Fruits and fruit products are an essential part of the human diet. Their health benefits are directly related to their content of valuable bioactive compounds, such as polyphenols, anthocyanins, or vitamins. Heat treatments allow the production of stable and safe products; however, their sensory quality and chemical composition are subject to significant negative changes. The use of emerging non-thermal technologies, such as HPP (High Pressure Processing), has the potential to inactivate the microbial load while exerting minimal effects on the nutritional and organoleptic properties of food products. HPP is an adequate alternative to heat treatments and simultaneously achieves the purposes of preservation and maintenance of freshness characteristics and health benefits of the final products. However, compounds responsible for antioxidant activity can be significantly affected during treatment and storage of HPP-processed products. Therefore, this article reviews the effect of HPP treatment and subsequent storage on the antioxidant and on the total phenolic, flavonoid, carotenoid, anthocyanin and vitamin contents of fruits and different processed fruit-based products.
- high hydrostatic pressure
- refrigerated or ambient storage
- fruit preparations
- antioxidant capacity
1. Introduction

2. Fruits and Their Antioxidant Activity
2.1. Determination of Antioxidant Activity/Capacity
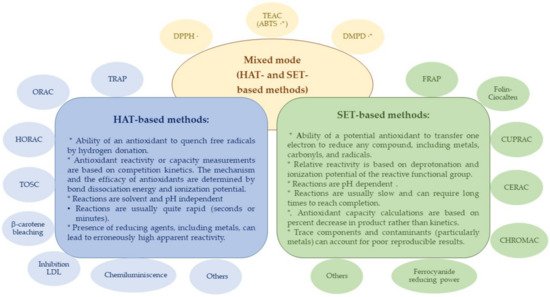
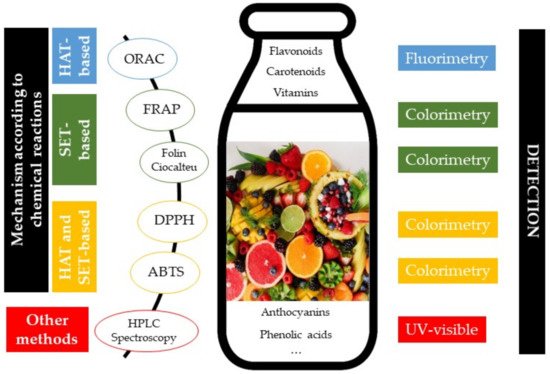
2.2. Vitamins and Phenolic Compounds Related to Antioxidant Capacity
3. Innovative Methods: High Pressure Processing for Fruit Treatment
3.1. Conventional methods
Traditional heat treatment methods, such as pasteurization and commercial sterilization, are widely used in the fruit processing industry for their effective preservative effect, due to the destruction of microorganisms and inactivation of enzymes. However, excessive treatment can lead to losses in nutritional quality and phytochemical content, as well as physicochemical, rheological and organoleptic properties of processed products [90].
Commercial sterilization is a more drastic thermal process, where the final product is microbiologically safe and guaranteed to be stable for long shelf-life. Pasteurization is a milder thermal process, whose main objectives are to ensure safety and prolong food shelf-life. It is a method that needs to be used with a complementary technology (such as cooling, acidification, reduction of water activity, and/or use of preservatives) to guarantee stability. Pasteurized products have low to medium stability and variable shelf-life, according to their characteristics and complementary conservation method [91].
Pasteurization is effective in inactivating some undesirable enzymes, such as POD and PPO, which can contribute to enzymatic browning and affect the quality of different fruit-based products. Normally, a short heat treatment at 80-100 °C is sufficient to inactivate some unwanted enzymes. Mild heat treatments (60 °C, 30 min) on pawpaw pulp achieved 70% inactivation of PPO [92]. On the other hand, some authors [93] found that when blueberry pureé was treated at different temperatures (40, 50, 60, 70, 80, 90, and 100 °C) for 20 min the PPO activity decreased with increasing temperature. The activity was inactive when the processing temperature was higher than 80 °C. In addition, β-glucosidase activity was significantly decreased with increasing temperature. However, undesirable changes in taste, color and aroma may occur after heat treatment [92,94-97]. In addition, high temperatures may reduce the nutritional value and functionality of bioactive compounds found in fruits and fruit-based products [95,98-100].
