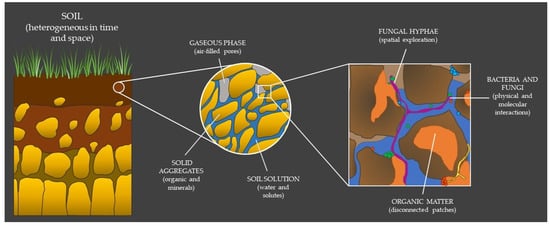
Understanding the fine scale heterogeneity of soil environments is a prerequisite for predicting and contextualizing the physiology of microorganisms and metabolic interactions among community members. Their dynamics, in all their shapes and forms, are the consequences of their adaptation in response to the micro-habitats they experience (Figure 1).
2. The Hidden Properties of Soil
Basic physical laws govern and dictate the properties of each of the microscopic components that compose soils; the surface-to-volume ratio increases with the decreasing dimensions of an object, leading to surface effects, such as surface tension, capillary forces, adhesion, and viscous drag.
Soil is a highly heterogeneous medium, consisting of a mixture of solid material, and of water- or air-filled pores
[1][4] (
Figure 1). Thus, soil can be theoretically interpreted, either as the (organized) arrangement of aggregates/particles in the soil (i.e., soil structure)
[2][3][5,6], or as the connectivity, tortuosity, and heterogeneity of the pore space between these soil components (i.e., soil architecture)
[4][7] (for a complete argumentation on soil structure versus soil architecture, see Baveye et al.
[5][3]). Albeit the hierarchical organization of aggregates highly depends on the amount of energy that is applied to take the soil apart, soils can be classified based on the distribution of their different aggregate species (i.e., soil texture). The aggregates are generally divided depending on their size, i.e., from (very) stable micro-aggregates (<2 µm), which are mainly composed of organic matter and clays, to less stable macro-aggregates, which are commonly composed of silt (2–63 µm) or sand (63 µm to 2 mm)
[6][8]. Physical and chemical processes driven by both biotic and abiotic factors cause changes in aggregate size and continuous particle rearrangement. These constant changes in spatial organization of the solids and voids affect the architecture and connectivity of the pore space, which, in turn, affects the distribution of water and gases
[7][9], as well as the diffusion of substrates (e.g., organic matter)
[8][9][10,11] and solutes (e.g., elements and ions)
[10][12] in soil.
Water is one of the most important, but at the same time is most variable component of soil. Soil’s water content depends on soil composition, rainfall, drainage, evaporation, temperature, and plant cover. Water is the medium connecting spatially separated areas in the soil matrix and becomes the solvent in which organic matter, microelements, and metabolites of different biological origins are dissolved or suspended (i.e., soil solution). The degree of water retention in soil microenvironments mainly depends on the soil pore neck size
[11][13]. In macro-pores, localized in and between macro-aggregates, water is often well drained, whereas it is fully retained in micro-pores (localized between micro-aggregates) due to capillary action
[11][12][13,14]. As a consequence, when alternating saturation/desiccation cycles that occur due to changes in precipitation or temperature, water status is more conserved in the micro-pores, generating fine water pockets rich in moisture that remain spatially disconnected from one another
[12][13][14][15][16][14,15,16,17,18].
Micro-pores are also important for the retention of important biological molecules, such as organic matter, proteins, and nucleic acids
[17][18][19][20][21][19,20,21,22,23]. As a result of the clay-cation exchange capacity (CEC)
[1][4], they can all be adsorbed and retained by the negative charges of clay in soil micro-aggregates. Indeed, Ranjard and Richaume
[12][14] found that organic matter is not homogenously distributed in soils, and higher concentrations (50–80%) were detected in micro-pores.
Furthermore, at the micro-scale level, micro-elements are to be considered for the growth and survival of microorganisms. Micro-elements are often present in the soil solution, as they are originating from both metabolically catalysed redox reactions as well as from abiotic chemical weathering of rock surfaces. They can diffuse in and out of the smallest pores, including the very narrow 1.8-nm-wide spaces between clay particles
[5][3]. These diffusion processes cause pH and element concentration gradients that are highly dependent on the abiotic properties of the soil (e.g., the mineral composition, morphology, and texture), the geochemistry of the surrounding fluids, as well as the activity of microorganisms secreting highly reactive organic acids (e.g., oxalic acids, citric acid)
[22][24].
