Non-steroidal anti-inflammatory drug-induced cardiovascular adverse events: A meta-analysis. J. Clin. Pharm. Ther. 2017, 42, 27–38.
- Bartekova, M.; Radosinska, J.; Jelemensky, M.; Dhalla, N.S. Role of cytokines and inflammation in heart function during health and disease. Heart Fail. Rev. 2018, 23, 733–758.
- Wilson, H.M.; Cheyne, L.; Brown, P.A.J.; Kerr, K.; Hannah, A.; Srinivasan, J.; Duniak, N.; Horgan, G.; Dawson, D.K. Characterization of the myocardial inflammatory response in acute stress-induced (Takotsubo) cardiomyopathy. JACC Basic Transl. Sci. 2018, 3, 766–778.
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891.
- Hu, J.; Wang, G.; Liu, X.; Zhou, L.; Jiang, M.; Yang, L. Polo-like kinase 1 (PLK1) is involved in toll-like receptor (TLR)-mediated TNF-α production in monocytic THP-A cells. PLoS ONE 2013, 8, e78832.
- Vazquez, E.; Navarro, M.; Salazar, Y.; Crespo, G.; Bruges, G.; Osorio, C.; Tortorici, V.; Vanegas, H.; López, M. Systemic changes following carrageenan-induced paw inflammation in rats. Inflamm. Res. 2015, 64, 333–342.
- Abdelall, E.K.A.; Lamie, P.F.; Ahmed, A.K.M.; El-Nahass, E.S. COX-1/COX-2 inhibition assays and histopathological study of the new designed anti-inflammatory agent with a pyrazolopyrimidine core. Bioorg. Chem. 2019, 86, 235–253.
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56.
- Casaro, M.; Souza, V.R.; Oliveira, F.A.; Ferreira, C.M. OVA-Induced Allergic Airway Inflammation Mouse Model. Methods Mol. Biol. 2019, 1916, 297–301.
- Kumar, A.D.; Prabhudeva, M.G.; Bharath, S.; Kumara, K.; Lokanath, N.K.; Kumar, K.A. Design and Amberlyst-15 mediated synthesis of novel thianyl-pyrazole carboxamides that potently inhibit Phospholipase A2 by binding to an allosteric site on the enzyme. Bioorg. Chem. 2018, 80, 444–452.
- Helal, M.H.; Salem, M.A.; Gouda, M.A.; Ahmed, N.S.; El-Sherif, A.A. Design, synthesis, characterization, quantum-chemical calculations and anti-inflammatory activity of novel series of thiophene derivatives. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 147, 73–83.
- Chaudhari, P.S.; Chitlange, S.S.; Nanda, R.K. Synthesis and Biological Evaluation of Novel 2-(4-acetyl-3-methyl-5-(arylamino)thiophen-2-yl)-3-arylquinazolin-4(3H)-one Derivatives as Potential Anti-inflammatory and Antioxidant Agents. Atiinflamm. Antiallergy Agents Med. Chem. 2018, 17, 102–114.
- Wang, X.; Song, K.; Li, L.; Chen, L. Structure-Based Drug Design Strategies and Challenges. Curr. Top. Med. Chem. 2018, 18, 998–1006.
- Shoichet, B.K.; Walters, W.P.; Jiang, H.; Bajorath, J. Advances in Computational Medicinal Chemistry: A Reflection on the Evolution of the Field and Perspective Going Forward. J. Med. Chem. 2016, 59, 4033–4034.
- Yu, W.; MacKerell, A.D., Jr. Computer-Aided Drug Design Methods. Methods Mol. Biol. 2017, 1520, 85–106.
- Sagaama, A.; Issaoui, N. Design, molecular docking analysis of an anti-inflammatory drug, computational analysis and intermolecular interactions energy studies of 1-benzothiophene-2-carboxylic acid. Comput. Biol. Chem. 2020, 88, 107348.
- Karthick, T.; Balachandran, V.; Perumal, S. Spectroscopic investigations, molecular interactions, and molecular docking studies on the potential inhibitor “thiophene-2-carboxylicacid”. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 141, 104–112.