Refrigerated storage can effectively slow the activation of the oxidases to stabilize the levels of antioxidants. After the pasteurization, storage temperature is an important factor that influences the qualities of fruit products during storage [101].
Due to their high moisture content, fruits are highly perishable; therefore, the use of other preservation methods is necessary to increase their shelf-life and reduce product wastage and environmental problem [26, 102]. Drying treatments are used to reduce the water activity of fresh fruits in order to extend their shelf-life. In addition to whole fruit or dried fruit pieces, new products such as fruit powder are also being developed [103,104]. Different methods are used in the food industry to reduce the moisture content of fruits such as solar dryer, microwave heating, infrared irradiation, fluidised drying, or vacuum drying [105]. The system applied plays a key role in the final moisture content, quality and shelf-life of the product obtained [106].
Freezing is a widely used long-term preservation method for fruits, where they retain attributes associated with freshness much better than other conventional preservation methods like sterilization and drying [107,108]. Frozen fruits retain physical structure, nutritional and sensory attributes. In recent years, the processing of fruit pulp and juice is becoming an important economic activity for the production sector, as it adds cost-effective value to fruits, and minimizes losses [109]. Frozen fruit pulps are an important source of raw material and bioactive compounds and are being frequently used in the food industry for the production of other fruit-based products [110].
3.2. Innovative methods: High Pressure Processing
Consumer demand for more natural products has led to an increase in the research on minimal preservation innovative techniques, such as High Pressure Processing (HPP), High Pressure Homogenization (HPH), and Pulsed Electric Fields (PEF)
Consumer demand for more natural products has led to an increase in the research on minimal preservation innovative techniques, such as High Pressure Processing (HPP), High Pressure Homogenization (HPH), and Pulsed Electric Fields (PEF)[90]
, among others. HPP can treat fruit products, previously packaged in flexible or rigid plastic bottles, at values ranging from 300 to 600 MPa, 0 °C to 90 °C. Usually, for commercial applications, it operates at room temperature (acting as a cold pasteurization technique). In HPP treatment, the pressure is applied uniformly, rapidly, independently of the shape, and according to Pascal’s principle. The products size results in fewer challenges when the production is scaled up[91]
. Another advantage is that HPP can assist in reducing the number of ingredients by eliminating the need for certain additives for product stability and preservatives used for food safety and shelf-life extension[92]
. Moreover, due to its limited effect on the covalent bonds of low molecular mass compounds such as vitamins, color, and flavor compounds, HPP treatment could preserve nutritional value and the sensory properties of fruits and vegetables. In addition, the primary structure of low-molecular-weight molecules (vitamins, amino acids, volatile compounds, pigments, etc.) is not affected, allowing a better retention of nutrients and sensory properties of foods. Nevertheless, the effect of HPP on vegetable products varies depending on processing conditions (pressure, hold time, and temperature) and food form (whole, pieces, juice, purée, mousse, or smoothie). Intrinsic factors such as pH or plant varieties also influence the process. Due to this, very often contradictory results can be found for the same matrix[32].
