It explores the renewable ways of obtaining methanol and its use in efficient energy systems for a net zero-emission carbon cycle, with a special focus on fuel cells. It investigates the different parts of the carbon cycle from a methanol and fuel cell perspective. In recent years, the potential for a methanol economy has been shown and there has been significant technological advancement of its renewable production and utilization. Even though its full adoption will require further development, it can be produced from renewable electricity and biomass or CO2 capture and can be used in several industrial sectors, which make it an excellent liquid electrofuel for the transition to a sustainable economy. By converting CO2 into liquid fuels, the harmful effects of CO2 emissions from existing industries that still rely on fossil fuels are reduced. The methanol can then be used both in the energy sector and the chemical industry, and become an all-around substitute for petroleum.
- methanol
- electrofuels
- power-to-X
- high temperature PEM
- fuel cell
- reforming
1. Introduction
in 2019 from 199 gCO2/kWhel in 2018 [8]. The drop is even more striking when compared to the emission levels of 2010 of 426 gCO2/kWhel
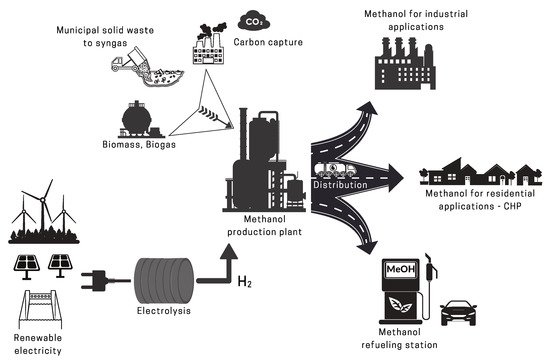
2. Methanol Production
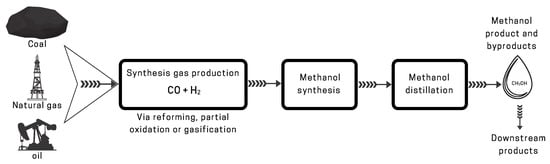
2.1. Traditional Methods
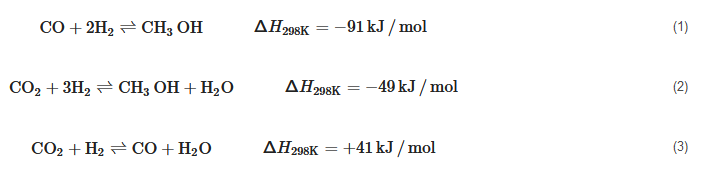
CO+2H2⇌CH3OHΔH298K=−91kJ/mol(1)
CO2+3H2⇌CH3OH+H2OΔH298K=−49kJ/mol(2)
CO2+H2⇌CO+H2OΔH298K=+41kJ/mol(3)
Due to the low one-pass conversion of COx (CO and CO
2
) to methanol of only about 5–15%, the recycling of the unconverted syngas is usually necessary with a recycle ratio of between 2:1 and 5:1[39]
. A typical methanol synthesis loop along with the sucessive distillation step is shown inFigure 3
. Before entering the methanol synthesis process, the composition of the synthesis gas is adjusted, for instance by adding CO2
according to the stoichiometric ratios in reactions () and (), resulting in a stoichiometric number SN = (mol H2
− mol CO2
)/(mol CO + mol CO2
), which is usually around 2 or slightly higher for methanol synthesis[32]. The synthesis gas after the composition adjustment is called makeup gas (MUG). A MUG compressor (or multi-stage MUG compressor) is usually required to fulfill the pressure requirement for the methanol synthesis process, for example, the produced MUG with a pressure of 50 bar and the methanol converter operated at 80 bar. After the MUG compressor, the syngas is mixed with the recycled gas, which then enters the methanol converter.
. The synthesis gas after the composition adjustment is called makeup gas (MUG). A MUG compressor (or multi-stage MUG compressor) is usually required to fulfill the pressure requirement for the methanol synthesis process, for example, the produced MUG with a pressure of 50 bar and the methanol converter operated at 80 bar. After the MUG compressor, the syngas is mixed with the recycled gas, which then enters the methanol converter.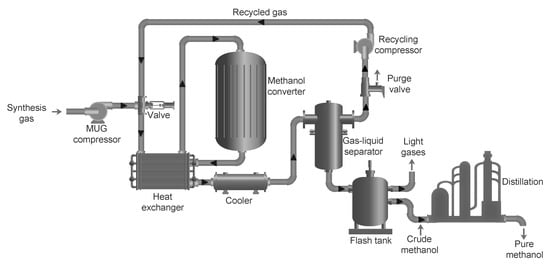
2.2. Renewable Methanol
| Step | Energy Conversion Route | Process Efficiency | Reference | ||
|---|---|---|---|---|---|
| Methanol | Hydrogen | Methanol | Hydrogen | ||
| 1 | Power Management, Conditioning and Transmission | 90% | [190] | ||
| 2 | H2 production by electrolysis | PEMEC → 78% | [191] | ||
| AEC → 74% | [192] | ||||
| SOEC → 96% | [71] | ||||
| 3 | Methanol synthesis | H2 compression | 79 (69–89)% | 75 (65–85)% | [83] |
| 4 | Fuel transportation | 99% | 95% | [193] | |
| 5 | Methanol Fuel cell system | Hydrogen Fuel cell system | 50% | 60% | [145][194] |
2.2.1. Technology Status and Prospects
2.2.2. Renewable CO2 Sources
2.2.3. Renewable H2 Sources
H2O(l)−→−−−electricityH2(g)+12O2(g).(4)

3. Methanol Use
Methanol is already one of the most important and widely traded chemical commodities, and its demand is growing rapidly with a compound annual growth rate (CAGR) of 6% between 2014 and 2019 [17]. This is mainly due to its versatility and the numerous industrial sectors it serves. As shown in Figure 4, it not only can be produced from various feedstock, but can also be used in several applications. In the following sections, an overview of its use in the chemical industry and the energy sector is given. Even though it is still extensively used in the chemical industry, this review focuses on its use in the energy sector, especially in fuel cells, as it investigates methanol as a renewable energy carrier.
3. Methanol Use
Figure 4. The methanol value chain. Adapted from [30,129].
3.1. Methanol
, it not only can be produced from various feedstock, but can also be used in several applications. In the following sections, an overview of its use in the chemical industry and the energy sector is given. Even though it is still extensively used in the chemical industry, this review focuses on its use in the energy sector, especially in fuel cells, as it investigates methanol as a renewable energy carrier.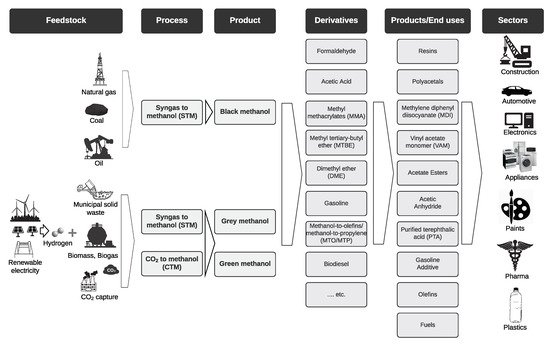
The chemical industry has long relied on fossil fuels for its processes. However, a transition driven by climate goals is underway to make the chemical industry more sustainable, both its raw materials and energy demand. Even though methanol is predominantly produced from fossil sources at the moment, the plethora of methanol chemical derivatives and products pave the way for a green methanol-based chemical industry. The fact that it is already one of the most important chemical commodities also makes it an ideal candidate for this transition and can help integrate fossil raw materials and biomass value chains [30].
For instance, lower C2–C4 olefins (ethylene and propylene) that are normally produced from crude oil and are the most important monomers for further petrolchemical and chemical synthesis processes can be produced from methanol [24]. The process of manufacturing lower olefins from methanol takes place on a mesoporous H-ZSM-5 zeolite catalyst at high temperatures of around 370–500 °C, a process that started in the late 1970s by Mobil Oil Corporation, with other similar processes that use different catalysts available in many plants around the globe today [24]. Moreover, methanol can also be used as a solvent and/or additive in various sectors, such as paint removers, wind-shield washers and it can be used as a fermentation substrate in microbial productions [17].
3.2. Mrethanol in Energy Systems
In recent years there has been a paradigm shift in the use of methanol. As recent as 2012, 85% of the methanol production was used in the chemical industry [22]. However, this is rapidly changing and the use of methanol in the energy sector now accounts for 40% of methanol consumption [17]. Below some of the uses of methanol in the energy sector are discussed.
