Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Luisa Galli.
Strongyloidiasis belongs to the group of neglected tropical diseases, due to diagnostic difficulties and the lack of systematic screening.
- Strongyloides stercoralis
- neglected tropical disease
- children
- international adoptions
- migrants
- screening
1. Introduction
Neglected tropical diseases (NTDs) are a group of various infections caused by bacteria, viruses, protozoa, and helminths, widespread in low- and middle-income countries, such as Africa, Asia, and South America [1]. In recent years, the increasing of migratory flow, adoption, and international travel has led to a rising relevance of NTDs also in developed countries [2]. In spite of this, NTDs are still often unrecognized, leading both to a delay in proper treatment and to an underestimate in their prevalence [3]. Globally, among the NTDs, strongyloidiasis caused by the soil-transmitted helminth Strongyloides stercoralis is one of the most commonly underdiagnosed, although a recent study estimated that more than 600 million people contract this infection globally [4].
At present, only few studies, mainly conducted in adults, have evaluated the prevalence of strongyloidiasis in immigrants, with conflicting results [5,6][5][6]. Literature is scarce about strongyloidiasis in childhood. Moreover, the majority of available studies have been conducted in endemic areas, but little is known about S. stercoralis prevalence in European and Italian children [7,8,9,10][7][8][9][10]. This is mainly due to the fact that a uniform and systematic screening for strongyloidiasis in children coming from high-burden countries, or in children with unexplained eosinophilia, is not usually performed.
This is a serious gap, considering that an increase of immigrants from Sub-Saharan Africa (SSA) has been reported in Italy, in addition to the foreign-born individuals already resident in our country (about 8% of the Italian population) [3,11][3][11]. Moreover, in the last 5 years, about 1 million children have migrated to Europe, without considering international adoption and children born of foreign parents [12].
In addition to the lack of a systematic screening for high-risk subjects, diagnostic difficulties are also present. In fact, coprological methods are not always able to identify the larvae, considering the intermittent fecal release of S. stercoralis [13,14][13][14]. Among the available tests, serology shows the highest detection rate in immunocompetent individuals, but it is poorly specific due to the possible cross-reaction with other parasites, being scarcely reliable in high-burden countries [15]. However, it is an excellent screening tool in low-endemic countries [15,16][15][16]. Regarding clinical signs and symptoms, strongyloidiasis can mimic other parasitic diseases. In fact, unspecific gastrointestinal, dermatological, and pulmonary manifestations are commonly reported, while failure to thrive could also be present in children [17,18][17][18]. Frequently, S. stercoralis infection can proceed asymptomatically, with peripheral eosinophilia being the sole sign [19]. Despite this, it is crucial to detect and treat both symptomatic and asymptomatic subjects, in order to prevent hyperinfection syndrome, which has a high mortality of around 87% [14,20][14][20]. Fortunately, strongyloidiasis can be easily treated with ivermectin, with high effectiveness and good tolerability [21]. A single dose of ivermectin is effective, thus increasing treatment adherence [22,23,24][22][23][24]. Regarding the pediatric age, ivermectin is approved in children over 15 kg, although a recent review highlights its high safety profile even below this weight [25]. Albendazole, which has been considered an alternative drug for treatment of strongyloidiasis, demonstrated unsatisfactory efficacy compared to ivermectin in several clinical trials [26,27,28,29][26][27][28][29].
2. Study Design
We retrospectively enrolled 2633 patients below 18 years of age, referred to the Infectious Diseases Unit of Anna Meyer tertiary care children’s University Hospital in Florence, Italy, between 1 January 2009 and 31 December 2020, and tested for S. stercoralis infection. Within our Infectious Disease Unit, S. stercoralis serology is usually performed in case of clinical suspicion or as part of migration health assessment. According to the local ethical board, all parents had signed, at the time of their first hospital access, an informed consent for children’s data inclusion in observational studies with anonymized data extraction. For each patient, data about age, gender, and country of birth were collected. Children were then divided in three groups depending on S. stercoralis serologic results: negative, borderline, or positive (Figure 1).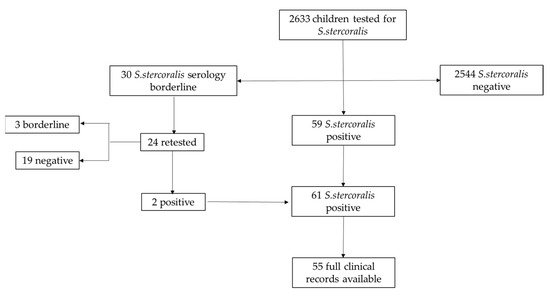
Figure 1. Flow chart of patients’ selection.