.4. Impact of HPP on Antioxidant Activity during Storage of Fruits and Fruit Products
4.1. Fruit, Pulp, and Purée
| Fruit Product | Treatment Conditions | Antioxidant Method (Units) | Main Effects after Storage | References |
|---|---|---|---|---|
| Cantaloupe purée | HPP-1: 300, 400 and 500 MPa, 5 min, 8 °C HPP-2: 300, 400 and 500 MPa, 5 min, 15 °C Storage: 10 days, 4 °C |
ORAC (μmol Trolox/100 g fresh weight) | The antioxidant capacity values of pressure treated purée were very stable during 10 days of storage at 4 °C. | [99] |
| Jujube pulp | HPP: 400, 500 and 600 MPa, 20 min TP: 100 °C, 10 min Storage: 40 days, 4 °C or 15 °C |
Ascorbic acid: Spectroscopy (mg AA/100 g of fresh weight) TPC: Folin-Ciocalteu (mg RE/100 g fresh weight) Total flavonoids: Spectroscopy (mg GAE/100 g fresh weight) DPPH (mg vitamin C equivalent/100 g fresh weight |
The ascorbic acid content of HPP-treated samples stored at 4 °C and 15 °C decreased with increasing storage time. The antioxidant capacity of HPP-treated and TP samples decreased during storage at 4 °C and 15 °C. However, compared to TP, HPP resulted in greater antioxidant capacity in jujube pulp (42% vs. 28%). |
[100] |
| Fresh Mango | HPP: 20, 40, 60 and 80 MPa, 10 min, 20 °C Storage: 16 days, 13 °C |
DPPH (g vitamin C equivalent/kg fresh weight) ABTS (g vitamin C equivalent/kg fresh weight) Vitamin C, total phenolics, flavonoids and carotenoids: Spectroscopy (mg/kg fresh weight) |
Increased bioactive substances (vitamin C, total phenolics, flavonoids, and carotenoids) and antioxidant activities (DPPH and ABTS). Mangoes treated with 20 MPa show higher antioxidant activity than that treated with 40, 60 and 80 MPa during storage. |
[101] |
| Persimmon pieces | HPP: 200 MPa, 3 and 6 min, 25 °C Storage: 21 days, 4 °C |
Total carotenoids: Spectroscopy (μg/100 g fresh weight) TPC: Folin-Ciocalteu (mg GAE/100 g fresh weight) DPPH (μmol Trolox/100 g fresh weight) |
During storage, antioxidant activity diminished in the two pressurized conditions. Total carotenoid content was not modified significantly up to 28 days in nontreated fruit. The degradation of phenolic compounds during storage in pressurized samples may explain the decrease in DPPH. |
[103] |
| Plum purée | HPP: 600 MPa, 230 s, 25 °C TP: 75 °C, 30 s Storage: 40 days, 4 °C |
Total anthocyanins: Spectroscopy (mg/100 g fresh weight) TPC: Folin-Ciocalteu (mg GAE/100 g fresh weight) ABTS (mg Trolox/100 g of fresh weight) |
The addition of ascorbic acid increased importantly the total antioxidant activity. At day 20 and 40 of storage, the purées with the highest total antioxidant activity and polyphenols content were those purées manufactured with ascorbic acid addition and processed by TP. |
[104] |
| Cupped strawberry | HPP: 400 MPa, 5 min TP: 75 °C, 20 min Storage: 45 days, 4 °C or 25 °C |
TPC: Folin-Ciocalteu (mg GAE/100 g of flesh) Total anthocyanins: Spectroscopy (mg/100 g of flesh) DPPH (mg Trolox/100 g flesh) FRAP (mg Trolox/100 g flesh) |
Reduction of antioxidant capacity in all samples (HPP and TP) during storage. Reduction in total phenolics, total anthocyanins and pulp antioxidant capacity, being more striking in the samples stored at 25 °C. Cupped strawberry stored at 4 °C showed higher contents of total phenols, total anthocyanins, and antioxidant capacity. |
[105] |
| Strawberry purée | HPP: 300 and 500 MPa, 1, 5 and 15 min, 0 °C and 50 °C TP: 90 °C, 15 min Storage: 12 weeks, 6 °C |
TPC: Folin-Ciocalteu (mg GAE/100 g fresh weight) Phenolic acids and flavonols compounds: HPLC-DAD (mg/100 g fresh weight) Anthocyanin: Spectroscopy (mg/100 g fresh weight) Total vitamin C: Spectroscopy (mg AA/100 g fresh weight) |
