The STAT proteins bind to specific response elements on the DNA in the nucleus, thereby inducing gene transcription. Based on their various functions, STAT proteins are essential in several health conditions such as autoimmune diseases and cancer. Despite their broad spectrum of activity, only STAT3 affects embryonic development, as shown in STAT3 knock-out mouse experiments.
- STAT
- prostate cancer
- metastasis
1. Introduction
The protein family “signal transducers and activators of transcription” (STATs) are transcription factors first described at the beginning of the 1990s while investigating the cytokine signaling pathways [1][2]. The protein family comprehends STAT1, STAT2, STAT3 , STAT4, STAT5a, STAT5b, and STAT6, encoded by different genes on different chromosomes [3]. These STAT proteins are involved in multiple biological processes such as immune response, mitogenesis, wound healing, cell survival, and cell growth by transmitting signals from the cell membrane into the nucleus [3][4]. The STAT proteins bind to specific response elements on the DNA in the nucleus, thereby inducing gene transcription. Based on their various functions, STAT proteins are essential in several health conditions such as autoimmune diseases and cancer [5][6]. Despite their broad spectrum of activity, only STAT3 affects embryonic development, as shown in STAT3 knock-out mouse experiments [7]. Different groups of signal proteins, including interleukins (e.g., IL-4 and IL-6), interferons (e.g., IFN-α/β), growth factors (e.g., EGF), and proteohormones (e.g., prolactin) activate STAT proteins [3].
In this review, we focus on the role of STAT proteins in metastatic prostate cancer (PCa) and summarize the current state of research regarding their involvement in metastatic castration-sensitive and castration-resistant PCa.
2. Prostate Cancer
PCa is the second most common cancer in men, with an estimated number of over 1.4 million new cases worldwide in 2020 and it is one of the leading causes of death in men, with an estimated 375,000 cancer-related deaths yearly [8]. PCa is androgen-dependent, requiring testosterone and its metabolite dihydrotestosterone (DHT) for growth and development. Consequently, androgen withdrawal causes reduced cell growth and induces apoptosis [9][10]. For locally confined PCa, radical prostatectomy and external beam radiotherapy are the treatment options with curative intent. However, in the setting of metastatic disease, metastatic hormone-sensitive PCa first-line therapy includes androgen deprivation therapy (ADT), or ADT combined with an antiandrogen (e.g., apalutamide, darolutamide, and enzalutamide), taxane-based chemotherapy (e.g., docetaxel, cabazitaxel), or the CYP17A1 inhibitor abiraterone [11]. Regrettably, after a median response duration of 18 months, most tumors develop ADT resistance and progress to castration-resistant PCa (CRPC) [12]. Therefore, treatment for CRPC includes antiandrogens, taxane-based chemotherapy, and abiraterone [11]. Since 2020, olaparib, a poly (ADP-ribose) polymerase (PARP)-inhibitor, has been EMA approved for the treatment of BRCA1 and BRCA2 positive mCRPC [11]. Unfortunately, despite treatment, the median survival of CRPC patients is only 19 months in average post castration resistance due to tumor progression and development of therapy resistance [13]. Therefore, new therapeutic targets and strategies are necessary for mCRPC.
3. Characterization of STAT Family of Proteins
The ND mediates protein–protein interactions, like dimerization and tetramerization, and nuclear import of STAT proteins [3]. The CCD is involved in the nuclear translocation processes and interactions with regulatory proteins such as IRF9, c-JUN, and SMRT [3][14][15][16]. The DBD is highly conserved in all STAT proteins [17][18]. The domain facilitates the association of STAT proteins with palindromic DNA-binding sites, primarily the “Interferon-Gamma Activated Sequence “(GAS) and the “Interferon-Stimulated Response Element “(ISRE), located in the promoter region of the target genes. Just as the ND and CCD, the DBD is involved in nuclear import and export of STAT [3]. The LD affects mainly the transcriptional activity of STAT proteins. However, the domain is also involved in the DNA-binding and nuclear export processes. The SH2 domain is essential for the binding of STATs to their membrane-bound receptors. This domain is phosphorylated at its tyrosine residues mediating protein dimerization [3][19]. Moreover, it is also involved in the nuclear export processes of STAT proteins. The Y segment is the smallest domain of STAT proteins and has a specific tyrosine motif for every member of the STAT protein family [3]. The tyrosine residues point outward in folded STAT proteins and can be phosphorylated, enabling STAT proteins to dimerize by their SH2 domains. The C-terminal TAD plays an essential role in mediating the transcriptional activity of STAT proteins. It is variable in its sequence and length and holds various conserved serine-phosphorylation sites, mandatory for the interaction with different coactivators [3]. Furthermore, it has a central role in the ubiquitin-mediated degradation of STAT proteins [20].
