Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Mohd Imran.
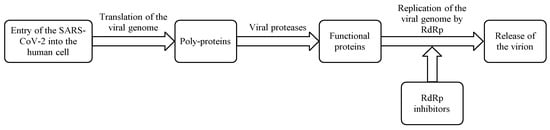
RdRp is an attractive target for developing therapies for COVID-19 as it plays a crucial role in the replication of SARS-CoV-2 (Scheme 1) and is well conserved between coronaviruses (RNA viruses).
- molnupiravir
- EIDD-2801
- MK-4482
- EIDD-1931
- patents
- SARS-CoV-2
- COVID-19
1. RNA-Dependent RNA-Polymerase (RdRp)
RdRp is an attractive target for developing therapies for COVID-19 as it plays a crucial role in the replication of SARS-CoV-2 (Scheme 1) and is well conserved between coronaviruses (RNA viruses). The multi-domain proteins contain less than 500 units of amino acids in length. The protein looks like a human cupped right hand with three subfolded domains constituting thumb, palm, and fingers. There is no known equivalent of RdRp in humans and it therefore produces no off-target untoward effects, making RdRp a selective target to develop RdRp inhibitors. Further, the availability of biochemical assays accelerates the development of RdRp inhibitors [12,13,14,15,16]. Two RdRp inhibitors, remdesivir (anti-Ebola virus experimental drug) [8] and favipiravir (anti-influenza drug) [9] have already been approved for COVID-19 treatment (Table 1). Both these broad-spectrum antiviral drugs have been shown to reduce the progression of COVID-19 and associated clinical symptoms along with a substantial decrease in recovery time [17,18,19]. Remdesivir is administered intravenously but many pharmaceutical companies are developing its acceptable and convenient oral dosage forms. On the other hand, favipiravir is an orally active antiviral drug but it shows a poor pharmacokinetic profile. Hence, some other possible RdRp inhibitors are being considered for COVID-19 treatment, which includes molnupiravir, galidesivir, ribavirin, sofosbuvir, and tenofovir [14,15]. Recently molnupiravir, an orally active RdRp inhibitor with a favorable pharmacokinetic profile, has received considerable attention owing to its ability to inhibit SARs-COV-2 replication, its quick clearance of SARs-COV-2, and the accompanying reduction in viral load and fast recovery time [20]. Molnupiravir reaches quantifiable concentration in 0.5 h between 600–1600 mg. Administration of a single dose produces mean Cmax values up to 13.2 ng/mL and shows median tmax between 0.25 and 0.75 h and a biological half-life (t1/2 )of 7 h. Its Cmax and area under the plasma concentration versus time curve (AUC) increases in a dose-proportional manner with no accumulation following multiple doses suggesting that molnupiravir has no accumulative toxicity. Administration of molnupiravir in a fed state shows a slight decrease in the rate of absorption but no decrease in overall exposure. Furthermore, it exhibits fewer adverse reactions and good tolerability. Based on the pharmacokinetic profile of molnupiravir, it can be inferred that molnupiravir has a quick onset of action, a wide therapeutic window, and excellent tolerance with a good safety profile. These attributes make molnupiravir a very useful therapeutic molecule against COVID-19 [21].
Scheme 1.
Mechanism of action of RdRp inhibitors.
Table 1. Approved RdRp inhibitors in clinical practice.