Air (gases) resides in between the fractions of the soil pore network that are filled with the soil solution. The soil atmosphere depends on the connectivity of the non-water-filled pores of soil with the atmosphere or with other open pores. It usually consists of varying amounts of gasses such as oxygen, carbon dioxide, nitrogen, and nitrogen dioxide, but also of volatile organic compounds (gasses with biotic origin). The composition of the soil atmosphere highly depends on the production or consumption of a specific gas by local organisms, the solubility and diffusion of the gases in the soil water and, consequently, also on the capacity for water retention of the soil itself, as described before
[11][23][13,25]. As an example, molecular oxygen has a very low solubility in water and its diffusion rate in water is 10,000-fold lower than in air
[23][25]. Hence, soil micropores filled with water will rapidly turn anoxic upon consumption, limiting the growth and survival of many microbes. On the other hand, micropores can also offer protective microhabitats against “toxic” gases
[24][26].
Independent of the soil component considered, the soil solution present in colloids can eventually diffuse out into wider pores, where it is readily available to microorganisms or is transported with the percolating water. It is also worth mentioning that filamentous fungi and plant roots stabilize the micro-aggregates of soil, and, in turn, together with other organisms living in the soil (e.g., nematodes, worms, larger animals), continuously increase soil solution conductivity through the soil. Nonetheless, the majority of substrates remain isolated and persist in the environment, possibly due to either physical protection or the separation from relevant enzymes
[17][19]. In this regard, given their even smaller size, exoenzymes released by bacteria, archaea, and fungi into the soil solution can diffuse in and out of tiny pores and have significant roles both in the total fitness of the microbial population as well as in geo-chemical nutrient cycles of an ecosystem
[25][27].
Respiration and decomposition rates differ considerably between sandy soils and soils rich in clay. This illustrates impressively how the dynamic interaction between the physical, air, and water components of soil directly influence the access, and thus the activity, of microbial populations to their substrate both in space and time. Sandy soils have lower surface-to-volume ratios than clay soils, meaning less water retention, but also lower segregation between microenvironments. In this context, soil heterogeneity has to be considered. For example, organic matter placed in different regions of the pore network was shown to be decomposed at different rates
[26][28] while a number of other studies reported increased respiration rates only after disruption of the soil structure in soils with high clay content (e.g.,
[27][29]). These results taken together suggest that both the local environmental conditions as well as the uneven distribution of microbial populations within the local environment strongly affect the mineralization rates
[26][28][29][28,30,31] and the range over which organisms can disperse and interact.
3. Bacterial–Fungal Interactions: A Harsh Existence
At the cell-to-cell level, bacteria and fungi interact at many different levels of intimacy that can be considered from two perspectives: in terms of physical associations and in terms of molecular communication
[30][32]. Physical associations can range from seemingly disordered polymicrobial communities (i.e., biofilms) to highly specific symbiotic associations of fungal hyphae and bacterial cells (i.e., ectosymbiotic or endosymbiotic). Molecular interactions, on the other hand, involve a complex and diverse set of chemicals and compounds, and the interaction can be contextualized as antibiosis, signaling, and chemotaxis, metabolite exchange, metabolic conversion, adhesion, protein secretion, genetic exchange, and physicochemical changes. More often than not, multiple mechanism of interaction can be employed by one microorganism. The multitude of interactions and their effect on the partners or surrounding environment is extensive and well reviewed in
[31][30][32][33][1,32,33,34].
Irrespective of the type of interaction, bacteria and fungi need to recognize each other prior to initiating any kind of target-oriented interaction. However, sensing and recognition mechanisms are often hindered by the particularity of soil and by the phenotypical characteristics of the organism (Figure 1).
When considering bacteria, they have their highest diversity in soil micro-aggregates, where they are either “swimming” in the soil solution, or attached to soil particles. This is not surprising, as it is in accordance with the higher concentrations of organic matter found in the micro-pores, the more stable conditions in water content, and a lower predation pressure by protozoan or other predators
[34][35][36][37][38][35,36,37,38,39]. One of the first studies focusing on the distribution of bacteria in soil revealed that specific bacterial populations are typically residing inside micro-aggregates, with 40–70% of these bacteria being localized in the 2–20 µm and in <2 µm size micro-pores
[12][14]. In some cases, cells can even penetrate pores smaller than themselves
[39][40]. In contrast, more recent studies reported that pores in the 30 to 150 µm size range harbor a greater abundance of specific bacterial groups
[40][41]. However, studies are scarce and only represent a snapshot in time, in a very dynamic environment. In fact, microbes explore a constantly changing environment in search of their
realized niche, let it be by either active exploration of soil by fungal hyphae or by chemotactic movement, or passive transportation in bacteria. Any potential in bacterial mobility is limited by surface tension, capillary forces, and viscous drag that increase the energy requirement for their motility, particularly in partially saturated pore networks. Motility was found to cease, virtually completely, when the thickness of the water film was smaller than 1.5 µm
[41][42]. For these reasons, microbial cells are often found as individual cells when associated with highly localized dissolved organic matter, or as patches of dense populations when linked to more conspicuous substrates
[42][43]. In bulk soil, the average distance between neighbouring bacterial cells was found to be around 12.46 µm, with inter-cell distances shorter near the soil surface (10.38 µm) than at depth (>18 µm), due to changes in cell densities
[43][44]. Simplified calculations suggests that, despite the very high number of cells and species in soil (10
8 cells per gram of soil), the number of neighbours that a single bacterial cell has within an interaction distance of ca. 20 µm is relatively limited (120 cells on average)
[43][44]. Similar crude estimates were also found when the surfaces of soil pores were used to calculate the exclusion zone of cells on soil aggregates (a radius of 178 µm)
[44][45].