The canonical JAK/STAT signaling pathway ( Figure 1 A) is mediated by the intracellular Janus kinases (JAK) [3]. In its inactivated state, STAT proteins are localized in the cytoplasm. The JAK proteins are associated with different transmembrane receptors. Extracellular ligand binding to these transmembrane receptors activates the JAKs leading to tyrosine residues autophosphorylation of activated JAK proteins and phosphorylation of tyrosine residues on the cytoplasmatic domain of their transmembrane receptors ( Figure 1 A). Those phospho-tyrosine residues serve as binding sites for the SH2 domain of the STAT proteins. Bound STAT proteins are subsequently activated by the JAK proteins’ phosphorylation of tyrosine residues in the STAT’s Y segment. After phosphorylation, STAT proteins dissociate from the receptor, homo- or heterodimerize with other STAT proteins, and translocate into the nucleus. Nuclear STAT complexes bind to the GAS or ISRE elements of the DNA, recruit comodulators and RNA polymerase II, triggering their target genes expression [3][21].
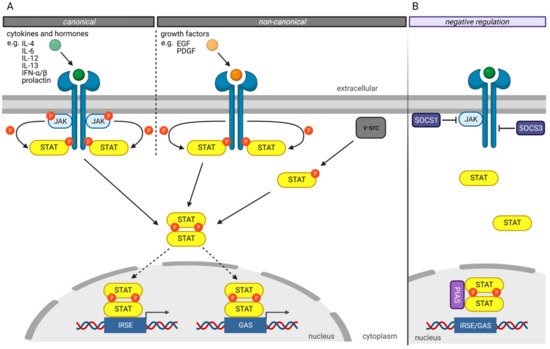
Several mechanisms have been developed to negatively regulate the JAK/STAT pathway to prevent hyperactivity of the STAT transcription factors ( Figure 1 B) [3]. The “Suppressor of cytokine signaling” (SOCS) proteins are crucial in negatively regulating the JAK/STAT pathway, whereby different mechanisms have been described [22][23]. For example, SOCS1 binds directly to JAK proteins and inhibits their catalytic activity [24]. In contrast, SOCS3 binds to JAK-proximal sites on transmembrane receptors, inhibiting JAK activity and simultaneously preventing STAT from binding to these receptors [24].
Another protein family involved in the negative regulation of the JAKT/STAT signaling pathway is the “Protein inhibitors of activated STAT” (PIAS) protein family [25]. PIAS proteins regulate the JAK/STAT signaling pathway by direct interaction of STAT proteins, recruitment of histone deacetylases to inhibit transcription, and modulation of the transcriptional activity of STAT proteins by SUMOylation [26][27]. Besides the direct inhibition of the transcriptional activity of STAT proteins, PIAS proteins prevent the DNA-binding of STAT proteins to the GAS and ISRE motives.
4. STAT Family Members in Prostate Cancer
In primary PCa, STAT3 is constitutively active as immunohistochemical analysis of primary PCa tissue revealed, while elevated levels of phosphorylated STAT3 correlate with higher Gleason scores [28]. Furthermore, STAT3 inhibition by small molecules such as Galiellalactone causes apoptosis-mediated tumor regression in vitro and reduces regional and distal lymph nodes metastases in vivo [28][29][30]. Moreover, in vitro experiments revealed that Galiellalactone reduces cell viability and invasion and induces apoptosis in metastatic PCa cell line DU145 [30].