34.2.1. Methanol in Internal Combustion Engines
3.1. Methanol in The Chemical Industry
3.2. Methanol in Energy Systems
3.2.1. Methanol in Internal Combustion Engines
3.2.2. Methanol in Fuel Cells
Direct Methanol Fuel Cells (DMFC)
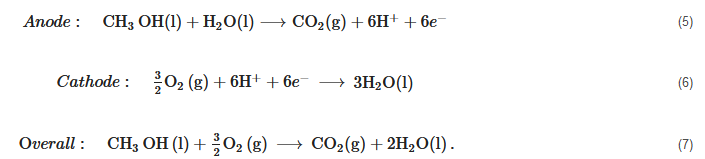
Methanol possesses some interesting properties that make it more performing compared to conventional fuels for internal combustion engine; high latent heat, fast-burning velocity, no carbon to carbon bonds, and high octane rating, and hence higher compression ratios and higher knock resistance for an increased engine efficiency [25,130]. In fact, its use in engines has been studied as far back as the beginning spark-ignition engines, where methanol was investigated for enhancing the engine performance [130]. Moreover, with its clean-burning and less explosive nature, methanol is the fuel of choice in most competitive motorsports, both due to its high performance and safety compared to other fuels [12,131]. However, methanol is corrosive to some metals and can cause swelling of rubber and plastic components, which calls for proper corrosion inhibitors and swelling resistant seals in methanol-based engines.
Despite its early use in internal combustion engines, it was only in the 1980s and 1990s with the California methanol fuel trials that its use as an alternative fuel in internal combustion engine was demonstrated at a larger scale, where 15,000 M85 (85% methanol) gasoline flex-fuel vehicles of various applications were operated [130]. The program started to reduce NOx emissions, mainly to prevent ozone depletion [132]. Even though the experiment was a technical success it was slowly abandoned in 2004, mainly because natural gas, which was the main source of methanol for the trails, was thought to be scarce and expensive at the time, which was later proven wrong with possibility of nowadays selling methanol from natural gas at half the price of gasoline [133]. Moreover, the oil companies came up with cleaner gasoline by blending it with Methyl Tertiary Butyl Ether (MTBE) to meet california’s demands, which however required methanol for its production and diverted it from being used as a substitute to gasoline [133].
High Temperature PEM Fuel Cells (HT-PEMFC)
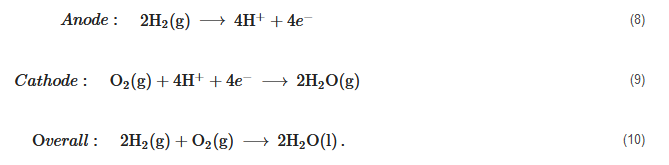
China, which is rich in coal but heavily relies on imported oil for its transportation sector, has recently decided to use methanol nationwide as a transportation fuel to tackle both energy security and air pollution challenges [18]. Driven by coal mining permits the Chinese methanol production from coal gasification has reached a production capacity able to cover half of China’s transportation fuel demand [130]. Similarly to the California trials of the 1990s, a methanol vehicle pilot program was conducted between 2012 and 2018 in 10 Chinese cities, where more than 1000 methanol vehicles were tested [18]. The program showed methanol’s feasibility as a viable transportation fuel with neither techno-economical nor safety issues, and today there are several hundreds of thousands of vehicles that run on pure methanol or methanol blends, including retrofitted vehicles [18]. Despite the coal mining pretext for the methanol production in big quantities in China, the fact that there are no major technical barriers to its use in internal combustion engines and its other numerous applications along with the increasing urgency for climate actions could lead to more investments and progress in renewable methanol production worldwide and establish it as the fuel of the future.
The International Maritime Organization (IMO) has set a new limit in which all sizes of ships will need to use fuel that meets the 0.50% m/m (mass by mass) SOx emissions from 1 January 2020, lowering it significantly from the current limit of 3.50% m/m for ships operating outside designated emission control areas [134]. Moreover, there is a plan to cut GHG emissions from international shipping in half by 2050 and successively eliminate them entirely [135]. These increasingly stringent emission limits and the fact that methanol is transported in huge amounts in ships is driving an increasing interest in the use of methanol for marine applications as well. At the moment there are a number of projects to test methanol-fueled vessels, including seven new dual-fuel engine chemical tankers, a retrofitted Stena Line ferry, a pilot boat by ScandiNaos, two retrofitted HT-PEMFC tourist boats and others research and development projects [136].
Recently, a study by A.P. Moller - Maersk and Lloyds Register identified alcohols, such as methanol and ethanol, along with biomethane and ammonia as the three main fuels to achieve a net-zero CO
2 emissions in the shipping industry [137]. They concluded that alcohols that can be produced from renewable hydrogen and CO
, CO2 from biomass or carbon capture have the advantage as they can use proven existing solutions for handling the low flash point and burning and are fully mixable in the vessel’s bunker tanks, creating bunkering flexibility.
Similarly, electrofuels can play an important role in decarbonizing aviation [138]. Goldmann et al. [11] investigated five electrofuels, including methanol for use in aviation and found that they can generally replace conventional kerosene-based fuels, but suffer from higher structural loads and potentially lower efficiencies. Moreover, kerosene-based fuels for international aviation remain tax-exempt due to Air Service Agreements (ASAs) made soon after the second world war [138]. Hence, for electrofuels to compete with kerosene-based fuels for aviation, the introduction of such taxes and more stringent fuel standards are required along with carbon pricing and increase in renewable electricity share for cheaper green hydrogen.
3.2.2. Methanol in
, and traces of CO and unconverted methanol-water mixture) directly in the fuel cell without any pre-purification, giving HT-PEMFCs big advantages in terms of fuel flexibility [149]. A comprehensive review of the core PBI-based HT-PEMFC technology and its components and their characterization can be found in our previous work [150]. Therefore, the focus of this review is not on the core HT-PEMFC technology but rather on the whole system and its role in the methanol economy.Fuel Cells
In fuel cells, methanol can be used either directly in direct methanol fuel cells (DMFC) or indirectly via methanol steam reforming into hydrogen-rich gas mixture in HT-PEMFCs. The two fuel cell types are described below.
Direct Methanol Fuel Cells (DMFC)
A direct methanol fuel cell is a variant of proton exchange membrane fuel cells (PEMFC) that use liquid methanol and water mixture instead of hydrogen to generate electricity via electrochemical reactions. This gives DMFC the advantage in terms of fuel handling as liquid mixture can be used without the complications of the hydrogen storage required in the case of low-temperature PEMFC or the added reforming system needed in HT-PEMFC systems. The reactions in a DMFC are given below:
Anode:CH3OH(l)+H2O(l)⟶CO2(g)+6H++6e−(5)
Cathode:32O2(g)+6H++6e−⟶3H2O(l)(6)
Overall:CH3OH(l)+32O2(g)⟶CO2(g)+2H2O(l).(7)
DMFCs are suitable for portable power generation due to their power range and rapid refueling characteristics [139]. Consequently, they are usually investigated to replace rechargeable batteries in portable applications. However, there are some practical issues, including their low efficiency of below 30% due to methanol cross-over through the membrane that limit their widespread application [140,141]. High methanol cross-over also means that the fuel cell exhaust contains methanol and possibly formaldehyde, which can cause health concern. To minimize the cross-over the fuel cell should be kept fully hydrated, which requires it to be humidified on both the anode and the cathode side, thereby adding system complexity, limiting the operating temperatures and lowering the achievable electrical efficiency. Moreover, there has been a mismatch between the pace at which they have been developing and the industry’s need for readily available solutions, which were met by advances in lithium-ion batteries and efficient microprocessors.
Recently, DMFCs have also been investigated for unmanned aerial vehicle (UAV) applications due to methanol’s high energy density [142,143,144]. However, their lower efficiency and lower power density means that higher methanol volume is needed for longer flight time, which inevitably compromises the UAVs payload.
High Temperature PEM Fuel Cells (HT-PEMFC)
Contrary to DMFCs, reformed methanol fuel cells are an efficient way of using methanol to produce energy, with up to 50% overall electrical efficiency [145]. A reformed methanol fuel cell (RMFC) or a high temperature PEM fuel cell (HT-PEMFC) is a proton exchange membrane fuel cell that operates at temperatures above 100 °C, typically between 160 °C and 180 °C. However, unlike low-temperature PEM fuel cells, it does not require liquid water for the proton conduction through the membrane. To achieve appropriate proton conduction at temperatures above the boiling point of water, it employs polybenzimidazole (PBI) membrane, able to conduct protons under anhydrous conditions when doped with phosphoric acid. Therefore, the need for reactant humidification and the associated water management that is crucial in low-temperature PEM fuel cells is eliminated, thereby avoiding the risk of cell flooding or drying. The reactions that take place in an HT-PEMFC are the same as those of a low-temperature PEM, and are given as follows:
Anode:2H2(g)⟶4H++4e−(8)
Cathode:O2(g)+4H++4e−⟶2H2O(g)(9)
Overall:2H2(g)+O2(g)⟶2H2O(l).(10)
The higher operating temperature of HT-PEMFC comes with an added advantage of higher tolerance to impurities in the fuel compared to their lower temperature counterparts [146,147,148]. In fact, it is possible to use the product gas of methanol steam reforming, known as reformate gas (H2, CO2, and traces of CO and unconverted methanol-water mixture) directly in the fuel cell without any pre-purification, giving HT-PEMFCs big advantages in terms of fuel flexibility [149]. A comprehensive review of the core PBI-based HT-PEMFC technology and its components and their characterization can be found in our previous work [150]. Therefore, the focus of this review is not on the core HT-PEMFC technology but rather on the whole system and its role in the methanol economy.
