Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Walter Quadros Ribeiro Junior.
Quinoa stands out as an excellent crop in the Cerrado region for cultivation in the off-season or irrigated winter season. Quinoa (Chenopodium quinoa Willd.) is a pseudocereal rich in natural antioxidants, flavonoids, and anthocyanins, and these compounds may protect plants against biotic and abiotic stress. Water stress increases leaf temperature, and reduces crop height, stomatal conductance, plant biomass, and yield. Here, we tested the effects of different water regimes on the agronomic characteristics, physiology, and grain quality of different elite quinoa genotypes under field conditions.
- Chenopodium quinoa
- water use efficiency
- phenolic compounds
- gas exchange
1. Introduction
Crop development and yield are affected by different environmental factors, and water restriction is the most important constraint on agricultural yield [1,2][1][2]. This is a particular problem in the Brazilian Cerrado, which has a tropical climate with an average of 1500 mm of rain, but where approximately 90% of precipitation occurs during the rainy season (from October to April). The rainy season is followed by a dry season (from May to September), during which the relative humidity is low, the evaporation very high, and precipitation is rare. There are three harvest periods in the Brazilian Cerrado: (1) The main crop season, which occurs during the wet season from October to January; (2) The off-season crop, which is grown at the end of wet season without irrigation, is planted between the months of January to March [3,4][3][4], and is harvested in May during the dry season; and (3) the winter season crop, which is cultivated under irrigated conditions, with the crop being both planted (April to May) and harvested (August to September) during the dry season. Both the off-season and winter season require careful selection of genotypes for grain production; drought-tolerant genotypes (DT) should be selected for the off-season crop, and high productivity per unit of applied water (PUAA) genotypes are needed for winter crops as they are grown under irrigation. Obtaining genotypes that are better adapted to stressful edaphoclimatic conditions in order to resist periods with water deficiency whilst maintaining the highest possible productivity for each condition is therefore of great importance in plant breeding programs [5].
Quinoa (Chenopodium quinoa Willd.) is a pseudocereal rich in natural antioxidants, flavonoids, and anthocyanins [6,7][6][7], and these compounds may protect plants against biotic and abiotic stress [8]. Water stress increases leaf temperature, and reduces crop height, stomatal conductance, plant biomass, and yield [9,10][9][10].
Quinoa has been cultivated for millennia under conditions of low rainfall, as it has physiological and morphological strategies to overcome water deficit [11]. Moreover, this crop has been cultivated in different agroclimatic zones as it is well adapted to a variety of different environments due to its high genetic diversity [11]. Quinoa has mainly been cultivated in Argentina, Bolivia, Chile, Colombia, Ecuador, and Peru, though high productivity has also been observed when planted in Kenya as well as the Himalayas and northern plains of India [12].
In Brazil, research carried out in Embrapa Cerrados led to the selection of the genotype BRS Piabiru [3], which is the first cultivar in use for quinoa grain cultivation that is adapted to Cerrado conditions. Although planting quinoa during the main crop is not recommended due to the high water availability during the harvesting period (which can potentially result in panicle seed germination [3,5][3][5]), quinoa is recommended for growth during the off-season or irrigated winter season due to its high water use efficiency, drought tolerance, and adaptation to different environmental conditions.
2. Discussions on Quinoa for the Brazilian Cerrado
2.1. Productivity and Productivity Per Unit of Applied Water (PUAA)
Irrigation water use efficiency refers to the yield obtained per unit of applied water [24][13] and is a fundamental physiological parameter that indicates the ability of crops to conserve water in a region under water stress due to drought resistance and high potential productivity [25][14]. In our study, the low WR of 150 mm resulted in lower PUAA because under severe water restriction the quinoa genotypes cannot express their productive potential, whilst at high WRs (above 480 mm) plants also had a lower PUAA due to an inability to absorb all supplied water and potentially an intolerance to excess water (Table 2). Under the high WR, genotypes showed high productivity; however, the grain dry matter per unit of applied water was low, indicating that there was no consistent relationship between crop yield and PUAA for this WR (Table 1 and Table 2). Thus, the 389 mm and 247 mm WRs showed the highest PUAA, but the highest productivity was observed under the WR 480 mm and 389 mm. Thus, WR 389 mm can be indicated for cultivating quinoa under an irrigated system in the Cerrado, as there is a trade-off in the relationship between productivity and water saving.
Table 1. Productivity (t ha−1) of 18 quinoa genotypes and BRS Piabiru under 4 water regimes.
Table 2. Productivity per unit of applied water (kg ha−1mm−1) of 18 quinoa genotypes and BRS Piabiru under 4 water regimes.
| Genotypes | Water Regime (mm) | |||
|---|---|---|---|---|
| 480 | 389 | 247 | 150 | |
| CPAC1 | 17.18 aB | |||
Quinoa plants can control the relationship between photosynthetic rate and transpiration, even with low leaf water potentials [26][15], and by limiting transpiration and inducing stomatal closure, they can increase PUAA and influence productivity under water stress [27][16]. In our study, with a 49% reduction in water applied throughout the crop cycle there was a 42% average yield loss over all genotypes (Table 1), indicating both drought tolerance and efficient water use, as this grain yield was obtained using half the water normally needed to meet the demands of the crop. Quinoa seeds can be obtained when little water is available in the vegetative stage, producing an average of 1.2 to 2.0 t ha−1 with half the required irrigation [28,29][17][18]. On the other hand, when a low irrigation strategy was used during all phenological stages this resulted in a 75% reduction in seed yield of the quinoa cultivar ‘Belen 2000’ [28,29][17][18].