In human PCa tissue, STAT5a/b is more frequently active in primary PCa during ADT and is also active in 95% of hormone-refractory PCa specimens with ADT [31]. In addition, STAT5 increases the transcriptional activity of the AR by influencing protein stability in PCa cells in vivo and in vitro [31][32]. Furthermore, active STAT5a/b protects antiandrogen-liganded AR from proteasomal degradation, while STAT5a/b knockdown combined with antiandrogen treatment enhances proteasomal degradation of AR followed by suppression of tumor growth [33].
in vitro studies revealed a possible role of STAT6 in cell survival as siRNA mediated STAT6-knockdown-induced apoptosis in metastatic PCa cell line DU145. Moreover, the STAT6 knockdown could be linked to the decreased ability of PCa cell lines to migrate, an essential step in tumor migration and metastasis [34]. Furthermore, IL-4 increased the clonogenic potential of primary PCa cell lines, which could be reversed by treatment with selective STAT6-inhibitor AS1517499 [35], indicating IL-4/STAT6 involvement in the clonogenic ability of PCa cells [36]. Additionally, several miRNAs were found acting as tumor suppressors while suppressing tumor growth, inducing apoptosis, or inhibiting metastasis in PCa by targeting STAT6, thereby underlining the cancer-promoting functioning of STAT6 in PCa [37][38][39]. Eventually, the transcriptional activity of STAT6 is up-regulated by AnxA2, which itself is suggested to be involved in PCa metastasis [40].
Current data describe STAT6 as an essential factor in metastasis in PCa, but its exact role in tumor progression is still largely unknown, and further investigation is needed.
References
- Schindler, C.; Fu, X.Y.; Improta, T.; Aebersold, R.; Darnell, J.E. Proteins of Transcription Factor ISGF-3: One Gene Encodes the 91-and 84-KDa ISGF-3 Proteins That Are Activated by Interferon α. Proc. Natl. Acad. Sci. USA 1992, 89, 7836–7839.
- Fu, X.Y.; Schindler, C.; Improta, T.; Aebersold, R.; Darnell, J.E. The Proteins of ISGF-3, the Interferon α-Induced Transcriptional Activator, Define a Gene Family Involved in Signal Transduction. Proc. Natl. Acad. Sci. USA 1992, 89, 7840–7843.
- Lim, C.P.; Cao, X. Structure, Function, and Regulation of STAT Proteins. Mol. Biosyst. 2006, 2, 536–550.
- Bromberg, J.F. Activation of STAT Proteins and Growth Control. BioEssays 2001, 23, 161–169.
- Lorenzini, T.; Dotta, L.; Giacomelli, M.; Vairo, D.; Badolato, R. STAT Mutations as Program Switchers: Turning Primary Immunodeficiencies into Autoimmune Diseases. J. Leukoc. Biol. 2017, 101, 29–38.
- Owen, K.L.; Brockwell, N.K.; Parker, B.S. Jak-Stat Signaling: A Double-Edged Sword of Immune Regulation and Cancer Progression. Cancers 2019, 11, 2002.
- Takeda, K.; Noguchi, K.; Shi, W.; Tanaka, T.; Matsumoto, M.; Yoshida, N.; Kishimoto, T.; Akira, S. Targeted Disruption of the Mouse Stat3 Gene Leads to Early Embryonic Lethality. Proc. Natl. Acad. Sci. USA 1997, 94, 3801–3804.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Isaacs, J.T. Resolving the Coffey Paradox: What Does the Androgen Receptor Do in Normal vs. Malignant Prostate Epithelial Cells? Am. J. Clin. Exp. Urol. 2018, 6, 55–61.
- Huggins, C.; Hodges, C.V. Studies on Prostatic Cancer: I. The Effect of Castration, of Estrogen and of Androgen Injection on Serum Phosphatases in Metastatic Carcinoma of the Prostate. 1941. J. Urol. 2002, 168, 9–12.
- European Association of Urology. Guidelines on Prostate Cancer; European Association of Urology: Arnhem, The Netherlands, 2021; Available online: https://uroweb.org/wp-content/uploads/EAU-EANM-ESTRO-ESUR-ISUP-SIOG-Guidelines-on-Prostate-Cancer-2021V4.pdf (accessed on 13 September 2021).
- Higano, C.S.; Crawford, E.D. New and Emerging Agents for the Treatment of Castration-Resistant Prostate Cancer. Urol. Oncol. Semin. Orig. Investig. 2011, 29 (Suppl. 6), 1–8.