A reformed methanol fuel system with all its main components is shown in Figure 5. The process starts in the catalytic burner, which uses some of the fuel to heat up the reformer. In the reformer, steam and methanol fed through an evaporator are heated up further to produce the reformate gas, which is then directed to the fuel cell to produce electricity. The evaporator recovers heat from the fuel cell stack exhaust air and coolant. In some cases the reformate gas can be cooled down in a separate heat exchanger to reach the fuel cell stack operating temperature. Once the systems is started, the reformate exhaust from the anode off-gas can be recycled in the burner.
5. The process starts in the catalytic burner, which uses some of the fuel to heat up the reformer. In the reformer, steam and methanol fed through an evaporator are heated up further to produce the reformate gas, which is then directed to the fuel cell to produce electricity. The evaporator recovers heat from the fuel cell stack exhaust air and coolant. In some cases the reformate gas can be cooled down in a separate heat exchanger to reach the fuel cell stack operating temperature. Once the systems is started, the reformate exhaust from the anode off-gas can be recycled in the burner.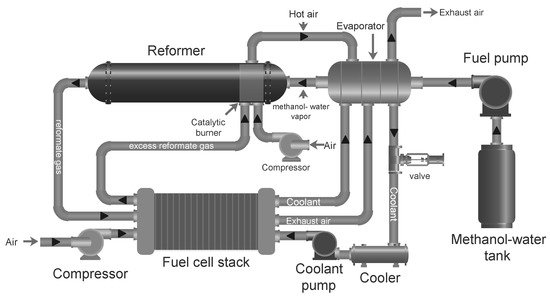
As can be seen in Figure 5, HT-PEMFC systems have the possibility of heat integration of hot and cold streams, where at least two reactors which require heat can be identified, i.e., the endothermic methanol steam reformer and the evaporator. On the other hand the fuel cell stack and the burner release heat, although the fuel cell stack requires heat during system start-up in order to reach operating temperature. In [151], a strategy to ensure proper heat integration over all the load ranges was suggested. The principal idea is to provide extra fuel to the burner whenever the heat provided by the depleted fuel and air at the stack outlets is not sufficient. It was found that below a certain load, anode stoichiometric ratio must be increased in order to provide enough heat to the burner to sustain both the reforming and fuel cell reactions. In other words, the amount of fuel mix that is sent to the fuel cell stack must be larger than what is strictly required for the electrochemical reactions to take place.
An alternative strategy for the warming up of the stack during the start up phase was investigated in [152], where alternating current is applied directly to the stack with a suitable frequency aimed at heating the stack’s main ohmic resistances (i.e. membrane and contact resistance). The strategy was found to reduce the start-up time compared to more conventional strategies based on external liquid coolant.
The use of different fuels requires different heat integration strategies and can affect the electrical efficiency of the system. In [151], it was shown that in an ideal case a methanol-fed system shows a better efficiency compared to a hydrogen-fed system. This can be attributed to the fact that in the system with the methanol reformer, the heat in the depleted gas at the stack outlet can be reused for the endothermic reforming process. However, it is worth noting that even though HT-PEMFC has a higher tolerance to CO in the reformate mixture, this can still reduce the cell performance and lifetime compared to a hydrogen-fed system.
M
The process of catalytically converting hydrocarbons into a hydrogen-rich gas mixture is known as reforming. The process requires an oxidizing agent and if water vapor is used for this purpose, the process is known as steam reforming, which is endothermic and requires permanent external heating to be sustained. If on the other hand, air is used as the oxidant the process is an exothermic one called partial oxidation, whereas if both water and air are used the process is called autothermal reforming, which is a combination of the two processes with net reaction enthalpy change of zero [153].
Methanol, which is the simplest alcohol with only one carbon atom has one of the lowest reforming temperatures of only 200–300 °C owing to the absence of a strong C–C bond [154,155]. The single carbon configuration gives it a high H/C ratio of 4:1, which results in high concentration of H
Methanol Reforming
2
in the reformate mixture for use in an HT-PEMFC. It is also liquid at ambient conditions with a boiling point of around 65 °C and completely miscible with water, and hence, easy to store and transport [22]. The most widely used reforming process in conjunction with HT-PEMFCs is methanol steam reforming (MSR) as it produces high concentration H2 per mole of methanol according to reaction (11), which is an algebraic sum of the water gas shift reaction (WGS) in reaction (12) and the methanol decomposition (MD) in reaction (13) [154,156,157].
per mole of methanol according to reaction (11), which is an algebraic sum of the water gas shift reaction (WGS) in reaction (12) and the methanol decomposition (MD) in reaction (13) [154][156][157].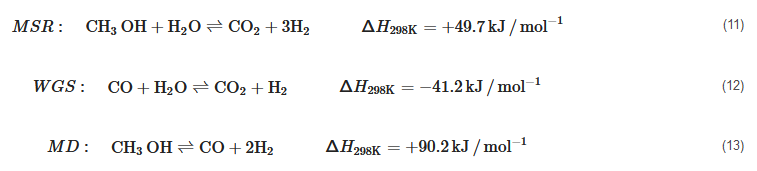
MSR:CH3OH+H2O⇌CO2+3H2ΔH298K=+49.7kJ/mol−1(11)
WGS:CO+H2O⇌CO2+H2ΔH298K=−41.2kJ/mol−1(12)
MD:CH3OH⇌CO+2H2ΔH298K=+90.2kJ/mol−1(13)
Copper-based catalysts, such as Cu/ZnO/Al
2
O3 are currently the most widely used commercial catalysts for hydrogen production for fuel cell systems with relatively good activity and selectivity, where Cu acts as the active component [158,159,160]. The reforming activity on Cu/ZnO/Al
are currently the most widely used commercial catalysts for hydrogen production for fuel cell systems with relatively good activity and selectivity, where Cu acts as the active component [158][159][160]. The reforming activity on Cu/ZnO/Al2
O3 starts at 160 °C [156], which would be ideal from a system integration point of view with an HT-PEMFC that typically works between 160–180 °C. However, even at around 200 °C around 8% of unconverted methanol slip can be present in the reformate mixture, an amount that is detrimental to HT-PEMFC’s performance and lifetime [161,162]. Therefore, it is only above 230 °C that a steam methanol reformer can produce a clean enough reformate mixture (≤2% methanol and ≤1% CO) that is usable in an HT-PEMFC without a cleanup process and without compromising the performance and durability of the fuel cell.
The CO concentration in the reformate mixture increases with increasing reforming temperature, while the unconverted methanol concentration decreases due to higher conversion rates at higher reforming temperatures. However, even though the harmful effect of CO on performance increases with increasing CO concentrations, an HT-PEMFC can tolerate higher CO concentrations of up to 5% with only small performance decay, especially at higher operating temperatures and lower current densities [150]. In comparison, low-temperature PEM fuel cell can only tolerate a few parts per million of CO concentrations in the anode feed. Nonetheless, the lower the CO concentrations in the reformate mixture the better it is for the fuel cell both in terms of performance and durability.
Therefore, since methanol slip and CO concentration have opposite trends with respect to reforming temperature, an optimal reforming temperature and proper control of the flow rates are required to obtain a proper reformate mixture. An experimental mapping of the methanol and CO concentrations in the reformate gas with respect to reformer temperature at the inlet are shown in Figure 6 [161].
starts at 160 °C [156], which would be ideal from a system integration point of view with an HT-PEMFC that typically works between 160–180 °C. However, even at around 200 °C around 8% of unconverted methanol slip can be present in the reformate mixture, an amount that is detrimental to HT-PEMFC’s performance and lifetime [161][162]. Therefore, it is only above 230 °C that a steam methanol reformer can produce a clean enough reformate mixture (≤2% methanol and ≤1% CO) that is usable in an HT-PEMFC without a cleanup process and without compromising the performance and durability of the fuel cell.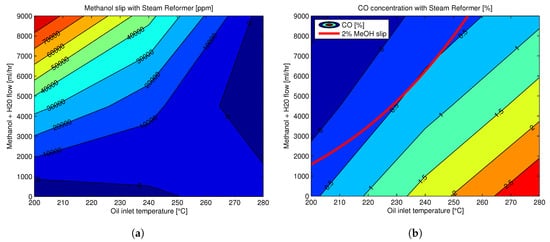
Figure 6.
a
b) CO concentration in reformate mixture at different reforming temperatures, with the red line showing the 2% methanol slip. Reprinted with permission from [161]; 2016, Aalborg Universitetsforlag.
In order to limit concentrations of undesired byproducts, including unconverted methanol in the reformate gas, catalysts for steam reforming should have high activity and fast kinetics at low temperature, high selectivity in order to suppress CO production, and good stability, and long lifetime [163]. Moreover, catalysts with well-dispersed and smaller crystallite size particles and higher specific surface area of copper generally result in better performance [164]. An extensive review of the influence of catalyst components and preparation methods on the performance of different Cu-based catalysts for MSR can be found in [163].