3. Geographic representation of participants’ country of origin
Overall, 2633 children evaluated at the Infectious Disease unit during the study period were screened for S. stercoralis infection. Overall, 1543 out of 2633 participants were male (58.6%) and the mean age was 7 years (IQR 4.19–10.18). Most of them (2288/2633, 86.7%) were referred to our center to perform screening for recent immigration or international adoption. Enrolled children were mainly from Oriental Europe and Balkan peninsula (682/2633, 25.9%), followed by Africa (472/2633, 18%), Latin America (493/2633, 18.7%), and Asia (443/2633, 16.8%). Only 345/2633 (13.1%) children were born in Italy, and among them 125/345 (36.2%) had foreign parents. Forty-six (46/2633, 1.7%) children came from other European countries. For 152/2633 (5.8%), mainly adopted children, we could not define the country of birth. The countries of origin of all participants are summarized in Figure 2.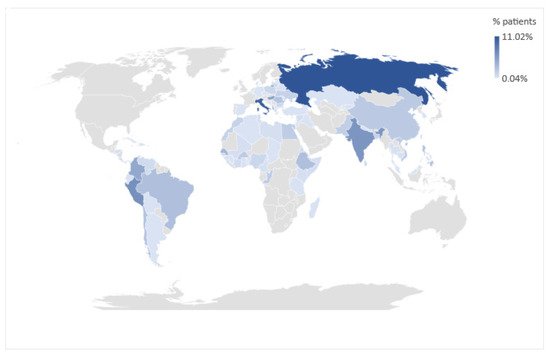
Figure 2. Geographic representation of participants’ country of origin.
References
- Hotez, P.J.; Aksoy, S.; Brindley, P.J.; Kamhawi, S. What constitutes a neglected tropical disease? PLoS Negl. Trop. Dis. 2020, 14, e0008001.
- Lingscheid, T.; Witzenrath, M.; Bouchaud, O.; Kern, P.; Da Cunha, J.S.; Beltrame, A.; Zammarchi, L.; Paul, M.; Kosina, P.; Marocco, S.; et al. Schistosomiasis in European Travelers and Migrants: Analysis of 14 Years TropNet Surveillance Data. Am. J. Trop. Med. Hyg. 2017, 97, 567–574.
- Zammarchi, L.; Gobbi, F.; Angheben, A.; Spinicci, M.; Buonfrate, D.; Calleri, G.; De Paola, M.; Bevilacqua, N.; Carrara, S.; Attard, L.; et al. Schistosomiasis, strongyloidiasis and Chagas disease: The leading imported neglected tropical diseases in Italy. J. Travel Med. 2019, 27.
- Buonfrate, D.; Bisanzio, D.; Giorli, G.; Odermatt, P.; Fürst, T.; Greenaway, C.; French, M.; Reithinger, R.; Gobbi, F.; Montresor, A.; et al. The Global Prevalence of Strongyloides stercoralis Infection. Pathogens 2020, 9, 468.
- Martelli, G.; Di Girolamo, C.; Zammarchi, L.; Angheben, A.; Morandi, M.; Tais, S.; Degani, M.; El Hamad, I.; Caligaris, S.; Ciannameo, A.; et al. Seroprevalence of five neglected parasitic diseases among immigrants accessing five infectious and tropical diseases units in Italy: A cross-sectional study. Clin. Microbiol. Infect. 2017, 23, 335.e1–335.e5.