Our work obtained higher yield values than others reported in the literature, and under all WRs the genotypes with high productivity were CPAC13, CPAC6, CPAC3, CPAC12, and CPAC17 and the genotypes with lower yield potential were CPAC19, CPAC11, and CPAC14 [30,31][19][20]. Under higher WR (480 mm) the genotypes did not differ, with the exception of CPAC11, which presented the lowest productivity and low PUAA; however, CPAC11 was also the only dwarf material used in this study (see below) (Figure 2, Table 1 and Table 2). For the 150 mm WR the CPAC17 genotype was superior to the other genotypes, and whilst productivity was altered there were no changes in efficiency between WR 150 and WR 389 mm (Table 1), meaning that it is a suitable genotype for use in situations with limited water availability such as the off-season.
Under high and intermediate water regimes, the highest PUAA was observed for CPAC3, CPAC6, and CPAC12 between WR 480 and WR 247 mm (Table 2) and considering that they were amongst the genotypes with highest productivity, these genotypes are suggested for the winter season. Specifically, CPAC6 exhibited reduced productivity only under the 247 mm WR (Table 1) and presented the highest PUAA of 26.7 kg ha−1 mm−1. This value is 32% higher than the PUAA of WR 480 mm (Table 2). CPAC13 presented higher productive potential and productivity than BRS Piabiru under a moderate water regime (389 mm), with 9.73 t ha−1 for CPAC13 and 8.14 t ha−1 for BRS Piabiru, respectively.
2.2. The Effects of Water Regime and Genotype on Grain Quality Indicators
In addition to productivity indicators of grain quality such as the concentrations of flavonoids and anthocyanins and 1000-grain weight should also be taken into account when selecting genotypes (Table 3 and Table 4, Figure S1). Our results show that the accumulation of flavonoids and anthocyanins in quinoa plants was more influenced by genotype than by the WRs. In particular, CPAC9 accumulated these compounds under both higher and lower water regimes and accumulated nearly double the concentration of the other genotypes under all WRs. In addition, CPAC9 is among the genotypes with greater productivity and PUAA under high and intermediate WRs (Table 1 and Table 2). The authors of [32][21], when studying the levels of flavonoids, phenolic acids, and betaines in the Andean grains of quinoa, kaniwa, and kiwicha, found flavonoid contents ranging from 36.2 to 144.3 mg/100 g, which are similar than those found here. In other crops such as peanuts, depending on the genotype and drought period, concentrations of phenolic compounds in seeds may be between 60 and 220 mg/100 g [33][22]. Further studies focusing on the biosynthesis of phenolic compounds and oxidation processes under water stress will provide more information on the genotypic variation of phenolic content in grains [34][23]. Grain weight is also affected by water restriction. With a water supply of 150 mm, there was a 14% decrease in TGW for the four genotypes analysed, similar to a previous study where TGW in irrigated plants was significantly higher (5.5 g) than in rainfed plants (4.2 g) [28,29][17][18]. Indeed, when water stress is applied during the grain filling period, it generally reduces the grain yield, the number of grains per plant, and the individual weight of the grains [35][24].
Table 3. Total flavonoid concentrations (mg/100 g) in grains of 18 quinoa genotypes and BRS Piabiru under 4 water regimes.
| Genotypes | Water Regime (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 480 | 389 | 247 | 150 | ||||||
| 22.2 aA | 16.43 cB | 10.41 cC | |||||||