Approved RdRp inhibitors in clinical practice.
| Drug’s Name | Dosage Forms (Route/Dose) |
Indications (Marketing Status) |
Countries |
|---|---|---|---|
| Remdesivir (Veklury®) |
Solution/Powder (Intravenous/200 mg loading dose, followed by 100 mg once daily for 5 to 10 days for adults) |
COVID-19 patients of ≥12 years requiring hospitalization (Prescription) |
Approved in >50 countries including USA, KSA, UAE and European Union |
| Favipiravir (FabiFlu®) |
Film-coated tablet (Oral/1800 mg/dose twice a day on the first day; followed by 800 mg/dose twice a day for 7–10 days for adults) |
COVID-19 (Prescription) |
Approved in many countries, including China, India, Russia and Japan |
2. Molnupiravir
Molnupiravir (MF: C13H19N3O7; MW: 329.31; CAS Registry Number: 2492423-29-5; Deleted CAS Registry Numbers: 2349386-89-4) is a pyrimidine ribonucleoside analog with a chemical name of ((2R,3S,4R,5R)-3,4-dihydroxy-5-(4-(hydroxyamino)-2-oxopyrimidin-1(2H)-yl) tetrahydrofuran-2-yl) methyl isobutyrate (Figure 1A). The other names of molnupiravir are EIDD-1931-isopropyl ester; EIDD-2801; MK-4482; uridine, 4-oxime, 5′-(2-methylpropanoate); and β-D-N4-hydroxycytidine-5′-isopropyl ester [21,22]. It is an orally active and directly acting antiviral interventional drug. Its efficacy and tolerability in COVID-19 patients has been investigated at doses of 200, 400, and 800 mg twice daily for five days.
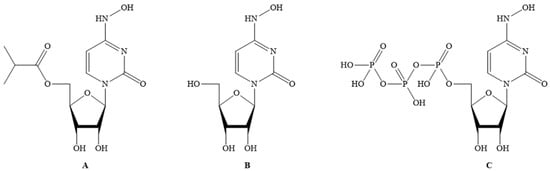
Figure 1.
(
A
) Molnupiravir; (
B
) EIDD-1931; (
C
) EIDD-1931-triphopsphate.
2.1. Mechanism of Action
Molnupiravir acts by inhibiting RdRp of SARS-CoV-2 to induce RNA mutagenesis in two steps. Molnupiravir is converted to EIDD-1931 (CAS Registry Number: 3258-02-4; Deleted CAS Registry Numbers: 85373-26-8; MF: C9H13N3O6; MW: 259.22; Melting Point (Sci-finder): 169–172 °C) (Figure 1B) in the body, which on phosphorylation by host kinases provides the EIDD-1931-triphosphate (CAS Registry Number: 34973-27-8; MF: C9H16N3O15P3; MW: 499.16; Other names: 4-Oxime uridine-5′-triphosphate, EIDD-2061, N4-Hydroxy-CTP, and N4-Hydroxycytidine triphosphate) (Figure 1C). This triphosphate acts as an alternate/competitive substrate for the RdRp enzyme of SARS-CoV-2. Therefore, RdRp generates mutated RNA copies for SARS-CoV-2. This process causes the inhibition of the normal functions of RdRp [21]. Molnupiravir is a better electron donor than electron acceptor, and hence this reducing property can contribute to the antiviral activity as it affects the conditions required for viral infection.
2.2. Discovery and Development
Molnupiravir is an isopropyl prodrug of EIDD-1931 (Figure 1B). The other names of EIDD-1931 are N-Hydroxycytidine (NHC), and N4-Hydroxycytidine. EIDD-1931 demonstrated inhibitory effects on the replication of many viruses, including coronaviruses with promising safety profiles. However, EIDD-1931 also demonstrated poor bioavailability in animal models. After oral treatment, EIDD-1931 was rapidly metabolized in the enterocytes of nonhuman primates. The uptake and the distribution profile of EIDD-1931 in mice are available in the literature [23]. Molnupiravir was developed to overcome the poor bioavailability issue of EIDD-2801 [21,22,24].
Molnupiravir has been developed by scientists at Emory University (USA), with financial support from the USA government [25]. An agreement to develop molnupiravir as an oral treatment for non-hospitalized COVID-19 patients has also been signed between Emory University, Ridgeback Biotherapeutics, Wayne & Wendy Holman, and Merck [26]. Molnupiravir was originally intended to treat alphavirus infections. At the time of the beginning of the pandemic, it was in pre-clinical testing for seasonal influenza. After the spread of COVID-19, the molnupiravir development program moved to the treatment of COVID-19 [25].