No vitamin C was detected in the thermally pasteurized purée after 8 weeks of storage, while complete degradation was observed after 4 weeks of storage for HPP-treated purée. HPP-preserved purée had higher content of polyphenols and color parameters compared to TP purée. |
[106] |
4.2. Fruit Juices
| Juice Products | Treatment Conditions | Antioxidant Method (Units) | Main Effects after Storage | References |
|---|---|---|---|---|
| Apple juice | HPP: 400 MPa, 15 min TP: 98 °C, 50 s Storage: 70 days |
DPPH (% inhibition) FRAP (% inhibition) Polyphenols: HPLC-DAD (μg epicatechin/mL) |
Antioxidant activity (DPPH) retention: 76% (TP) and 73% (HPP). Antioxidant activity (FRAP) retention 77% (TP) and 76% (HPP). Samples TP and HPP decreased polyphenol content by 33% and 53%, respectively. |
[108] |
| Apple juice | HPP: 300 (3 pulses 5 min), 450 and 600 MPa, 5 min Storage: 90 days, 4 °C |
DPPH (μM Trolox) ABTS (μM Trolox) TPC: Folin-Ciocalteu (mg GAE/L) Ascorbic acid: HPLC-DAD (mg/L) |
Antioxidant activity (DPPH) increased up to 6.4% after HPP (600 MPa) and decreased with storage up to 21.5%. Antioxidant activity (ABTS) increased by 19.0% after HPP (600 MPa) and decreased with storage up to 36.0%. TPC increased after HPP, by a maximum of 6.1% at the highest pressure used. HPP treatment significantly decreased the total vitamin C content of apple juice between 2.5% and 30.9%. | [109] |
| Apple juice with Sabah snake grass leaves | HPP: 300, 400 and 500 MPa, 5 min, 25 °C Storage: 36 days, 4 °C |
DPPH (mmol/g) FRAP (mmol FeSO4/g) TPC: Folin-Ciocalteu (g GAE/kg on a fresh weight basis) |
5.5–6.8% decrease in HPP samples. Almost unaltered: 1.2% decrease in HPP samples. Significantly lower amount of TPC than the untreated samples from 4–2%, but a decrease up to 10.8% in HPP samples after storage. |
[110] |
| Aronia juice | HPP: 200, 400 and 600 MPa, 15 min, 26–38 °C Storage: 80 days, 4 °C |
ABTS (mmol Trolox equivalents/mL) FRAP (mmol Fe2+ equivalents/mL) TPC: Folin-Ciocalteu (mg catechin/mL) |
During storage, the pressurized juices demonstrated ABTS and FRAP values higher by 14% and 5% as compared to the untreated juices. Juice HPP yielded a 12% drop (200 MPa) an 8% drop (600 MPa) yielded compared to the untreated samples and 36% drop at the end of storage. |
[111] |
| Cherry juice | HPP: 400 and 550 MPa, 5 and 2 min, rt TP: 70 °C, 30 s Storage: 28 days, 4 °C |
DPPH (IC50, mL juice/mL DPPH) TPC: Folin-Ciocalteu (mg GAE/mL) Anthocyanins: Spectroscopy (mg CGE/L) |
TP samples had the lowest antioxidant activity after 28 days A decline of about 26% for HPP samples and 20% for TP ones during storage. Both HPP treatments significantly increased the anthocyanins content (up to 15.5%) while TP was slightly decreasing them. |
[112] |
| Elephant Apple juice | HPP: 600 MPa, 5 min, 35 °C TP: 80 °C, 60 s Storage: 60 days, 4 °C |
DPPH (µmol Trolox/g, % inhibition) TPC: Folin-Ciocalteu (mg GAE/kg) Flavonoids: Spectroscopy (mg quercetin/kg) |
In HPP samples, the antioxidant activity was maintained during storage but decreases in untreated samples (8.3%) and in TP samples (29.3%). TPC in control samples decreased 25.9% after 10 days storage and 25.9% for TP (60 days), while increasing up to 18.2% for HPP (60 days). A decrease was occurred in untreated samples after 10 days (27.2%) and also after TP (38.7) and HPP (37.7) during 60 days storage. |
[113] |
| Jabuticaba juice | HPP: 200, 400 and 600 MPa, 5 min, rt TP: 90 °C, 60 s Storage: 28 days, 4 °C |
ABTS (% antiradical activity) DPPH (% inhibition) Reducing power TPC: Folin-Ciocalteu (mg GAE/100 mL) Total flavonoids: Spectroscopy (mg quercetin equivalents/100 mL) Anthocyanins: Spectroscopy (mg/100 mL) |