| CPAC1 | 0.80 cA | 0.76 dA | 0.76 cA | 0.80 dA | |||||
| CPAC2 | 15.89 aB | 21.0 aA | 13.02 dB | 13.0 cB | |||||
| CPAC2 | 0.63 dA | 0.68 dA | 0.62 dA | 0.40 cB | CPAC3 | 18.59 aB | 20.8 aB | 25.29 aA | 13.5 cD |
| CPAC3 | 0.63 dD | 1.0 bB | 0.83 cC | 1.16 cA | CPAC4 | 16.89 aB | 21.15 aA | 21.1 bA | 13.03 cC |
| CPAC4 | 0.59 dB | 0.65 eB | 0.59 dB | 0.76 dA | CPAC5 | 16.9 aB | 21.0 aA | 23.9 bA | |
| CPAC5 | 12.1 cC | ||||||||
| 0.74 cA | 0.59 eB | 0.54 dB | 0.70 dA | CPAC6 | 17.7 aB | 22.72 aA | 23.0 aA | 16.4 bB | |
| CPAC6 | 0.60 dB | 0.73 dA | 0.65 dB | 0.84 dA | CPAC8 | 18.01 aA | 18.18 bA | 18.23 cA | 10.52 bB |
| CPAC8 | 0.58 dB | 1.07 bA | 0.55 dB | 0.57 eB | CPAC9 | 17.73 aB | 18.01 bB | 23.3 aA | 14.10 cC |
| CPAC9 | 1.72 aC | 1.89 aB | 1.44 aD | 2.05 aA | CPAC10 | 17.83 aB | 21.49 aA | 20.6 bA | 15.38 cB |
| CPAC10 | 1.16 bA | 0.73 dB | 0.76 cB | 0.91 dB | CPAC11 | 11.1 bC | 18.0 bB | 21.89 bA | |
| CPAC11 | 15.85 bA | ||||||||
| 0.61 dA | 0.48 fA | 0.54 dA | 0.61 eA | CPAC12 | 18.0 aB | 20.1 aB | 23.56 aA | 17.24 bD | |
| CPAC12 | 0.55 dB | 0.68 dA | 0.50 dB | 0.65 eB | CPAC13 | 19.83 aB | 25.9 aA | 19.1 bB | 17.6 bB |
| CPAC13 | 0.66 dB | 0.63 eB | 0.50 dC | 0.73 dA | CPAC14 | 12.69 aB0 | 16.3 bA | 15.71 cA | 10.1 cC |
| CPAC14 | 0.83 cB | 0.82 cB | 0.68 cC | 0.94 cA | BRS Piabiru | 15.79 aB | 20.92 aA | 21.88 bA | 14.37 cB |
| BRS Piabiru | 0.89 cB | 1.08 bA | 0.61 dD | 0.74 dC | CPAC16 | 17.74 aA | 19.8 bA | 19.18 bA | |
| CPAC16 | 15.57 bA | ||||||||
| 0.72 cA | 0.65 eA | 0.81 cA | 0.80 dA | CPAC17 | 18.58 aB | 21.70 aA | 19.44 bB | 24.25 aA | |
| CPAC17 | 1.03 bB | 0.86 cCCPAC18 | 18.66 aB | 23.56 aA | 16.89 cB | 14.71 cC | |||
| CPAC19 | 16.08 aA | 16.50 bA | 11.6 dB | 10.25 cB | |||||
| 0.79 cC | 1.18 bA | ||||||||
| CPAC18 | 0.68 dA | 0.61 eA | 0.72 cA | 0.60 eA | |||||
| CPAC19 | 0.64 dA | 0.63 eA | 0.71 cA | CPAC20 | 17.82 aA | 18.82 bA | 15.94 cA | 11.89 cB | |
| 0.66 eA | |||||||||
| CPAC20 | |||||||||
| 1.13 bA | |||||||||
| 0.99 bA | |||||||||
| 1.06 bA | |||||||||
| 1.05 cA | |||||||||
| Genotypes | Water Regime (mm) | ||||||||
| 480 | 389 | 247 | 150 | ||||||
| CPAC1 | 7.82 aA | 8.34 bA | 4.06 bB | 1.56 bC | |||||
| CPAC2 | 8.32 aA | 7.89 bA | 3.94 cB | 1.83 bC | |||||
| CPAC3 | 8.02 aA | 7.81 bB | 6.25 aC | 1.94 bD | |||||
| CPAC4 | 8.40 aA | 8.23 bA | 5.21 aB | 1.95 bC | |||||
| CPAC5 | 8.17 aA | 7.90 bA | 5.22 aB | 1.94 bC | |||||
| CPAC6 | 8.50 aA | 8.84 aA | 5.68 aB | 2.46 aC | |||||
| CPAC8 | 8.64 aA | 7.07 cB | 4.50 aC | 1.58 bD | |||||
| CPAC9 | 8.21 aA | 7.01 cB | 5.75 aC | 2.11 bD | |||||
| CPAC10 | 8.56 aA | 8.36 bA | 5.12 aB | 2.61 aC | |||||
| CPAC11 | 5.66 bB | 6.80 cA | 5.40 aB | 2.38 aC | |||||
| CPAC12 | 8.57 aA | 7.83 bA | 5.82 aB | 2.58 aC | |||||
| CPAC13 | 8.85 aA | 9.73 aA | 4.17 bB | 2.60 aC | |||||
| CPAC14 | 9.21 aA | 6.51 cB | 3.88 bB | 1.61 bB | |||||
| BRS Piabiru | 7.58 aA | 8.14 bA | 5.40 aB | 1.84 bC | |||||
| CPAC16 | 8.51 aA | 7.46 cA | 4.74 aB | 2.33 aC | |||||
| CPAC17 | 8.92 aA | 8.44 bA | 4.80 aB | 3.64 aC | |||||
| CPAC18 | 8.96 Aa | 9.16 aA | 4.75 aB | 2.21 bC | |||||
| CPAC19 | 7.71 aA | 6.42 cB | 2.53 cC | 1.79 bC | |||||
| CPAC20 | 7.97 aA | 7.08 cA | 3.93 bB | 2.09 bC | |||||
Means followed by the same lowercase letter (column) or uppercase letter (line), do not differ according to the Scott–Knott test at a 5% probability.
Means followed by the same lowercase letter (column) or uppercase letter (line) do not differ according to the Scott–Knott test at a 5% probability.
Means followed by the same lowercase letter (column) or uppercase letter (line) do not differ according to the Scott–Knott test at 5% probability.