Catalyst deactivation generally happens due to thermal sintering above 300 °C with recommended operating temperatures of below 260 °C [164]. Coke deposition due to iron or other transition metals, wax or carbon formation due to silica reaction with alumina, and catalyst poisoning with sulfur and chloride content in the reactants are other deactivation mechanisms that have been observed for such catalysts in methanol steam reforming [165]. In order to maintain the chemical and thermal stability of Cu/ZnO-based catalysts in industrial application, Al
2
O3 or other substitutes are often added as stabilizers or promoters [165,166].
or other substitutes are often added as stabilizers or promoters [165][166].To overcome the problem of active phase sintering, the metals in groups 8 to 10 of the periodic table have also been studied as catalysts with higher thermal stability and similar selectivity compared to Cu-based catalysts [163]. Moreover, increasing the steam to carbon ratio in the feed of methanol-steam mixture decreases the catalyst deactivation rate [167], since the absence of water vapor can lead to coke formation that in turn can block catalyst pores and exacerbate their deactivation [168,169].
HT-PEMFC Technology Status and Prospects
Conventional packed-bed reformers use catalysts in the form of pellets due to relatively low cost of preparation and ease of operation [170], despite being characterized by larger thermal gradient between the tube wall and the interior catalyst bed [155]. Recently, advanced micro-processing is making it increasingly possible to manufacture well-structured micro-channel reactors with wall-coated catalysts, which present fewer heat and mass transfer limitations [154]. Catalytic membrane reactor (CMR) with palladium-based membranes is another novel technology with improved process efficiency, where methanol steam reforming occurs on the tube side and hydrogen is permeated through a membrane and continuously collected on the shell side allowing to shift the reaction equilibrium towards the products, thereby improving methanol conversion [171].
at 180–200 °C. However, they reported instability of the single cell at high current density.HT-PEMFC Technology Status and Prospects
Lotrič et al. [146] compared the heat-integrated systems capable of producing 25 W of gross electric power for use in portable applications. The results indicated that the system of integrated HT-PEMFC stack and methanol steam reformer was feasible and had higher system efficiency than the system with LT-PEMFC stack. Ribeirinha et al. [147] studied the thermal integration of a low-temperature methanol steam reforming cell with an HT-PEMFC in a combined stack arrangement. In addition, the degradation of the membrane electrode assembly (MEA) due to the methanol in the reformate stream was observed and investigated by using EIS analysis.Reformed methanol fuel cells, like all fuel cells, are scalable and versatile and can be used in a variety of applications. They are investigated for stationary application; such as backup power for telecom application [145], uninterrupted power unit (UPS) [172], combined heat and power (CHP) generation for residential applications [173], and for portable power generation; as well as in automotive applications, both as auxiliary power units (APU) [172] for boats and road vehicles [174] and as range extenders [175,176].
A number of projects at demonstration and some at commercialization level have shown the potential for reformed methanol-fueled HT-PEM fuel cells in different applications. In automotive applications, reformed methanol fuel cell systems have been predominately used as range extenders in combination with batteries. In the European project ARTEMIS [177], the possibility to use a HT-PEM power train on a light-duty, commercial vehicle was investigated. Another example is in the danish municipality of Aalborg, where a methanol filling station was opened in 2015 to refill methanol fuel cell cars including a prototype of a Fiat 500 powered by a 5kW HT-PEM Stack, developed ad hoc for the demonstration project [175]. Recently, a Denmark-based company Blue World Technologies has started the development of a methanol-fueled HT-PEM fuel cell system for automotive application [178]. The company is betting on the technology and has a partnership with a Chinese electric vehicle manufacturer AIWAYS to supply the market with methanol fuel cell cars [176].
In the maritime sector, a project by the name e4ship funded by the German government demonstrated a 90kW system based on a methanol HT-PEMFC stack on the ferryboat Mariella from the Viking line. The system compromises 5KW fuel cell modules with methanol-reforming units for conversion of methanol into hydrogen [179]. In another demonstration project in the municipality of Essen in Germany a touristic vessel on the Lake Baldeneysee was powered by a 35 kW HT-PEM fuel cell system fueled by methanol [180]. For both these projects the fuel cells were developed by the Denmark-based fuel cell manufacturer SerEnergy.
HT-PEM fuel cells for residential CHP applications have also seen some development in the past few years. Demonstration projects have mainly used natural gas as a fuel for the already available distribution infrastructure. With little adaptation of the reforming process, these systems can operate with a variety of fuels, including methanol. Besides, HT-PEM fuel cells have the advantage of providing high-quality heat, which matches with the residential application requirements. In the Eurostars project HyRIS a micro CHP system was developed by the Germany-based Fuel Cell Research Center, ZBT, and the company Advent Technologies in Greece [181]. In another European demonstration project called ene-Field more than 1000 CHP units, including HT-PEMFC-based ones, have been tested in 10 European countries in the years between 2012–2017 [182]. The HT-PEMFC systems for residential use in this project delivers 300 W electric power and 600 W thermal power, where the small energy output offers cost-saving compared to the conventional energy supplier [183].
In UPS applications, methanol-fueled HTPEM systems are developed and commercialized by Serenergy [172]. The systems are targeted mainly for telecom applications in remote regions where conventional diesel generators are currently used.
For the commercial success of HT-PEMFCs, further development of more resistant materials and improved system integration and control designs are necessary to increase their lifetime. Bipolar plates with excellent corrosion resistance, low bulk density and high electrical conductivity [184], and methanol steam reformer which maximizes the heat transfer with a uniform flow distribution and low-pressure drop [185], are significant to reduce the balance-of-plant component number, size, mass, and ultimately cost.
The efficient heat integration is another key issue that needs to be addressed. An integrated stack-reformer system was demonstrated by Pan et al. [186] but with low hydrogen yield due to the lower operating temperature of the reformer at 200 °C. Ji et al. [187] tested an internal reforming fuel cell (IRMF) with methanol solution and air and achieved power density of 0.45–0.55 W/cm2
at 180–200 °C. However, they reported instability of the single cell at high current density.
Lotrič et al. [146] compared the heat-integrated systems capable of producing 25 W of gross electric power for use in portable applications. The results indicated that the system of integrated HT-PEMFC stack and methanol steam reformer was feasible and had higher system efficiency than the system with LT-PEMFC stack. Ribeirinha et al. [147] studied the thermal integration of a low-temperature methanol steam reforming cell with an HT-PEMFC in a combined stack arrangement. In addition, the degradation of the membrane electrode assembly (MEA) due to the methanol in the reformate stream was observed and investigated by using EIS analysis.
References
- Shindell, D.; Smith, C.J. Climate and air-quality benefits of a realistic phase-out of fossil fuels. Nature 2019, 573, 408–411.
- Smith, C.J.; Forster, P.M.; Allen, M.; Fuglestvedt, J.; Millar, R.J.; Rogelj, J.; Zickfeld, K. Current fossil fuel infrastructure does not yet commit us to 1.5 °C warming. Nat. Commun. 2019, 10.
- Shindell, D.; Faluvegi, G.; Seltzer, K.; Shindell, C. Quantified, localized health benefits of accelerated carbon dioxide emissions reductions. Nat. Clim. Chang. 2018, 1–5.
- Weindl, I.; Lotze-Campen, H.; Popp, A.; Müller, C.; Havlík, P.; Herrero, M.; Schmitz, C.; Rolinski, S. Livestock in a changing climate: production system transitions as an adaptation strategy for agriculture. Environ. Res. Lett. 2015, 10, 094021.
- Lelieveld, J.; Klingmüller, K.; Pozzer, A.; Burnett, R.T.; Haines, A.; Ramanathan, V. Effects of fossil fuel and total anthropogenic emission removal on public health and climate. Proc. Natl. Acad. Sci. USA 2019, 116, 7192–7197.
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918.
- IEA. Global Energy & CO2 Status Report 2019. Available online: https://www.iea.org/reports/global-energy-and-co2-status-report-2019/emissions (accessed on 17 January 2020).
- ENERGINET. Rekordlav CO2-udledning fra danskernes elforbrug i 2019. Available online: https://energinet.dk/Om-nyheder/Nyheder/2020/01/16/Rekord-lav-CO2udledning-fra-danskernes-elforbrug-i-2019 (accessed on 17 January 2020).
- Frensch, S.H.; Olesen, A.C.; Simon Araya, S.; Kær, S.K. Model-Supported Analysis of Degradation Phenomena of a PEM Water Electrolysis Cell under Dynamic Operation. ECS Trans. 2018, 85, 37–45.
- Larscheid, P.; Lück, L.; Moser, A. Potential of new business models for grid integrated water electrolysis. Renew. Energy 2018, 125, 599–608.
- Goldmann, A.; Sauter, W.; Oettinger, M.; Kluge, T.; Schröder, U.; Seume, J.R.; Friedrichs, J.; Dinkelacker, F. A study on electrofuels in aviation. Energies 2018, 11, 392.
- Hobson, C.; Márquez, C. Renewable Methanol Report; Technical report; ATA Markets Intelligence S.L. on behalf of the Methanol Institute: Madrid, Spain, 2018.
- Goeppert, A.; Czaun, M.; Surya Prakash, G.K.; Olah, G.A. Air as the renewable carbon source of the future: An overview of CO2 capture from the atmosphere. Energy Environ. Sci. 2012, 5, 7833–7853.