- Wade, C.A.; Kyprianou, N. Profiling Prostate Cancer Therapeutic Resistance. Int. J. Mol. Sci. 2018, 19, 904.
- Zhang, X.; Wrzeszczynska, M.H.; Horvath, C.M.; Darnell, J.E. Interacting Regions in Stat3 and C-Jun That Participate in Cooperative Transcriptional Activation. Mol. Cell. Biol. 1999, 19, 7138–7146.
- Horvath, C.M.; Stark, G.R.; Kerr, I.M.; Darnell, J.E. Interactions between STAT and Non-STAT Proteins in the Interferon-Stimulated Gene Factor 3 Transcription Complex. Mol. Cell. Biol. 1996, 16, 6957–6964.
- Collum, R.G.; Brutsaert, S.; Lee, G.; Schindler, C. A Stat3-Interacting Protein (StIP1) Regulates Cytokine Signal Transduction. Proc. Natl. Acad. Sci. USA 2000, 97, 10120–10125.
- Decker, T.; Kovarik, P.; Meinke, A. GAS Elements: A Few Nucleotides with a Major Impact on Cytokine-Induced Gene Expression. J. Interf. Cytokine Res. 1997, 17, 121–134.
- Smith, P.L.; Lombardi, G.; Foster, G.R. Type I Interferons and the Innate Immune Response—More than Just Antiviral Cytokines. Mol. Immunol. 2005, 42, 869–877.
- Shuai, K.; Horvath, C.M.; Huang, L.H.T.; Qureshi, S.A.; Cowburn, D.; Darnell, J.E. Interferon Activation of the Transcription Factor Stat91 Involves Dimerization through SH2-Phosphotyrosyl Peptide Interactions. Cell 1994, 76, 821–828.
- Wang, D. A Small Amphipathic Alpha -Helical Region Is Required for Transcriptional Activities and Proteasome-Dependent Turnover of the Tyrosine-Phosphorylated Stat5. EMBO J. 2000, 19, 392–399.
- Kornberg, R.D. Eukaryotic Transcriptional Control. Trends Cell Biol. 1999, 9, M46–M49.
- Alexander, W.S.; Starr, R.; Metcalf, D.; Nicholson, S.E.; Farley, A.; Elefanty, A.G.; Brysha, M.; Kile, B.T.; Richardson, R.; Baca, M.; et al. Suppressors of Cytokine Signaling (SOCS): Negative Regulators of Signal Transduction. J. Leukoc. Biol. 1999, 66, 588–592.
- Hilton, D.J. Negative Regulators of Cytokine Signal Transduction. Cell. Mol. Life Sci. 1999, 55, 1568–1577.
- Krebs, D.L.; Hilton, D.J. SOCS Proteins: Negative Regulators of Cytokine Signaling. Stem Cells 2001, 19, 378–387.
- Schmidt, D.; Müller, S. PIAS/SUMO: New Partners in Transcriptional Regulation. Cell. Mol. Life Sci. 2003, 60, 2561–2574.
- Shuai, K. Regulation of Cytokine Signaling Pathways by PIAS Proteins. Cell Res. 2006, 16, 196–202.
- Shuai, K.; Liu, B. Regulation of Gene-Activation Pathways by Pias Proteins in the Immune System. Nat. Rev. Immunol. 2005, 5, 593–605.
- Mora, L.B.; Buettner, R.; Seigne, J.; Diaz, J.; Ahmad, N.; Garcia, R.; Bowman, T.; Falcone, R.; Fairclough, R.; Cantor, A.; et al. Constitutive Activation of Stat3 in Human Prostate Tumors and Cell Lines: Direct Inhibition of Stat3 Signaling Induces Apoptosis of Prostate Cancer Cells. Cancer Res. 2002, 62, 6659–6666.
- Hellsten, R.; Johansson, M.; Dahlman, A.; Dizeyi, N.; Sterner, O.; Bjartell, A. Galiellalactone Is a Novel Therapeutic Candidate against Hormone-Refractory Prostate Cancer Expressing Activated Stat3. Prostate 2008, 68, 269–280.