2.3. Low Water Availability Leads to Reduced Plant Height
The heights of the quinoa genotypes in this study ranged from 0.99 to 1.53 m, which are superior to those in literature [36][25]. Our results support previous studies demonstrating that a reduction in irrigation resulted in a significant decrease in plant height by 0.55 and 0.80 m for Bolivian quinoa [37][26]. Here, we detected a decrease in cultivated quinoa plant height under 70% irrigation deficit in relation to control plants, similar to that observed by [38][27]. The reduction of plant size and leaf area under stress conditions is directly related to a decline in dry mass when compared to plants maintained under adequate soil water potential conditions [39][28]. The low water potential of the soil limits water absorption capacity, and this quickly suppresses the rate of cell expansion and division of growing tissues [40][29]. Moreover, under severe water deficit stomata close and the consequent reduction of photosynthesis results in a decrease in dry mass production [39][28]. Plant height can also be used as a criterion to determine the susceptibility of quinoa genotypes to drought, as the longer the cycle, the larger the plant. Short-to-medium cycle cultivars are desirable, and would provide the possibility of more crops per year in irrigated systems [2] and enable off-season and winter planting. A shorter crop cycle also represents a method of escape from water stress under Cerrado conditions, besides contributing to the definition of the sowing season, such that the grain maturation occurs when the humidity is reduced, thus avoiding seed deterioration [2]. Amongst the studied genotypes, CPAC11 was significantly shorter. Whilst this is ideal for avoiding lodging, this genotype also showed the lowest productivity in most WRs under the planting density we employed. This may be related to reduced cell expansion, which results in a reduction in leaf area and, consequently, less photoassimilates for translocation to the grain [41][30]. In addition, water deficit affects carbohydrate utilization, altering the efficiency with which photoassimilates are used in the growth and development of new plant organs [41][30]. This genotype (CPAC11), however, may not have expressed its productive potential considering that it is the only dwarf material used in this study (Figure 2) and may need a higher planting density per square meter.
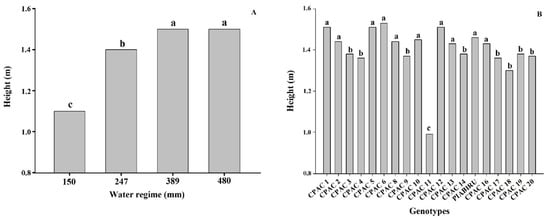

Figure 2. Height of different quinoa genotypes under 4 water regimes. (A) Effect of 4 water regimes in plant height in 20 quinoa genotypes. (B) Means followed by the same lowercase letter do not differ according to the Scott–Knott test at 5% probability.
2.4. Physiological Parameters
Abiotic stresses generally result in reduced rates of photosynthesis and transpiration that can ultimately contribute to reduced growth and productivity [41][30], making assessment of physiological parameters in the field an important tool in screening for stress tolerance. For this reason, we analysed a number of physiological parameters in a subset of the quinoa genotypes. Remote sensing of leaf temperature by thermal imaging can be a reliable way to detect changes in the physiological state of plants in response to different biotic and abiotic stresses [42][31]. Indeed, canopy temperature has been used successfully in breeding programs for drought-prone environments [43[32][33],44], as genotypes that maintain transpiration will tend to have lower canopy temperature than other genotypes under the same environmental conditions in the field [45][34]. Here, reduced water availability led to increased canopy temperature, reflecting the stomatal closure and reduced transpiration that we detected using gas exchange analysis. However, we were not able to detect differences between the genotypes using this parameter (Figure S2), despite the fact that differences in transpiration were detected between genotypes at each WR (Table 5). It may be the case that larger differences in transpiration between genotypes are required to result in alterations in leaf temperature that can be measured using this technique.
Table 5. Stomatal conductance (gs–mol m−2 s−1), transpiration rate (E–mmol m−2 s−1 Internal carbon (Ci-μmol m−2 s−1), and photosynthetic rate (A-μmol m−2 s−1) of 4 quinoa genotypes (CPAC4, CPAC9, CPAC11, and Piabiru), under 4 water regimes (150, 247, 389, 480 mm).
| Genotypes | Water Regime (mm) | ||||
|---|---|---|---|---|---|
| 150 | 247 | 389 | 480 | ||
| gs | CPAC4 | 0.034 cD | 0.235 bC | 0.470 bB | 0.583 bA |
| CPAC11 | 0.040 cC | 0.232 bB | 0.358 cA | 0.342 cA | |
| BRS Piabiru | 0.058 aD | 0.218 bC | 0.516 abB | 0.629 aA | |
| CPAC19 | 0.050 bC | 0.329 aB | 0.552 aA | 0.594 abA | |
| E | CPAC4 | 1.45 bcD | 6.15 aC | 8.96 aB | 9.88 aA |
| CPAC11 | 1.20 cD | 4.87 bC | 6.43 cA | 5.67 cB | |
| BRS Piabiru | 3.11 aD | 4.76 bC | 8.20 aB | 9.46 aA | |