- Brown, A.; Le Feuvre, P. Technology Roadmap: Delivering Sustainable Bioenergy; International Energy Agency (IEA): Paris, France, 2017; p. 94.
- Cui, X.; Kær, S.K. Thermodynamic analyses of a moderate-temperature carbon dioxide hydrogenation to methanol via reverse water gas shift process with in situ water removal. Ind. Eng. Chem. Res. 2019.
- Alberico, E.; Nielsen, M. Towards a methanol economy based on homogeneous catalysis: Methanol to H2 and CO2 to methanol. Chem. Commun. 2015, 51, 6714–6725.
- Methanol Institute. Methanol|Methanol Institute. Available online: https://www.methanol.org (accessed on 10 December 2019).
- Zhao, K. A Brief Review of China’s Methanol Vehicle Pilot and Policy; Technical report; Methanol Institute: Alexandria, VA, USA, 2019.
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Beyond Oil and Gas: The Methanol Economy; Wiley-VCH: Hoboken, NJ, USA, 2006.
- Olah, G.A. Towards Oil Independence Through Renewable Methanol Chemistry. Angew. Chem. Int. Ed. 2013, 52, 104–107.
- Offermanns, H.; Schulz, K.; Brandes, E.; Schendler, T. Methanol Utilisation Technologies. In Methanol: The Basic Chemical and Energy Feedstock of the Future: Asinger’s Vision Today; Bertau, M., Offermanns, H., Plass, L., Schmidt, F., Wernicke, H.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 327–601.
- Ott, J.; Gronemann, V.; Pontzen, F.; Fiedler, E.; Grossmann, G.; Kersebohm, D.B.; Weiss, G.; Witte, C. Methanol. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012.
- El-Zeftawy, A.M. Focus on the Chemical Value of Methanol. J. Univ. Eng. Sci. 1995, 7, 209–254.
- Khadzhiev, S.N.; Kolesnichenko, N.V.; Ezhova, N.N. Manufacturing of lower olefins from natural gas through methanol and its derivatives (review). Pet. Chem. 2008, 48, 325–334.
- Zhen, X.; Wang, Y. An overview of methanol as an internal combustion engine fuel. Renew. Sustain. Energy Rev. 2015, 52, 477–493.
- Dalena, F.; Senatore, A.; Basile, M.; Knani, S.; Basile, A.; Iulianelli, A. Advances in Methanol Production and Utilization, with Particular Emphasis toward Hydrogen Generation via Membrane Reactor Technology. Membranes 2018, 8, 98.
- Alvarado, M. The Changing Face of the Global Methanol Industry; Technical report; IHS: London, UK, 2016.
- Market Watch. Methanol Market 2019 Analysis and Technological Innovation by Leading Key Players 2026. Available online: https://www.marketwatch.com/press-release/methanol-market-2019-analysis-and-technological-innovation-by-leading-key-players-2026-2019-08-07 (accessed on 18 January 2020).
- Sheldon, D. Methanol Production - A Technical History. Johns. Matthey Technol. Rev. 2017, 61, 172–182.
- Bertau, M.; Offermanns, H.; Plass, L.; Schmidt, F.; Wernicke, H.J. Methanol: The Basic Chemical and Energy Feedstock of the Future: Asinger’s Vision Today; Springer: Berlin/Heidelberg, Germany, 2014.
- Dalena, F.; Senatore, A.; Marino, A.; Gordano, A.; Basile, M.; Basile, A. Chapter 1—Methanol Production and Applications: An Overview. In Methanol; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–28.
- Wernicke, H.J.; Plass, L.; Schmidt, F. Methanol Generation. In Methanol: The Basic Chemical and Energy Feedstock of the Future: Asinger’s Vision Today; Bertau, M., Offermanns, H., Plass, L., Schmidt, F., Wernicke, H.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 51–301.
- Rostrup-Nielsen, J.; Christiansen, L.J. Concepts in Syngas Manufacture. In Catalyst Science Series; Imerial Collage Press: London, UK, 2011; Volume 10, p. 379.
- Cui, X.; Kær, S.K. Two-dimensional thermal analysis of radial heat transfer of monoliths in small-scale steam methane reforming. Int. J. Hydrog. Energy 2018, 43, 11952–11968.
- Lange, J.P. Methanol synthesis: a short review of technology improvements. Catal. Today 2001, 64, 3–8.
- Bell, D.; Towler, B.; Fan, M. Coal Gasification and Its Applications, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; p. 416.
- Liu, K.; Song, C.; Subramani, V. Hydrogen and Syngas Production and Purification Technologies; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 1–533.
- Bozzano, G.; Manenti, F. Efficient methanol synthesis: Perspectives, technologies and optimization strategies. Prog. Energy Combust. Sci. 2016, 56, 71–105.
- Barbieri, G.; Marigliano, G.; Golemme, G.; Drioli, E. Simulation of CO2 hydrogenation with CH3OH removal in a zeolite membrane reactor. Chem. Eng. J. 2002, 85, 53–59.
- Riaz, A.; Zahedi, G.; Klemeš, J.J. A review of cleaner production methods for the manufacture of methanol. J. Clean. Prod. 2013, 57, 19–37.
- Palma, V.; Meloni, E.; Ruocco, C.; Martino, M.; Ricca, A. State of the Art of Conventional Reactors for Methanol Production. In Methanol Science and Engineering; Angelo, B., Francesco, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 29–51.
- Blug, M.; Leker, J.; Plass, L.; Günther, A. Methanol Generation Economics. In Methanol: The Basic Chemical and Energy Feedstock of the Future: Asinger’s Vision Today; Bertau, M., Offermanns, H., Plass, L., Schmidt, F., Wernicke, H.J., Eds.; Springer: Berlin, Germany, 2014; pp. 603–618.
- Dahl, P.J.; Ostergaard, J. Process for Production of DME from Crude Methanol. U.S. Patent 10,011,548, 3 July 2018.
- Isayama, Y.; Saka, S. Biodiesel production by supercritical process with crude bio-methanol prepared by wood gasification. Bioresour. Technol. 2008, 99, 4775–4779.
- Zhang, J.; Liang, S.; Feng, X. A novel multi-effect methanol distillation process. Chem. Eng. Process. Process. Intensif. 2010, 49, 1031–1037.
- Cui, C.; Xi, Z.; Liu, S.; Sun, J. An enumeration-based synthesis framework for multi-effect distillation processes. Chem. Eng. Res. Des. 2019, 144, 216–227.
- Cui, C.; Sun, J.; Li, X. A hybrid design combining double-effect thermal integration and heat pump to the methanol distillation process for improving energy efficiency. Chem. Eng. Process. Process. Intensif. 2017, 119, 81–92.
- Gahleitner, G. Hydrogen from renewable electricity: An international review of power-to-gas pilot plants for stationary applications. Int. J. Hydrog. Energy 2013, 38, 2039–2061.
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176.
- Serenergy A/S. Methanol Production. Available online: http://www.serenergy.com (accessed on 10 December 2019).
- Gallucci, F.; Paturzo, L.; Basile, A. An experimental study of CO2 hydrogenation into methanol involving a zeolite membrane reactor. Chem. Eng. Process. Process. Intensif. 2004, 43, 1029–1036.
- Dehghani, Z.; Bayat, M.; Rahimpour, M. Sorption-enhanced methanol synthesis: Dynamic modeling and optimization. J. Taiwan Inst. Chem. Eng. 2014, 45, 1490–1500.
- Arora, A.; Iyer, S.S.; Bajaj, I.; Faruque Hasan, M.M. Optimal Methanol Production via Sorption-Enhanced Reaction Process. Ind. Eng. Chem. Res. 2018, 57, 14143–14161.
- Bos, M.; Brilman, D. A novel condensation reactor for efficient CO2 to methanol conversion for storage of renewable electric energy. Chem. Eng. J. 2015, 278, 527–532.
- Bukhtiyarova, M.; Lunkenbein, T.; Kähler, K.; Schlögl, R. Methanol Synthesis from Industrial CO2 Sources: A Contribution to Chemical Energy Conversion. Catal. Lett. 2017, 147, 416–427.
- Liu, X.M.; Lu, G.Q.; Yan, Z.F.; Beltramini, J. Recent Advances in Catalysts for Methanol Synthesis via Hydrogenation of CO and CO2. Ind. Eng. Chem. Res. 2003, 42, 6518–6530.
- Saeidi, S.; Amin, N.A.S.; Rahimpour, M.R. Hydrogenation of CO2 to value-added products—A review and potential future developments. J. CO2 Util. 2014, 5, 66–81.
- Ganesh, I. Conversion of carbon dioxide into methanol—A potential liquid fuel: Fundamental challenges and opportunities (a review). Renew. Sustain. Energy Rev. 2014, 31, 221–257.
- Jadhav, S.G.; Vaidya, P.D.; Bhanage, B.M.; Joshi, J.B. Catalytic carbon dioxide hydrogenation to methanol: A review of recent studies. Chem. Eng. Res. Des. 2014, 92, 2557–2567.