2.2.1. Pre-Clinical Studies
Molnupiravir demonstrated potent anti-influenza activity and good oral bioavailability in mice/ferrets/nonhuman primates [22]. This study also highlighted that molnupiravir shows antiviral effects because of its mutagenic property towards the influenza virus. The study further demonstrated a therapeutic window of >1713 (antiviral efficacy vs. cytotoxicity) and suggested further clinical studies of molnupiravir for influenza treatment. The pre-clinical study of molnupiravir in the animal model also showed its oral effectiveness against coronaviruses, including SARS-CoV and MERS-CoV [27]. This study supported the mutagenic property for SARS-CoV and MERS-CoV. The effectiveness of EIDD-1931 against remdesivir resistant virus was also demonstrated, which indicates that molnupiravir might be active against a broader range of viruses than remdesivir. Remdesivir inadequately controls SARS-CoV-2 transmission [28]. However, molnupiravir proved effective at reducing SARS-CoV-2 infection and blocking transmission in ferrets. Accordingly, molnupiravir has been suggested as a countermeasure to prevent community transmission of SARS-CoV-2 [28].
2.2.2. Clinical Studies
The Phase 1 clinical data (pharmacokinetics, safety, and tolerability) of molnupiravir has recently been published [21]. The study was conducted in subjects over the age range of 19–60 years with a mean body mass index (BMI) of 24.4–25.4 kg/m2 in which male individuals were prominent. The pharmacokinetic profile of molnupiravir was evaluated in single and multiple-dose administrations in phase-1 randomized, double-blinded, and placebo-controlled clinical trials. In addition, the effect of food on drug pharmacokinetics was evaluated. The study revealed that molnupiravir absorbed well in plasma in the concentration range of 50–1600 mg in a dose-dependent manner. The rate of absorption of molnupiravir was quite low in the fed state. However, during exposure for longer durations, the absorption rate of both the fed and unfed states was similar. The main observed adverse event was headache. Molnupiravir did not exhibit any negative effects on vital functions and electrocardiogram data and had no clinically significant impact on hematological parameters. Molnupiravir has been considered quite safe at the dose levels of 50–1600 mg. The plasma t1/2 of molnupiravir is dose-dependent and ranges between 0.907 and 7.08 h. The tolerated dose was 50–800 mg BID in a multiple-ascending dose study and 50–1600 mg in a single dose study for 5.5 days. The effective dose for SARS-CoV-2 in humans was reported to be 200–800 mg. Accumulation of the drug was not seen in a multiple-dose study and molnupiravir has been proclaimed to be a promising pharmacological intervention for viral respiratory infections based on previous animal studies. Taken together, the study revealed that molnupiravir is well-tolerated and has dose-dependent pharmacokinetics when administered in healthy individuals at clinically relevant concentrations. It is expected that molnupiravir treatment may be 800 mg capsules two times a day for five days. A summary of the phase 1 clinical trials of molnupiravir is presented in Table 2.
Table 2. Summary of the phase 1 clinical trials of molnupiravir.
Summary of the phase 1 clinical trials of molnupiravir.
| Type of Study | Total Participants | Dose | Pharmacokinetic Data |
|---|---|---|---|
| Interventional, phase 1, randomized, double-blind, placebo-controlled study | 130 | (i) A total of 64 subjects received a single oral dose of 50 to 1600 mg molnupiravir or placebo in the single-ascending-dose part. | Mean C |
Molnupiravir has completed its Phase 2 studies. However, the data of Phase 2 studies are not publicly available.
2.2.3. Current Clinical Trials
Molnupiravir is undergoing seven clinical studies [www.clinicaltrials.gov] (accessed on 9 August 2021). A summary of these clinical studies is provided in Table 3.