| CPAC19 | 1.71 bD | 5.83 aC | 7.27 bB | 8.38 bA | |
| Ci | CPAC 4 | 144.0 aC | 195.3 aB | 239.9 bA | 241.2 bA |
| CPAC11 | 112.6 bC | 198.6 aB | 253.6 aA | 259.2 aA | |
| BRS Piabiru | 129.0 abD | 191.3 aC | 257.0 aB | 270.3 aA | |
| CPAC19 | 82.5 cC | 198.4 aB | 255.9 aA | 256.9 abA | |
| A | CPAC4 | 4.9 cD | 19.0 cC | 22.0 bB | 28.1 bA |
| CPAC11 | 5.9 bC | 20.5 bcB | 22.9 bA | 20.0 cB | |
| BRS Piabiru | 9.0 aD | 22.4 bC | 32.9 aB | 35.4 aA | |
| CPAC19 | 8.9 aC | 27.0 aB | 32.9 aA | 34.3 aA | |
| Genotypes | Water Regime (mm) | ||||
| 480 | 389 | 247 | 150 | ||
| CPAC1 | 81.59 cB | 101.62 dA | 66.21 dC | 88.34 dA | |
| CPAC2 | 95.97 bA | 97.23 dA | 85.71 dA | 92.21 dA | |
| CPAC3 | 98.83 bA | 51.54 eB | 97.12 cA | 113.25 cA | |
| CPAC4 | 80.02 cB | 98.31 dA | 91.64 cA | 96.23 dA | |
| CPAC5 | 84.07 cB | 103.0 dA | 74.58 dB | 86.80 dB | |
| CPAC6 | 83.65 cC | 115.23 cA | 82.81 dC | 100.05 dB | |
| CPAC8 | 96.95 bB | 110.72 dA | 114.25 bA | 93.58 dB | |
| CPAC9 | 215.22 aB | 171.32 aC | 205.11 aB | 226.02 aA | |
| CPAC10 | 85.48 cC | 110.0 dB | 96.32 cC | 210.81 bA | |
| CPAC11 | 89.00 cB | 118.59 cA | 100.97 cB | 113.25 cA | |
| CPAC12 | 96.17 bB | 120.98 cA | 84.82 dB | 112.51 cA | |
| CPAC13 | 99.47 bA | 111.33 dA | 84.73 dB | 102.33 dA | |
| CPAC14 | 115.19 bA | 119.74 cA | 84.94 dB | 104.71 cA | |
| BRS Piabiru | 104.15 bA | 105.89 dA | 101.98 cA | 104.56 cA | |
| CPAC16 | 105.09 bB | 124.80 cA | 99.48 cB | 110.93 cB | |
| CPAC17 | 115.73 bC | 145.76 bA | 97.05 cC | 118.86 cB | |
| CPAC18 | 88.27 cB | 62.76 eC | 94.25 cB | 113.57 cA | |
| CPAC19 | 109.63 bB | 119.74 cA | 102.59 cB | 100.01 dB | |
| CPAC20 | 119.93 bB | 139.03 bA | 114.40 bB | 115.41 cB | |
Means followed by the same lowercase letter (column) or uppercase letter (line) do not differ according to the Scott–Knott test at a 5% probability.
Table 4. Total anthocyanin concentrations (mg/100 g) in grains of 18 quinoa genotypes and BRS Piabiru under 4 water regimes.
Means followed by the same lowercase letter (column) or uppercase letter (line) do not differ according to the Tukey test at a 5% probability.
Stomatal closure is a common response to water restriction where water deficit affects guard cell turgidity and stomatal aperture, resulting in decreases in the rates of transpiration and assimilation of CO2 and increased leaf temperature [46,47,48][35][36][37]. Gas exchange parameters such as net photosynthesis, transpiration and stomatal conductance are therefore sensitive indicators of water deficit in plants that are useful in the evaluation of genotypes adapted for growing in environments with limited water availability. Our data are consistent with previous studies, since the photosynthetic rate presented a behaviour similar to that observed for stomatal conductance and transpiration, reflecting the opening and closing of stomata under different water conditions [49][38], and quinoa is known to suffer both stomatal and mesophyll limitations under drought stress [50][39] These results also suggest that the reduction in photosynthetic rates in the most stressed water regimes are related to partial stomatal closure and a consequent reduction in CO2 assimilation (Table 5). Water stress is considered to be severe when stomatal conductance values are below 0.1 mol.m−2 s−1 [47][36], and therefore in this study all genotypes were under severe stress in the 150 mm WR (Table 5). Similar values for stomatal conductance and effects of water restriction in quinoa have been reported elsewhere; for example stomatal conductance decreased from 0.213 mol.m−2 s−1 under irrigation to 0.091 mol.m−2 s−1 under water deficit in one study [51][40] whilst an investigation of 10 genotypes in the field without irrigation that received around 160 mm of water reported values between 1.0 and 0.18 mol.m−2 s−1 and a clear relationship between maintenance of stomatal conductance and higher photosynthetic rate [52][41].
Analysis of gas exchange also revealed differences in the responses of the genotypes to alterations in water availability and relationships with productivity. CPAC4 and CPAC11 appear most sensitive to water restriction as they presented the lowest net photosynthesis under the 150 mm and 247 mm regimes. Interestingly though, whilst CPAC19 showed greater photosynthesis under all water regimes, this was not reflected in greater productivity, indicating that other factors, such as plant morphology and the capacity to use photoassimilates for grain filling also has an important impact. This is also seen in the relationship between water regime, photosynthesis and productivity for individual genotypes; for example, the increased photosynthesis shown by CPAC4 between WR389 and WR480 did not translate into greater productivity, reinforcing the importance of parameters such as PUAA for selecting genotypes under irrigated conditions (Table 1, Table 2 and Table 5).