- Dang, S.; Yang, H.; Gao, P.; Wang, H.; Li, X.; Wei, W.; Sun, Y. A review of research progress on heterogeneous catalysts for methanol synthesis from carbon dioxide hydrogenation. Catal. Today 2019, 330, 61–75.
- Roy, S.; Cherevotan, A.; Peter, S.C. Thermochemical CO2 Hydrogenation to Single Carbon Products: Scientific and Technological Challenges. ACS Energy Lett. 2018, 3, 1938–1966.
- Marlin, D.S.; Sarron, E.; Sigurbjörnsson, Ó. Process Advantages of Direct CO2 to Methanol Synthesis. Front. Chem. 2018, 6, 1–8.
- Pontzen, F.; Liebner, W.; Gronemann, V.; Rothaemel, M.; Ahlers, B. CO2-based methanol and DME - Efficient technologies for industrial scale production. Catal. Today 2011, 171, 242–250.
- Joo, O.S.; Jung, K.D.; Moon, I.; Rozovskii, A.Y.; Lin, G.I.; Han, S.H.; Uhm, S.J. Carbon dioxide hydrogenation to form methanol via a reverse-water-gas- shift reaction (the CAMERE process). Ind. Eng. Chem. Res. 1999, 38, 1808–1812.
- Joo, O.S.; Jung, K.D.; Yonsoo, J. CAMERE Process for methanol synthesis from CO2 hydrogenation. Stud. Surf. Sci. Catal. 2004, 153, 67–72.
- Anicic, B.; Trop, P.; Goricanec, D. Comparison between two methods of methanol production from carbon dioxide. Energy 2014, 77, 279–289.
- Samimi, F.; Rahimpour, M.R. Direct Methanol Fuel Cell. In Methanol Science and Engineering; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 381–397.
- Zheng, Y.; Wang, J.; Yu, B.; Zhang, W.; Chen, J.; Qiao, J.; Zhang, J. A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): Advanced materials and technology. Chem. Soc. Rev. 2017, 46, 1427–1463.
- Graves, C.; Ebbesen, S.D.; Mogensen, M. Co-electrolysis of CO2 and H2O in solid oxide cells: Performance and durability. Solid State Ionics 2011, 192, 398–403.
- Andika, R.; Nandiyanto, A.B.D.; Putra, Z.A.; Bilad, M.R.; Kim, Y.; Yun, C.M.; Lee, M. Co-electrolysis for power-to-methanol applications. Renew. Sustain. Energy Rev. 2018, 95, 227–241.
- Jensen, S.H.; Graves, C.; Chen, M.; Hansen, J.B.; Sun, X. Characterization of a planar solid oxide cell stack operated at elevated pressure. J. Electrochem. Soc. 2016, 163, F1596–F1604.
- Al-Kalbani, H.; Xuan, J.; García, S.; Wang, H. Comparative energetic assessment of methanol production from CO2: Chemical versus electrochemical process. Appl. Energy 2016, 165, 1–13.
- Sun, X.; Chen, M.; Jensen, S.H.; Ebbesen, S.D.; Graves, C.; Mogensen, M. Thermodynamic analysis of synthetic hydrocarbon fuel production in pressurized solid oxide electrolysis cells. Int. J. Hydrog. Energy 2012, 37, 17101–17110.
- Ebbesen, S.D.; Hansen, J.B.; Mogensen, M.B. Biogas upgrading using SOEC with a Ni-ScYSZ electrode. In ECS Transactions; Electrochemical Society Inc.: Pennington, NJ, USA, 2013; Volume 57, pp. 3217–3227.
- Yoneima, T.; Fukushima, K.; Saito, N.; Nakashima, K. Effect of Sulfur on the Sintering of Nickel Particles. Mater. Trans. 2016, 57, 1374–1377.
- Brett, D.J.L.; Atkinson, A.; Cumming, D.; Ramírez-Cabrera, E.; Rudkin, R.; Brandon, N.P. Methanol as a direct fuel in intermediate temperature (500–600 °C) solid oxide fuel cells with copper based anodes. Chem. Eng. Sci. 2005, 60, 5649–5662.
- Skafte, T.L.; Guan, Z.; Machala, M.L.; Gopal, C.B.; Monti, M.; Martinez, L.; Stamate, E.; Sanna, S.; Garrido Torres, J.A.; Crumlin, E.J.; et al. Selective high-temperature CO2 electrolysis enabled by oxidized carbon intermediates. Nat. Energy 2019, 4, 846–855.
- Saito, M.; Takeuchi, M.; Fujitani, T.; Toyir, J.; Luo, S.; Wu, J.; Mabuse, H.; Ushikoshi, K.; Mori, K.; Watanabe, T. Advances in joint research between NIRE and RITE for developing a novel technology for methanol synthesis from CO2 and H2. Appl. Organomet. Chem. 2000, 14, 763–772.
- Toyir, J.; Miloua, R.; Elkadri, N.; Nawdali, M.; Toufik, H.; Miloua, F.; Saito, M. Sustainable process for the production of methanol from CO2 and H2 using Cu/ZnO-based multicomponent catalyst. Phys. Procedia 2009, 2, 1075–1079.
- Doss, B.; Ramos, C.; Atkins, S. Optimization of Methanol Synthesis from Carbon Dioxide and Hydrogen: Demonstration of a Pilot-Scale Carbon-Neutral Synthetic Fuels Process. Energy Fuels 2009, 23, 4647–4650.
- Deerberg, G.; Oles, M.; Schlögl, R. The Project Carbon2Chem®. Chemie-Ingenieur-Technik 2018, 90, 1365–1368.
- Power2Met. Available online: https://energiforskning.dk/en/node/9313 (accessed on 10 December 2019).
- Brynolf, S.; Taljegard, M.; Grahn, M.; Hansson, J. Electrofuels for the transport sector: A review of production costs. Renew. Sustain. Energy Rev. 2018, 81, 1887–1905.
- Klenert, D.; Mattauch, L.; Combet, E.; Edenhofer, O.; Hepburn, C.; Rafaty, R.; Stern, N. Making carbon pricing work for citizens. Nat. Clim. Chang. 2018, 8, 669–677.
- Ramstein, C.; Dominioni, G.; Ettehad, S.; Lam, L.; Quant, M.; Zhang, J.; Mark, L.; Nierop, S.; Berg, T.; Leuschner, P.; et al. State and Trends of Carbon Pricing 2019; The World Bank: Washington, DC, USA, 2019.
- Laude, A.; Ricci, O.; Bureau, G.; Royer-Adnot, J.; Fabbri, A. CO2 capture and storage from a bioethanol plant: Carbon and energy footprint and economic assessment. Int. J. Greenh. Gas Control. 2011, 5, 1220–1231.
- Lin, W.C.; Chen, Y.P.; Tseng, C.P. Pilot-scale chemical–biological system for efficient H2S removal from biogas. Bioresour. Technol. 2013, 135, 283–291.
- Bao, Z.; Yu, F. Catalytic Conversion of Biogas to Syngas via Dry Reforming Process. Adv. Bioenergy 2018, 3, 43–76.
- Kumar, N.; Shojaee, M.; Spivey, J.J. Catalytic bi-reforming of methane: From greenhouse gases to syngas. Curr. Opin. Chem. Eng. 2015, 9, 8–15.
- Hansen, J.B.; Fock, F.; Lindboe, H.H. Biogas upgrading: By steam electrolysis or co-electrolysis of biogas and steam? ECS Trans. 2013, 57, 3089–3097.
- International Energy Agency (IEA). Technology Roadmap: Carbon Capture and Storage; Technical report; IEA: Paris, France, 2013.
- Odeh, N.A.; Cockerill, T.T. Life cycle GHG assessment of fossil fuel power plants with carbon capture and storage. Energy Policy 2008, 36, 367–380.
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443.
- Pérez-Fortes, M.; Schöneberger, J.C.; Boulamanti, A.; Tzimas, E. Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. Appl. Energy 2016, 161, 718–732.
- Holloway, S. Safety of the underground disposal of carbon dioxide. Energy Convers. Manag. 1997, 38, S241–S245.
- Ehteshami, S.M.M.; Chan, S.H. The role of hydrogen and fuel cells to store renewable energy in the future energy network - potentials and challenges. Energy Policy 2014, 73, 103–109.
- Barbir, F. PEM electrolysis for production of hydrogen from renewable energy sources. Solar Energy 2005, 78, 661–669.
- Kiaee, M.; Cruden, A.; Infield, D.; Chladek, P. Improvement of power system frequency stability using alkaline electrolysis plants. Proc. Inst. Mech. Eng. Part J. Power Energy 2013, 227, 115–123.
- Guinot, B.; Montignac, F.; Champel, B.; Vannucci, D. Profitability of an electrolysis based hydrogen production plant providing grid balancing services. Int. J. Hydrog. Energy 2015, 40, 8778–8787.
- Mohammadi, A.; Mehrpooya, M. A comprehensive review on coupling different types of electrolyzer to renewable energy sources. Energy 2018, 158, 632–655.
- Sherif, S.; Barbir, F.; Veziroglu, T. Wind energy and the hydrogen economy—Review of the technology. Solar Energy 2005, 78, 647–660.