A completely different story is depicted when filamentous fungi are considered. Fungi actively explore the soil pore space through hyphal spread, and cope well with heterogeneous distribution of nutrients
[45][46]. They can cross air–water interfaces and nutrient-depleted spots to gain access to distant nutrient resources. The extensive mycelial architecture enables fungi to easily and efficiently re-allocate useful compounds to substrate-limited regions, to the benefit of exploratory colonisation of more unfavourable habitats
[46][47].
A relevant feature of microbial interaction related to mycelial growth is that hyphae serve as dispersal vectors for motile bacteria (i.e., fungal highways)
[47][48] and, hence, allow for bacterial colonization of new micro-habitats
[48][49][49,50]. Moreover, the mycelia of fungi and oomycetes enhance bacterial activity by nutrient and water transfer (excretion) from the hyphae to the bacterial cells, thus enabling bacterial growth in otherwise too oligotrophic habitats
[50][51], or enhancing microbial activity in dry soils
[51][52]. An important example of such a symbiotic interaction is extraradical mycelia of arbuscular mycorrhizal and other mycorrhizal fungi in the rhizosphere of plants, where a large array of bacteria can directly consume fungal exudates released into the environment
[33][34]. The mutualistic relationship can also be reciprocal: fungi take advantage of their bacterial partners to improve their carbon source pool in a mechanism named bacterial farming
[52][53]. Fungal hyphae can also become an ideal hotspot for horizontal gene transfer
[53][54][55][54,55,56] or bacterial prey populations
[56][57], as they facilitate dispersal and preferential contact of bacteria in the hyphosphere. In this regard, it should be mentioned that, apart from fungal hyphae, plant roots and dead organisms also behave as hot spots for highly interactive (e.g., competition, pathogeny, mutualism, predation) microbial communities. Nonetheless, these many physical interactions between bacteria and hyphae-forming fungi may represent short-lived associations, as microscale communities frequently assemble and disassemble by migration, attachment, and detachment from surfaces and cells.
In this quite lonesome and unforgiving scenario, physical contact between cells is often not realizable. Hence, molecular compounds are employed and secreted in the soil solution to sense potential partners in their surroundings. Importantly, the release of molecular signals represent a cost, both energetic and elemental, and hence are regulated to maximize the fitness of the organism
[25][27]. As molecules diffuse freely from any pore and are easily removed from the microenvironments, molecular interactions can only occur at relatively short distances. On leaf surfaces, for example, interactions among bacteria have been found to occur principally in the 5 to 20 µm range
[57][58], whereas in soil, where local patches of cells may reside in a pocket of soil solution, diffusible metabolites can reach neighbouring cells up to 100 µm away
[43][58][44,59]. The interactive dynamics are very different in soil crusts, biofilms, or mats where cells are well physically constrained and in direct contact with each other (e.g.,
[59][60]).
Sensing and recognition of fungi and bacteria includes also solution-independent ways. Volatile organic compounds (VOCs) are one important quorum sending vehicle-enabling communication over longer distances, especially in vision of the spatial separation between soil particles often occurring in unsaturated water conditions
[60][61][61,62].
Finally, it is worth mentioning that bacteria and fungi can also indirectly interact by modifying their microenvironment in ways that can positively or negatively affect their partners (i.e., niche modulation)
[33][34]. For example, the acidification or the neutralization of pH values influences the solubility of soil nutrients (e.g., phosphorus
[22][24]), thus inhibiting or stimulating overall bacterial growth and metabolism
[62][63][64][63,64,65]. Nutrient depletion is one example of the many ways of indirect interaction. Iron depletion by the excretion of bacterial or fungal siderophores can negatively affect the performance of surrounding organisms, including plants
[65][66][66,67].
In terms of BFI in different scenarios of soil heterogeneity, there is a limited number of studies available. They show both a random distribution of soil microorganisms and a high degree of microbial networking. We will now discuss methodologies and techniques well-suitable for observing microbial interactions and we hope to stimulate the reader to further expand the research in this field.