- Canesin, G.; Evans-Axelsson, S.; Hellsten, R.; Sterner, O.; Krzyzanowska, A.; Andersson, T.; Bjartell, A. The STAT3 Inhibitor Galiellalactone Effectively Reduces Tumor Growth and Metastatic Spread in an Orthotopic Xenograft Mouse Model of Prostate Cancer. Eur. Urol. 2016, 69, 400–404.
- Tan, S.H.; Dagvadorj, A.; Shen, F.; Gu, L.; Liao, Z.; Abdulghani, J.; Zhang, Y.; Gelmann, E.P.; Zellweger, T.; Culig, Z.; et al. Transcription Factor Stat5 Synergizes with Androgen Receptor in Prostate Cancer Cells. Cancer Res. 2008, 68, 236–248.
- Thomas, C.; Zoubeidi, A.; Kuruma, H.; Fazli, L.; Lamoureux, F.; Beraldi, E.; Monia, B.P.; MacLeod, A.R.; Thüroff, J.W.; Gleave, M.E. Transcription Factor Stat5 Knockdown Enhances Androgen Receptor Degradation and Delays Castration-Resistant Prostate Cancer Progression in Vivo. Mol. Cancer Ther. 2011, 10, 347–359.
- Hoang, D.T.; Gu, L.; Liao, Z.; Shen, F.; Talati, P.G.; Koptyra, M.; Tan, S.H.; Ellsworth, E.; Gupta, S.; Montie, H.; et al. Inhibition of Stat5a/b Enhances Proteasomal Degradation of Androgen Receptor Liganded by Antiandrogens in Prostate Cancer. Mol. Cancer Ther. 2015, 14, 713–726.
- Das, S.; Roth, C.P.; Wasson, L.M.; Vishwanatha, J.K. Signal Transducer and Activator of Transcription-6 (STAT6) Is a Constitutively Expressed Survival Factor in Human Prostate Cancer. Prostate 2007, 67, 1550–1564.
- Nagashima, S.; Yokota, M.; Nakai, E.I.; Kuromitsu, S.; Ohga, K.; Takeuchi, M.; Tsukamoto, S.I.; Ohta, M. Synthesis and Evaluation of 2-{Amino}pyrimidine-5-Carboxamide Derivatives as Novel STAT6 Inhibitors. Bioorganic Med. Chem. 2007, 15, 1044–1055.
- Nappo, G.; Handle, F.; Santer, F.R.; McNeill, R.V.; Seed, R.I.; Collins, A.T.; Morrone, G.; Culig, Z.; Maitland, N.J.; Erb, H.H.H. The Immunosuppressive Cytokine Interleukin-4 Increases the Clonogenic Potential of Prostate Stem-like Cells by Activation of STAT6 Signalling. Oncogenesis 2017, 6, e342.
- Liu, D.; Tao, T.; Xu, B.; Chen, S.; Liu, C.; Zhang, L.; Lu, K.; Huang, Y.; Jiang, L.; Zhang, X.; et al. MiR-361-5p Acts as a Tumor Suppressor in Prostate Cancer by Targeting Signal Transducer and Activator of Transcription-6(STAT6). Biochem. Biophys. Res. Commun. 2014, 445, 151–156.
- Xu, B.I.N.; Lu, X.; Zhao, Y.; Liu, C.; Huang, X.; Chen, S.; Zhu, W.; Zhang, L.; Chen, M. MicroRNA-135a Induces Prostate Cancer Cell Apoptosis via Inhibition of STAT6. Oncol. Lett. 2019, 17, 1889–1895.
- Wang, N.; Tao, L.; Zhong, H.; Zhao, S.; Yu, Y.; Yu, B.; Chen, X.; Gao, J.; Wang, R. MiR-135b Inhibits Tumour Metastasis in Prostate Cancer by Targeting STAT6. Oncol. Lett. 2016, 11, 543–550.
- Das, S.; Shetty, P.; Valapala, M.; Dasgupta, S.; Gryczynski, Z.; Vishwanatha, J.K. Signal Transducer and Activator of Transcription 6 (STAT6) Is a Novel Interactor of Annexin A2 in Prostate Cancer Cells. Biochemistry 2010, 49, 2216–2226.