- Asundi, A.; Beliavsky, A.; Liu, X.J.; Akaberi, A.; Schwarzer, G.; Bisoffi, Z.; Mendez, A.R.; Shrier, I.; Greenaway, C. Prevalence of strongyloidiasis and schistosomiasis among migrants: A systematic review and meta-analysis. Lancet Glob. Health 2019, 7, e236–e248.
- Khieu, V.; Schaer, F.; Marti, H.; Sayasone, S.; Duong, S.; Muth, S.; Odermatt, P. Diagnosis, Treatment and Risk Factors of Strongyloides stercoralis in Schoolchildren in Cambodia. PLoS Negl. Trop. Dis. 2013, 7, e2035.
- Forrer, A.; Khieu, V.; Schär, F.; Hattendorf, J.; Marti, H.; Neumayr, A.; Char, M.C.; Hatz, C.; Muth, S.; Odermatt, P. Strongyloides stercoralis is associated with significant morbidity in rural Cambodia, including stunting in children. PLoS Negl. Trop. Dis. 2017, 11, e0005685.
- Hailu, T.; Nibret, E.; Amor, A.; Munshea, A.; Anegagrie, M. Efficacy of Single Dose Ivermectin Against Strongyloides stercoralis Infection Among Primary School Children in Amhara National Regional State. Infect. Dis. Res. Treat. 2020, 13.
- Amor, A.; Rodriguez, E.; Saugar, J.M.; Arroyo, A.; López-Quintana, B.; Abera, B.; Yimer, M.; Yizengaw, E.; Zewdie, D.; Ayehubizu, Z.; et al. High prevalence of Strongyloides stercoralis in school-aged children in a rural highland of north-western Ethiopia: The role of intensive diagnostic work-up. Parasites Vectors 2016, 9, 617.
- Zammarchi, L.; Tilli, M.; Botta, A.; Buonfrate, D.; Bartoloni, A.; Boccalini, S. Strategies for management of strongyloidiasis in migrants from Sub-Saharan Africa recently arrived in Italy: A cost-effectiveness analysis. Travel Med. Infect. Dis. 2020, 36, 101561.
- Von Both, U. Children on the move—A call for active screening in migrants. Lancet Child Adolesc. Health 2020, 4, 174–175.
- Schaer, F.; Trostdorf, U.; Giardina, F.; Khieu, V.; Muth, S.; Marti, H.; Vounatsou, P.; Odermatt, P. Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl. Trop. Dis. 2013, 7, e2288.
- Siddiqui, A.A.; Berk, S.L. Diagnosis ofStrongyloides stercoralisInfection. Clin. Infect. Dis. 2001, 33, 1040–1047.
- Requena-Méndez, A.; Chiodini, P.; Bisoffi, Z.; Buonfrate, D.; Gotuzzo, E.; Muñoz, J. The Laboratory Diagnosis and Follow Up of Strongyloidiasis: A Systematic Review. PLoS Negl. Trop. Dis. 2013, 7, e2002.
- Agbata, E.N.; Morton, R.L.; Bisoffi, Z.; Bottieau, E.; Greenaway, C.; Biggs, B.-A.; Montero, N.; Tran, A.; Rowbotham, N.; Arevalo-Rodriguez, I.; et al. Effectiveness of Screening and Treatment Approaches for Schistosomiasis and Strongyloidiasis in Newly-Arrived Migrants from Endemic Countries in the EU/EEA: A Systematic Review. Int. J. Environ. Res. Public Health 2018, 16, 11.
- Olsen, A.; Van Lieshout, L.; Marti, H.; Polderman, T.; Polman, K.; Steinmann, P.; Stothard, R.; Thybo, S.; Verweij, J.J.; Magnussen, P. Strongyloidiasis—The most neglected of the neglected tropical diseases? Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 967–972.
- Buonfrate, D.; Marrone, R.; Silva, R.; Mirisola, C.; Ragusa, A.; Mistretta, M.; Perandin, F.; Bisoffi, Z. Prevalence of Strongyloidiasis in a Cohort of Migrants in Italy and Accuracy of a Novel ELISA Assay for S. stercoralis Infection, a Cross-Sectional Study. Microorganisms 2021, 9, 401.