- Bellotti, D.; Rivarolo, M.; Magistri, L.; Massardo, A. Thermo-economic comparison of hydrogen and hydro-methane produced from hydroelectric energy for land transportation. Int. J. Hydrog. Energy 2015, 40, 2433–2444.
- Yilmaz, F.; Ozturk, M.; Selbas, R. Thermodynamic performance assessment of ocean thermal energy conversion based hydrogen production and liquefaction process. Int. J. Hydrog. Energy 2018, 43, 10626–10636.
- Khosravi, A.; Syri, S.; Assad, M.; Malekan, M. Thermodynamic and economic analysis of a hybrid ocean thermal energy conversion/photovoltaic system with hydrogen-based energy storage system. Energy 2019, 172, 304–319.
- Balta, M.T.; Hepbasli, A. Potential methods for geothermal-based hydrogen production. Int. J. Hydrog. Energy 2010, 35, 4949–4961.
- Tolga Balta, M.; Dincer, I.; Hepbasli, A. Thermodynamic assessment of geothermal energy use in hydrogen production. Int. J. Hydrog. Energy 2009, 34, 2925–2939.
- Acar, C.; Dincer, I. Comparative assessment of hydrogen production methods from renewable and non-renewable sources. Int. J. Hydrog. Energy 2014, 39, 1–12.
- Mori, D.; Hirose, K. Recent challenges of hydrogen storage technologies for fuel cell vehicles. Int. J. Hydrog. Energy 2009, 34, 4569–4574.
- Acar, C.; Dincer, I. Review and evaluation of hydrogen production options for better environment. J. Clean. Prod. 2019, 218, 835–849.
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611.
- Ramea, K. An integrated quantitative-qualitative study to monitor the utilization and assess the perception of hydrogen fueling stations. Int. J. Hydrog. Energy 2019.
- Santos, D.M.; Sequeira, C.A.; Figueiredo, J.L. Hydrogen production by alkaline water electrolysis. Quimica Nova 2013, 36, 1176–1193.
- Kreuter, W.; Hofmann, H. Electrolysis: The important energy transformer in a world of sustainable energy. Int. J. Hydrog. Energy 1998, 23, 661–666.
- Feng, Q.; Yuan, X.Z.; Liu, G.; Wei, B.; Zhang, Z.; Li, H.; Wang, H. A review of proton exchange membrane water electrolysis on degradation mechanisms and mitigation strategies. J. Power Sour. 2017, 366, 33–55.
- Rashid, M.; Al Mesfer, M.K.; Naseem, H.; Danish, M. Hydrogen Production by Water Electrolysis: A Review of Alkaline Water Electrolysis, PEM Water Electrolysis and High Temperature Water Electrolysis. Int. J. Eng. Adv. Technol. (IJEAT) 2015, 4, 2249–8958.
- Ursúa, A.; Gandía, L.M.; Sanchis, P. Hydrogen production from water electrolysis: Current status and future trends. Proc. IEEE 2012, 100, 410–426.
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390.
- David, M.; Ocampo-Martínez, C.; Sánchez-Peña, R. Advances in alkaline water electrolyzers: A review. J. Energy Storage 2019, 23, 392–403.
- Chisholm, G.; Cronin, L. Hydrogen From Water Electrolysis. In Storing Energy: With Special Reference to Renewable Energy Sources; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 315–343.
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrog. Energy 2013, 38, 4901–4934.
- Marshall, A.; Børresen, B.; Hagen, G.; Tsypkin, M.; Tunold, R. Hydrogen production by advanced proton exchange membrane (PEM) water electrolysers-Reduced energy consumption by improved electrocatalysis. Energy 2007, 32, 431–436.
- Al Shakhshir, S.; Cui, X.; Frensch, S.; Kær, S.K. In-situ experimental characterization of the clamping pressure effects on low temperature polymer electrolyte membrane electrolysis. Int. J. Hydrog. Energy 2017, 42, 21597–21606.
- Li, N.; Araya, S.S.; Kær, S.K. The effect of Fe3+ contamination in feed water on proton exchange membrane electrolyzer performance. Int. J. Hydrog. Energy 2019, 44, 12952–12957.
- Slavcheva, E.; Radev, I.; Bliznakov, S.; Topalov, G.; Andreev, P.; Budevski, E. Sputtered iridium oxide films as electrocatalysts for water splitting via PEM electrolysis. Electrochim. Acta 2007, 52, 3889–3894.
- Slavcheva, E.; Borisov, G.; Lefterova, E.; Petkucheva, E.; Boshnakova, I. Ebonex supported iridium as anode catalyst for PEM water electrolysis. Int. J. Hydrog. Energy 2015, 40, 11356–11361.
- Paulose, M.; Mor, G.K.; Varghese, O.K.; Shankar, K.; Grimes, C.A. Visible light photoelectrochemical and water-photoelectrolysis properties of titania nanotube arrays. J. Photochem. Photobiol. Chem. 2006, 178, 8–15.
- Lindquist, S.E.; Fell, C. Fuels—Hydrogen Generators|Photoelectrolysis. Encycl. Electrochem. Power Sources 2009, 369–383.
- Ahmed, M.; Dincer, I. A review on photoelectrochemical hydrogen production systems: Challenges and future directions. Int. J. Hydrog. Energy 2019, 44, 2474–2507.
- Ausfelder, F.; Beilmann, C.; Bertau, M.; Bräuninger, S.; Heinzel, A.; Hoer, R.; Koch, W.; Mahlendorf, F.; Metzelthin, A.; Peuckert, M.; et al. Energy Storage as Part of a Secure Energy Supply. ChemBioEng Rev. 2017, 4, 144–210.
- Verhelst, S.; Turner, J.W.; Sileghem, L.; Vancoillie, J. Methanol as a fuel for internal combustion engines. Prog. Energy Combust. Sci. 2019, 70, 43–88.
- Methanol as a Vehicle Fuel | Methanex Corporation. Available online: https://www.methanex.com/about-methanol/methanol-vehicle-fuel (accessed on 10 December 2019).
- Gavaghan, H. Technology: California cleans up its cars with methanol|New Scientist. Available online: https://www.newscientist.com/article/mg12517073-900-technology-california-cleans-up-its-cars-with-methanol/ (accessed on 25 January 2020).
- Fuel Freedom Foundation. When California had 15,000 methanol cars—Fuel Freedom Foundation. Available online: https://www.fuelfreedom.org/when-california-had-15000-methanol-cars/ (accessed on 10 December 2019).
- International Maritime Organization (IMO). Sulphur 2020—Cutting Sulphur Oxide Emissions. Available online: http://www.imo.org/en/MediaCentre/HotTopics/Pages/Sulphur-2020.aspx (accessed on 10 December 2019).
- International Maritime Organization (IMO). Energy efficiency and the reduction of GHG emissions from ships. Available online: http://www.imo.org/en/MediaCentre/HotTopics/GHG/Pages/default.aspx (accessed on 10 December 2019).
- Gregory Dolan. Overview of Global Methanol Fuel Blending; Technical report; Methanol Institute: Alexandria, VA, USA, 2019.
- A.P. Moller—Maersk and Lloyds Register. Alcohol, Biomethane and Ammonia are the Best-Positioned Fuels to Reach Zero Net Emissions. Available online: https://www.maersk.com/news/articles/2019/10/24/alcohol-biomethane-and-ammonia-are-the-best-positioned-fuels-to-reach-zero-net-emissions (accessed on 10 December 2019).
- Transport & Environment (TE). Roadmap to Decarbonising European Aviation; Technical report; European Federation for Transport & Environment AISBL: Brussels, Belgium, 2018.
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989.
- Kamarudin, S.; Achmad, F.; Daud, W. Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int. J. Hydrog. Energy 2009, 34, 6902–6916.
- Icardi, U.; Specchia, S.; Fontana, G.; Saracco, G.; Specchia, V. Compact direct methanol fuel cells for portable application. J. Power Sour. 2008, 176, 460–467.
- Kang, K.; Park, S.; Cho, S.O.; Choi, K.; Ju, H. Development of Lightweight 200-W Direct Methanol Fuel Cell System for Unmanned Aerial Vehicle Applications and Flight Demonstration. Fuel Cells 2014, 14, 694–700.
- Gong, A.; Verstraete, D. Fuel cell propulsion in small fixed-wing unmanned aerial vehicles: Current status and research needs. Int. J. Hydrog. Energy 2017, 42, 21311–21333.
- González-Espasandín, Ó.; Leo, T.J.; Navarro-Arévalo, E. Fuel cells: A real option for unmanned aerial vehicles propulsion. Sci. World J. 2014, 2014.
- SerEnergy A/S. The Benefits of Methanol Fuel Cells. Available online: https://serenergy.com/ (accessed on 10 December 2019).
- Lotrič, A.; Sekavčnik, M.; Hočevar, S. Effectiveness of heat-integrated methanol steam reformer and polymer electrolyte membrane fuel cell stack systems for portable applications. J. Power Sour. 2014, 270, 166–182.