Table 3. Interventional randomized clinical trials on molnupiravir (EIDD-2801, MK-4482) for the treatment of COVID-19.
| Sponsor (Status) |
Phase (Number Enrolled) (Interventions) |
NCT Number (Other IDs) |
Start Date (SD)/Completion Date (CD)/Last Update (LU) | |
|---|---|---|---|---|
| Merck Sharp & Dohme Corp. (Active, not recruiting) |
2/3 (304) (Molnupiravir/Placebo) | max up to 13.2 ng/mL and median tmax 0.25 and 0.75 h for doses in between 600–1600 mg. Excretion in urine (0.002%) for >800 mg dose. Geometric mean terminal elimination half-lives (t1/2) = 0.91–1.29 h postdose of drug up to 800 mg dose. Median t1/2 for 1200 and 1600 mg doses = 1.75 and 1.50 h |
||
| NCT04575584 | (4482-001, 2020-003367-26, MK-4482-001, PHRR201210-003189, jRCT2031200404) |
SD: 19 October 2020 CD: 10 August 2021 LU: 7 May 2021 |
(ii) A total of 55 subjects received twice-daily (BID) doses of 50 to 800 mg molnupiravir or placebo for 5.5 days in the multiple-ascending-dose part. | Median t |
| Merck Sharp & Dohme Corp.(Recruiting) | max in all dose cohorts of between 1.00 and 1.75 h postdose across both Days 1 and 6. At the 800-mg BID dose level, the mean t1/2 = 7.08 h AUCτ = 0.938–1.16; Cmax= 0.843–1.10 at all dose levels |
|||
| 2/3 (1850) (Molnupiravir/Placebo) |
NCT04575597 (4482-002, 2020-003368-24, MK-4482-002, PHRR201209-003186, RCT2031210148) |
SD: 19 October 2020 CD: 19 April 2022 LU: 5 August 2021 |
(iii) A total of 10 subjects received a single dose of 200 mg in the fed state followed by a single dose of 200 mg molnupiravir in the fasted state after a washout period of 14 days, or vice versa. | Mean Cmax |
| Merck Sharp & Dohme Corp. (Not yet recruiting) | —approximately 36% lower in the fed state compared to the fasted state AUCinf—similar for both fed and fasted states Mean t1/2 in fed and fasted treatments = 1.09 and 0.977 h |
|||
| 3 (1332) (Molnupiravir/Placebo) |
NCT04939428 (4482-013, 2021-000904-39, MK-4482-013) |
SD: 16 August 2021 CD: 3 April 2022 LU: 4 August 2021 |
(iv) One subject in the multiple-ascending-dose part received 800 mg molnupiravir BID for three days. | It was discontinued by the investigators. |
| Ridgeback Biotherapeutics | |||
| (Recruiting) | 2 (96) (EIDD-2801/Placebo) |
NCT04405739 (EIDD-2801-2004) |
SD: 16 June 2020 CD: 8 December 2021 LU: 20 May 2021 |
| Ridgeback Biotherapeutics (Completed) |
1 (130) (EIDD-2801/Placebo) |
NCT04392219 (EIDD-2801-1001, 2020-001407-17) |
SD: 10 April 2020 CD: 11 August 2020 LU: 19 July 2021 |
| Ridgeback Biotherapeutics (Completed) |
2 (204) (EIDD-2801/Placebo) |
NCT04405570 (EIDD-2801-2003) |
SD: 16 June 2020 CD: 21 February 2021 LU: 23 February 2021 |
| University of Liverpool (Recruiting) |
1/2 (600) (EIDD-2801/Nitazoxanide/VIR-7832/VIR-7831/Placebo) |
NCT04746183 (UoL001542) |
SD: 3 July 2020 CD: 30 April 2022 LU: 20 May 2021 |
The discovery and development timeline of molnupiravir has been summarized in Scheme 2.

Scheme 2.
Summary of the development of molnupiravir.