Gas exchange measurements may not always be able to detect the deleterious effects of water restriction on chloroplast function parameters such as the effective quantum yield of photosystem II potentially useful tools [53][42]. Here we detected decreased F’v/F’m with low water availability (WR 150 mm, Figure S3); this decrease in plants stressed by drought, in comparison with well-hydrated plants, is mainly due to the lack of CO2 inside the leaf and it is under this WR that we detected large decreases in Ci for all genotypes (Table 5). However, this parameter responded little to the median level of stress (389 mm), despite the fact that this WR provoked changes in several gas exchange parameters (Table 5), and furthermore we did not detect any differences between the genotypes (Figure S3). Indeed, measurements of chlorophyll a fluorescence tend to have low sensitivity to mild stresses, for example, 18 days of suspension of irrigation were required to reduce Fv/Fm in two greenhouse-grown quinoa genotypes [53][42]. In contrast to F’v/F’m, chlorophyll indices proved to be unaffected by WR but showed differences between genotypes (Table S1), and lack of an effect of drought and flooding on chlorophyll in quinoa has previously been reported [52][41]. Despite the lack of an effect of stress on chlorophyll, these indices may prove useful for selection of genotypes due to the fact that chlorophyll abundance is typically positively related to photosynthetic potential and productivity [54][43]. In this sense of the four genotypes analysed CPAC19 stood out due to greater total chlorophyll and a lower chlorophyll a:b ratio that may indicate greater light absorption capacity by photosystem II [55][44].
A lack of water in the soil increases the risk of the rate of transpiration exceeding the rate of water absorption and transport, resulting in a situation of water deficit. Partial stomatal closure can reduced transpiration, but under water stress, plants often also accumulate compatible solutes or osmoprotectors including proline, glycine, betaine, and sugars [56][45]. The accumulation of compatible solutes reduces cellular osmotic potential, thereby permitting water absorption and maintaining turgor pressure and physiological processes [57][46]. The accumulation of proline may therefore be an important characteristic for the selection of drought-tolerant plants [58][47] and indeed seed or leaf treatment of quinoa plants with free proline can increase growth under water stress [59][48] whilst a number of studies have connected compatible proline accumulation with drought and salt tolerance in this species [49][38]. However, whilst we detected increased proline concentrations in quinoa in response to water stress, this only occurred under the most severe water regimes, meaning that it could not be used to discriminate between the genotypes under water regimes that reflect Cerrado conditions (Table S2). Despite morphological alterations, stomatal control and osmotic adjustment water restriction may eventually affect leaf water status. Leaf relative water content (RWC) can therefore be used to indicate the balance between water supply and transpiration [60][49], and in the case of F’v/F’m, we did not detect differences between the genotypes for this parameter (Figure S4). However, RWC did serve to indicate the degree of stress to which the plants were subject, as the RWC values detected below 389 mm correspond to those associated with the beginning of wilting (Figure S4, [20][50] and are similar to those observed in greenhouse grown plants during suspension of irrigation [53][42].
3. Conclusions
Through experiments performed under different water regimes here we have shown that quinoa has excellent potential for planting as an off-season and winter crop in the Cerrado region. Several genotypes presented advantages in relation to the currently used BRS Piabiru genotype; the choice of genotype will depend on farming practices, nutritional content, and weather conditions. CPAC13 and CPAC6 are particularly suited to growth as a winter crop under irrigated conditions, and CPAC17 under off-season rain-fed conditions, whilst CPAC9 appears advantageous in terms of phenolic compounds in the grains. The accumulation of flavonoids and anthocyanins in quinoa genotypes was more influenced by quinoa genotype than by the WRs. Analysis of physiological parameters provided information regarding the mechanisms involved in stress tolerance in different quinoa genotypes, which is essential if we are to develop strategies to maintain or increase plant productivity in environments with water limitation. The results of this work show that the water regimes for quinoa can be reduced without a significant reduction in grain yield. This increase in dry matter accumulation efficiency per unit of water applied in quinoa means it is a crop that can be cultivated under Cerrado conditions, for both the off-season and winter season, under relatively low levels of irrigation whilst obtaining high yields. This fact, coupled with proper water management this can result in higher yield per area, which is desirable for areas under irrigated cultivation where irrigation is a costly practice.References
- Scheelbeek, P.F.; Bird, F.A.; Tuomisto, H.L.; Green, R.; Harris, F.B.; Joy, E.J.M.; Chalabi, Z.; Allemn, E.; Haines, A.; Dangour, A.D. Effect of environmental changes on vegetable and legume yields and nutritional quality. Proc. Natl. Acad. Sci. USA 2018, 115, 6804–6809.
- Soares, G.F.; Ribeiro, W.Q., Jr.; Pereira, L.F.; Lima, C.A.; Soares, D.D.S.; Muller, O.; Rascher, U.; Ramos, M.L.G. Characterization of wheat genotypes for drought tolerance and water use efficiency. Sci. Agric. 2021, 78, e20190304.
- Spehar, C.R.; Santos, R.L.B. Quinoa BRS Piabiru: Alternativa para diversificar os sistemas de produção de grãos. Pesq. Agropec. Bras. 2001, 37, 889–893.
- Garcia, R.A.; Ceccon, G.; Sutier, G.A.D.; Santos, A.L.F.D. Soybean-corn succession according to seeding date. Pesq. Agropec. Bras. 2018, 53, 22–29.
- Präger, A.; Munz, S.; Nkebiwe, P.M.; Mast, B.; Graeff-Hönninger, S. Yield and quality characteristics of different quinoa (Chenopodium quinoa Willd.) cultivars grown under field conditions in Southwestern Germany. Agronomy. 2018, 8, 197.