- Ribeirinha, P.; Alves, I.; Vázquez, F.V.; Schuller, G.; Boaventura, M.; Mendes, A. Heat integration of methanol steam reformer with a high-temperature polymeric electrolyte membrane fuel cell. Energy 2017, 120, 468–477.
- Thomas, S.; Araya, S.S.; Frensch, S.H.; Steenberg, T.; Kær, S.K. Hydrogen mass transport resistance changes in a high temperature polymer membrane fuel cell as a function of current density and acid doping. Electrochim. Acta 2019, 317, 521–527.
- Andreasen, S.J.; Kær, S.K.; Justesen, K.K.; Sahlin, S.L. High Temperature PEM Fuel Cell Systems, Control and Diagnostics. In High Temperature Polymer Electrolyte Membrane Fuel Cells; Springer International Publishing: Cham, Switzerland, 2016; pp. 459–486.
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kær, S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrog. Energy 2016, 41, 21310–21344.
- Andreasen, S.J. Design and Control of High Temperature PEM Fuel Cell System. Ph.D. Thesis, Aalborg University, Aalborg, Denmark, 2009.
- Kurz, T.; Küfner, F.; Gerteisen, D. Heating of Low and High Temperature PEM Fuel Cells with Alternating Current. Fuel Cells 2018, 18, 326–334.
- Geissler, K.; Newson, E.; Vogel, F.; Truong, T.B.; Hottinger, P.; Wokaun, A. Autothermal methanol reforming for hydrogen production in fuel cell applications. Phys. Chem. Chem. Phys. 2001, 3, 289–293.
- Iulianelli, A.; Ribeirinha, P.; Mendes, A.; Basile, A. Methanol steam reforming for hydrogen generation via conventional and membrane reactors: A review. Renew. Sustain. Energy Rev. 2014, 29, 355–368.
- Karim, A.; Bravo, J.; Gorm, D.; Conant, T.; Datye, A. Comparison of wall-coated and packed-bed reactors for steam reforming of methanol. Catal. Today 2005, 110, 86–91.
- Peppley, B.A.; Amphlett, J.C.; Kearns, L.M.; Mann, R.F. Methanol–steam reforming on Cu/ZnO/Al2O3. Part 1: the reaction network. Appl. Catal. Gen. 1999, 179, 21–29.
- Peppley, B.A.; Amphlett, J.C.; Kearns, L.M.; Mann, R.F. Methanol-steam reforming on Cu/ZnO/Al2O3 catalysts. Part 2. A comprehensive kinetic model. Appl. Catal. Gen. 1999, 179, 31–49.
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Steady-state isotopic transient kinetic analysis of steam reforming of methanol over Cu-based catalysts. Appl. Catal. Environ. 2009, 88, 490–496.
- Huang, C.Y.; Sun, Y.M.; Chou, C.Y.; Su, C.C. Performance of catalysts CuO–ZnO–Al2O3, CuO–ZnO–Al2O3–Pt–Rh, and Pt-Rh in a small reformer for hydrogen generation. J. Power Sour. 2007, 166, 450–457.
- Purnama, H.; Ressler, T.; Jentoft, R.E.; Soerijanto, H.; Schlögl, R.; Schomäcker, R. CO formation/selectivity for steam reforming of methanol with a commercial CuO/ZnO/Al2O3 catalyst. Appl. Catal. Gen. 2004, 259, 83–94.
- Sahlin, S.L. Characterization and Modeling of a Methanol Reforming Fuel Cell System. Ph.D. Thesis, Aalborg University, Aalborg, Denmark, 2016.
- Simon Araya, S.; Juhl Andreasen, S.; Venstrup Nielsen, H.; Knudsen Kær, S. Investigating the effects of methanol-water vapor mixture on a PBI-based high temperature PEM fuel cell. Int. J. Hydrog. Energy 2012, 37, 18231–18242.
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for methanol steam reforming—A review. Appl. Catal. Environ. 2010, 99, 43–57.
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol Steam Reforming for Hydrogen Production. Chem. Rev. 2007, 107, 3992–4021.
- Twigg, M.V.; Spencer, M.S. Deactivation of copper metal catalysts for methanol decomposition, methanol steam reforming and methanol synthesis. Top. Catal. 2003, 22, 191–203.
- Breen, J.P.; Ross, J.R.H. Methanol reforming for fuel-cell applications: development of zirconia-containing Cu–Zn–Al catalysts. Catal. Today 1999, 51, 521–533.
- Valdés-Solís, T.; Marbán, G.; Fuertes, A.B. Nanosized catalysts for the production of hydrogen by methanol steam reforming. Catal. Today 2006, 116, 354–360.
- Agarwal, V.; Patel, S.; Pant, K. H2 production by steam reforming of methanol over Cu/ZnO/Al2O3 catalysts: transient deactivation kinetics modeling. Appl. Catal. Gen. 2005, 279, 155–164.
- Cao, W.; Chen, G.; Li, S.; Quan, Y. Methanol-steam reforming over a ZnO–Cr2O3/CeO2–ZrO2/Al2O3 catalyst. Chem. Eng. J. 2006, 119, 93–98.
- Lee, M.T.; Greif, R.; Grigoropoulos, C.P.; Park, H.G.; Hsu, F.K. Transport in packed-bed and wall-coated steam-methanol reformers. J. Power Sour. 2007, 166, 194–201.
- Saidi, M. Performance assessment and evaluation of catalytic membrane reactor for pure hydrogen production via steam reforming of methanol. Int. J. Hydrog. Energy 2017, 42, 16170–16185.
- Mobile Hybrid Power shows APU with Serenergy HTPEM. Fuel Cells Bull. 2013, 2013, 8.
- Arsalis, A.; Nielsen, M.P.; Kær, S.K. Modeling and off-design performance of a 1kWe HT-PEMFC (high temperature-proton exchange membrane fuel cell)-based residential micro-CHP (combined-heat-and-power) system for Danish single-family households. Energy 2011, 36, 993–1002.
- Liu, Y.; Lehnert, W.; Janßen, H.; Samsun, R.C.; Stolten, D. A review of high-temperature polymer electrolyte membrane fuel-cell (HT-PEMFC)-based auxiliary power units for diesel-powered road vehicles. J. Power Sour. 2016, 311, 91–102.
- First methanol filling station opens to serve range-extender cars. Fuel Cells Bull. 2015, 2015, 7–8.
- Blue World Technologies signs strategic cooperation agreement with Chinese electric vehicle manufacturer AIWAYS—Blue World Technologies. Available online: https://www.blue.world/blue-world-technologies-signs-strategic-cooperation-agreement-with-chinese-electric-vehicle-manufacturer-aiways/ (accessed on 10 December 2019).
- Millo, F.; Caputo, S.; Piu, A. Analysis of a HT-PEMFC range extender for a light duty full electric vehicle (LD-FEV). Int. J. Hydrog. Energy 2016, 41, 16489–16498.
- Blue World Technologies. Available online: https://www.blue.world (accessed on 10 December 2019).
- Gas Fuelled Ships onboard Fuel Cell powered Viking Mariella. Available online: https://www.motorship.com/gfsconference/latest-news101/unique-venue-revealed-another-first-for-gfs!2 (accessed on 10 December 2019).
- Serenergy. The first methanol fuel cell powered vessel in Germany is now sailing the waters of lake Baldeneysee. Available online: https://serenergy.com/the-first-methanol-fuel-cell-powered-vessel-in-germany-is-now-sailing-the-waters-of-lake-baldeneysee/ (accessed on 10 December 2019).
- ZBT, Helbio, Advent demo HTPEM stack in CHP configuration. Fuel Cells Bull. 2013, 2013, 11.
- Nielsen, E.R.; Prag, C.B.; Bachmann, T.M.; Carnicelli, F.; Boyd, E.; Walker, I.; Ruf, L.; Stephens, A. Status on Demonstration of Fuel Cell Based Micro-CHP Units in Europe. Fuel Cells 2019, 19, 340–345.
- Elcore to Install 135 Micro-CHP Fuel Cells in Europe under ene.field Project. Available online: http://www.fuelcelltoday.com/news-archive/2013/august/elcore-to-install-135-micro-chp-fuel-cells-in-europe-under-enefield-project (accessed on 10 December 2019).
- Oh, M.H.; Yoon, Y.S.; Park, S.G. The electrical and physical properties of alternative material bipolar plate for PEM fuel cell system. Electrochim. Acta 2004, 50, 777–780.
- Ribeirinha, P.; Boaventura, M.; Lopes, J.C.B.; Sousa, J.M.; Mendes, A. Study of different designs of methanol steam reformers: Experiment and modeling. Int. J. Hydrog. Energy 2014, 39, 19970–19981.
- Pan, C.; He, R.; Li, Q.; Jensen, J.O.; Bjerrum, N.J.; Hjulmand, H.A.; Jensen, A.B. Integration of high temperature PEM fuel cells with a methanol reformer. J. Power Sour. 2005, 145, 392–398.
- Ji, F.; Yang, L.; Li, Y.; Sun, H.; Sun, G. Performance enhancement by optimizing the reformer for an internal reforming methanol fuel cell. Energy Sci. Eng. 2019, 1–11.