- Buonfrate, D.; Angheben, A.; Gobbi, F.; Muñoz, J.; Requena-Mendez, A.; Gotuzzo, E.; Mena, M.A.; Bisoffi, Z. Imported Strongyloidiasis: Epidemiology, Presentations, and Treatment. Curr. Infect. Dis. Rep. 2012, 14, 256–262.
- Buonfrate, D.; Requena-Mendez, A.; Angheben, A.; Muñoz, J.; Gobbi, F.; Ende, J.V.D.; Bisoffi, Z. Severe strongyloidiasis: A systematic review of case reports. BMC Infect. Dis. 2013, 13, 78.
- Bisoffi, Z.; Buonfrate, D.; Montresor, A.; Mendez, A.R.; Muñoz, J.; Krolewiecki, A.; Gotuzzo, E.; Mena, M.A.; Chiodini, P.; Anselmi, M.; et al. Strongyloides stercoralis: A Plea for Action. PLoS Negl. Trop. Dis. 2013, 7, e2214.
- Buonfrate, D.; Salas-Coronas, J.; Muñoz, J.; Maruri, B.T.; Rodari, P.; Castelli, F.; Zammarchi, L.; Bianchi, L.; Gobbi, F.; Fernández, I.; et al. Multiple-dose versus single-dose ivermectin for Strongyloides stercoralis infection (Strong Treat 1 to 4): A multicentre, open-label, phase 3, randomised controlled superiority trial. Lancet Infect. Dis. 2019, 19, 1181–1190.
- Gann, P.H.; Neva, F.A.; Gam, A.A. A Randomized Trial of Single- and Two-Dose Ivermectin versus Thiabendazole for Treatment of Strongyloidiasis. J. Infect. Dis. 1994, 169, 1076–1079.
- Henriquez-Camacho, C.; Gotuzzo, E.; Echevarria, J.; Jr, A.C.W.; Terashima, A.; Samalvides, F.; Perez-Molina, J.A.; Plana, M.N. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst. Rev. 2016, 1, CD007745.
- Jittamala, P.; Monteiro, W.; Smit, M.R.; Pedrique, B.; Specht, S.; Chaccour, C.J.; Dard, C.; Del Giudice, P.; Khieu, V.; Maruani, A.; et al. A systematic review and an individual patient data meta-analysis of ivermectin use in children weighing less than fifteen kilograms: Is it time to reconsider the current contraindication? PLoS Negl. Trop. Dis. 2021, 15, e0009144.
- Suputtamongkol, Y.; Premasathian, N.; Bhumimuang, K.; Waywa, D.; Nilganuwong, S.; Karuphong, E.; Anekthananon, T.; Wanachiwanawin, D.; Silpasakorn, S. Efficacy and Safety of Single and Double Doses of Ivermectin versus 7-Day High Dose Albendazole for Chronic Strongyloidiasis. PLoS Negl. Trop. Dis. 2011, 5, e1044.
- Nontasut, P.; Muennoo, C.; Sa-nguankiat, S.; Fongsri, S.; Vichit, A. Prevalence of strongyloides in Northern Thailand and treat-ment with ivermectin vs. albendazole. Southeast Asian J. Trop. Med. Public Health 2005, 36, 442–444.
- Datry, A.; Hilmarsdottir, I.; Mayorga-Sagastume, R.; Lyagoubi, M.; Gaxotte, P.; Biligui, S.; Chodakewitz, J.; Neu, D.; Danis, M.; Gentilini, M. Treatment of Strongyloides stercoralis infection with ivermectin compared with albendazole: Results of an open study of 60 cases. Trans. R. Soc. Trop. Med. Hyg. 1994, 88, 344–345.
- Marti, H.; Hatz, C.; Chwaya, H.M.; Haji, H.J.; Mgeni, A.F.; Ameir, J.S.; Savioli, L. A Comparative Trial of a Single-Dose Ivermectin Versus Three Days of Albendazole for Treatment of Strongyloides Stercoralis and Other Soil-Transmitted Helminth Infections in Children. Am. J. Trop. Med. Hyg. 1996, 55, 477–481.
More