- Saad-Allah, K.M.; Youssef, M.S. Phytochemical and genetic characterization of five quinoa (Chenopodium quinoa Willd.) genotypes introduced to Egypt. Physiol. Mol. Biol. Plants 2018, 24, 617–629.
- Wang, D.; Cao, D.; Yao, Y.; Wang, J.; Li, Z.; Liu, B. Understanding the checmial foundation and genetic mecanism of the black grain trait in quinoa by integrating metabolome and transcriptome analyses. Biotechnol. Biotechnol. Equip. 2020, 34, 1095–1103.
- Razzaghi, F.; Bahadori-Ghasroldashti, M.R.; Henriksen, S.; Sepaskhah, A.R.; Jacobsen, S.E. Physiological characteristics and irrigation water productivity of quinoa (Chenopodium quinoa Willd.) in response to deficit irrigation imposed at different growing stages—A field study from Southern Iran. J. Agron. Crop Sci. 2020, 206, 390–404.
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17.
- Jayme-Oliveira, A.; Ribeiro, W.Q.; Ramos, M.L.G.; Ziviani, A.C.; Jakelaitis, A. Amaranth, quinoa, and millet growth and development under different water regimes in the Brazilian Cerrado. Pesq. Agropec. Bras. 2017, 52, 561–571.
- Bazile, D.; Pulvento, C.; Verniau, A.; Al-Nusairi, M.S.; Ba, D.; Breidy, J.; Hassan, L.; Mohammed, M.I.; Mambetov, O.; Otambekova, M.; et al. Worldwide evaluations of quinoa: Preliminary results from post international year of quinoa FAO projects in nine countries. Front. Plant Sci. 2016, 7, 850.
- FAO. The State of Food Insecurity in the World 2014; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021; Available online: http://www.fao.org/publications/sofi/en/ (accessed on 11 March 2021).
- Howell, T.A. Irrigation Efficiency. In Encyclopedia of Soil Science, 2nd ed.; CRC: Boca Raton, FL, USA, 2003.
- Zhang, M.; Duan, L.; Tian, X.; He, Z.; Li, J.; Wang, B.; Li, Z. Uniconazole-induced tolerance of soybean to water deficit stress in relation to changes in photosynthesis, hormones and antioxidant system. J. Plant Physiol. 2007, 164, 709–717.
- Vacher, J.J. Responses of two main Andean crops, quinoa (Chenopodium quinoa Willd) and papa amarga (Solanum juzepczukii Buk.) to drought on the Bolivian Altiplano: Significance of local adaptation. Agric. Ecosyst. Environ. 1998, 68, 99–108.
- Yousfi, S.; Serret, M.D.; Araus, J.L. Shoot δ(15)N gives a better indication than ion concentration or Δ(13)C of genotypic differences in the response of durum wheat to salinity. Funct. Plant Biol. 2009, 36, 144–155.
- Geerts, S.; Raes, D.; Garcia, M.; Condori, O.; Mamani, J.; Miranda, R.; Cusicanqui, J.; Taboada, C.; Yucra, E.; Vacher, J. Could deficit irrigation be a sustainable practice for quinoa (Chenopodium quinoa Willd.) in the Southern Bolivian Altiplano? Agric. Water Manag. 2008, 95, 909–917.
- Geerts, S.; Raes, D.; Garcia, M.; Vacher, J.; Mamani, R.; Mendoza, J.; Huanca, R.; Morales, B.; Miranda, R.; Cusicanqui, J.; et al. Introducing deficit irrigation to stabilize yields of quinoa (Chenopodium quinoa Willd.). Eur. J. Agron. 2008, 28, 427–436.
- Martínez, E.A.; Veas, E.; Jorquera, C.; San Martín, R.; Jara, P. Re-introduction of quinoa into Arid Chile: Cultivation of two lowland races under extremely low irrigation. J. Agron. Crop Sci. 2009, 195, 1–10.
- Delgado, A.I.; Palacios, J.H.; Betancourt, C. Evaluation of 16 genotypes of sweet quinoa (Chenopodium quinoa Willd.) in the municipality of Iles, Nariño (Colombia). Agron. Colomb. 2009, 27, 159–167.
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.M.; Mattila, P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133.
- Aninbon, C.; Jogloy, S.; Vorasoot, N.; Patanothai, A.; Nuchadomrong, S.; Senawong, T. Effect of end of season water deficit on phenolic compounds in peanut genotypes with different levels of resistance to drought. Food Chem. 2016, 196, 123–129.
- Martini, D.; Taddei, F.; Nicoletti, I.; Ciccoritti, R.; Corradini, D.; D’Egidio, M.G. Effects of genotype and environment on phenolic acids content and total antioxidant capacity in durum wheat. Cereal Chem. 2014, 91, 310–317.
- Gámez, A.L.; Soba, D.; Zamarreño, Á.M.; García-Mina, J.M.; Aranjuelo, I.; Morales, F. Effect of water stress during grain filling on yield, quality and physiological traits of Illpa and Rainbow quinoa (Chenopodium quinoa Willd.) cultivars. Plants 2019, 8, 173.
- Maliro, M.F.; Guwela, V.F.; Nyaika, J.; Murphy, K.M. Preliminary studies of the performance of quinoa (Chenopodium quinoa Willd.) genotypes under irrigated and rainfed conditions of central Malawi. Front. Plant Sci. 2017, 8, 227.
- Talebnejad, R.; Sepaskhah, A.R. Effect of deficit irrigation and different saline groundwater depths on yield and water productivity of quinoa. Agric. Water Manag. 2015, 159, 225–238.
- Yang, A.; Akhtar, S.S.; Amjad, M.; Iqbal, S.; Jacobsen, S.E. Growth and physiological responses of quinoa to drought and temperature stress. J. Agron. Crop Sci. 2016, 202, 445–453.
- Endres, L. Photosynthesis and water relations in Brazilian sugarcane. Open Agric. J. 2010, 4, 31–37.
- Shao, H.B.; Chu, L.Y.; Shao, M.A.; Jaleel, C.A.; Hong-mei, M. Higher plant antioxidants and redox signaling under environmental stresses. Comptes Rendus Biol. 2008, 331, 433–441.
- Jordan, W.R. Whole Plant Response to Water Deficits: An Overview. Limit. Effic. Water Use Crop. Prod. 1983, 289–317.
- Li, C.; Liu, S.; Berninger, F. Picea seedlings show apparent acclimation to drought with increasing altitude in the eastern Himalaya. Trees Struct. Funct. 2004, 18, 277–283.
- Balota, M.; Payne, W.A.; Evett, S.R.; Peters, T.R. Morphological and physiological traits associated with canopy temperature depression in three closely related wheat lines. Crop Sci. 2008, 48, 1897–1910.
- Jones, H.G.; Serraj, R.; Loveys, B.R.; Xiong, L.; Wheaton, A.; Price, A.H. Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct. Plant Biol. 2009, 36, 978–989.
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009, 112, 119–123.
- Waseem, M.; Ali, A.; Tahir, M.; Nadeem, M.A.; Ayub, M.; Tanveer, A.; Ahmad, R.; Hussain, M. Mechanism of drought tolerance in plant and its management through different methods. Cont. J. Agric. Sci. 2011, 5, 10–25.
- Flexas, J.; Diaz-Espejo, A.; Gago, J.; Gallé, A.; Galmés, J.; Gulías, J.; Medrano, H. Photosynthetic limitations in Mediterranean plants: A review. Environ. Exp. Bot. 2014, 103, 12–23.
- Tatagiba, S.D.; Pezzopane, J.E.M.; Reis, E.F. Fotossíntese em Eucalyptus sob diferentes condições edafoclimáticas. Rev. Eng. Agric. RevEng 2015, 23, 336–345.
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa Abiotic Stress Responses: A Review. Plants 2018, 7, 106.
- Killi, D.; Haworth, M. Diffusive and metabolic constraints to photosynthesis in quinoa during drought and salt stress. Plants 2017, 6, 49.
- Hussin, S.; Khalifa, W.; Geissler, N.; Koyro, H.W. Influence of the root endophyte Piriformospora indica on the plant water relations, gas exchange and growth of Chenopodium quinoa at limited water availability. J. Agron. Crop Sci. 2017, 203, 373–384.
- González, J.A.; Gallardo, M.; Hilal, M.B.; Rosa, M.D.; Prado, F.E. Physiological responses of quinoa (Chenopodium quinoa) to drought and waterlogging stresses: Dry matter partitioning. Bot. Stud. 2009, 50, 35–42.
- Morales, A.; Zurita-Silva, A.; Maldonado, J.; Silva, H. Transcriptional responses of Chilean quinoa (Chenopodium quinoa Willd.) under water deficit conditions uncovers ABA-independent expression patterns. Front. Plant Sci. 2017, 8, 216.
- O’Neill, P.M.; Shanahan, J.F.; Schepers, J.S. Use of chlorophyll fluorescence assessments to differentiate corn hybrid response to variable water conditions. Crop Sci. 2006, 46, 681–687.
- Belous, O.; Klemeshova, K.; Malyarovskaya, V. Photosynthetic Pigments of Subtropical Plants. In Photosynthesis—From Its Evolution to Future Improvements in Photosynthetic Efficiency Using Nanomaterials; IntechOpen Limited: London, UK, 2018; pp. 31–52.
- Nounjan, N.; Chansongkrow, P.; Charoensawan, V.; Siangliw, J.L.; Toojinda, T.; Chadchawan, N.; Chansongkrow, P.; Charoensawan, V.; Siangliw, J.L.; Toojinda, T.; et al. High performance of photosynthesis and osmotic adjustment are associated with salt tolerance ability in rice carrying drought tolerance QTL: Physiological and co-expression network analysis. Front. Plant Sci. 2018, 9, 1135.
- Pintó-Marijuan, M.; Munné-Bosch, S. Ecophysiology of invasive plants: Osmotic adjustment and antioxidants. Trends Plant Sci. 2013, 18, 660–666.
- Ben Rejeb, K.; Abdelly, C.; Savouré, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. PPB 2014, 80, 278–284.
- Yaqoob, H.; Akram, N.A.; Iftikhar, S.; Ashraf, M.; Khalid, N.; Sadiq, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Seed pretreatment and foliar application of proline regulate morphological, physio-biochemical processes and activity of antioxidant enzymes in plants of two cultivars of quinoa (Chenopodium quinoa Willd.). Plants 2019, 8, 588.
- Lugojan, C.; Ciulca, S. Evaluation of relative water content in winter wheat. For. Biotechnol. 2011, 15, 173–177.
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428.
More