Higher values for ABTS: 55–31% (600–400 MPa). No differences between HPP and TP for DPPH. The reductive power decreased significantly after storage. The control decreased significantly with increasing storage time, but the TPCs of HPP and TP samples remained constant. A significant decrease of total flavonoids with increasing storage time ranging from 27.9 to 43.3%. No significant changes for the samples treated at 400 and 600 MPa. Control, TP, and HPP-200 decreased anthocyanin content by 20.9%, 19.1, and 10.8%, respectively. | [114] |
| Korla pear juice | HPP: 500 MPa, 10 min, rt Pretreatment: UF TP: 110 °C, 8.6 s Storage: 56 days, 4 °C |
DPPH (mmols Trolox/mL) FRAP (mmols Trolox/mL) TPC: Folin-Ciocalteu (mg GAE/100 mL) Ascorbic acid: HPLC (µg/mL) |
Antioxidant capacity (DPPH) in HPP- and TP-treated juices were decreased by 48.85% and 50.40%, respectively. Antioxidant capacity (FRAP) in HPP- and TP-treated juices were decreased by 8.57% and 11.36%, respectively. No significant difference of total phenols and ascorbic acid between UF pre-treated and UF-HPP juice, while they were significantly decreased by 4.73% and 13.43%, respectively, after UF-TP treatment. | [115] |
| Maoberry juice | HPP: 400 and 600 MPa, 10 min, 25–33 °C TP: 90 °C, 1 min Storage: 28 days, 4 °C |
DPPH (% of inhibition) FRAP (mmol Fe (II)/mL) TPC: Folin-Ciocalteu (mg GAE/100 mL) Ascorbic acid: HPLC-DAD (mg/100 mL) Anthocyanins: Spectroscopy (mg/L) |
Pressurized products retained higher antioxidant activity (75.1%) than pasteurized samples (65.0%) after total storage. A double reduction in FRAP values (49.4% against 25.7%) was obtained by TP compared to HPP treatment during storage. Total phenols were relatively stable under HPP, whilst a significant decrease of these compounds was found in TP at zero time (9.9%) and at 28 days (14.2%), respectively. Pressure levels had no effect on the loss of ascorbic acid. Anthocyanins decreased up to 10.5% in HPP and 24.3% in TP after 28 storage days. | [116] |
| Mulberry juice | HPP: 500 MPa, 5 min HPCD: 15 MPa, 55 °C, 10 min TP: 110 °C, 8.6 s Storage: 28 days, 4 °C and 25 °C |
DPPH (mmols Trolox/L juice) FRAP (mmols Trolox/L juice) TPC: Folin-Ciocalteu (mg GAE/L) Anthocyanins: Spectroscopy (mg/L) |
No differences among the untreated, the HPP-treated, and the TP treated juices immediately after the treatments. A slight increase in antioxidant capacity in the TP-treated juice. An increase in HPCD samples by 16%; no changes for HPP samples. After 28 days of storage at 4 and 25 °C, the concentration of TPC in all the juices gained a significant increase. After entire storage at 4 and 25 °C, the anthocyanins content in the TP-treated juice was decreased by 8% and 29%, respectively, and in the HPP-treated juice was decreased by 13% and 30%, respectively. |
[117] |
| Orange juice | HPP: 550 MPa, 70 s, 18 °C TP: 70 °C, 70 s Storage: 36 days, 4 °C |
DPPH (mL/mg) TPC: Folin-Ciocalteu (mg GAE/100 mL juice) Flavonoids: Spectroscopy (mg rutin equivalent/100 mL) Anthocyanins: Spectroscopy (mg CGE/100 mL) Carotenoids: Spectroscopy (mg β-carotene equivalent/100 mL) |
Both treatments caused a decrease (26% TP and 13% HPP) in antioxidant activity.TPC decrease of 25.4% in TP samples and of 10.7% in HPP samples after 36 days. Increase of 26.3% after 4 days and 10.3% after 36 days for TP samples and decrease of 39.3% for HPP samples after storage. Similar decrease in HPP (14.5%) and TP (17.6%) samples after 36 days. A decrease in both treatments after 36 days: 22.3% (TP) and 26.6% (HPP). |
[118] |
| Orange juice | HPP: 520 MPa, 6 min, 60 °C TP: 95 °C, 30 s Storage: 90 days, 4 °C * |
ABTS (µmol Trolox/100 mL juice) Ascorbic acid: Spectroscopy (mg AA/100 mL) TPC: Folin-Ciocalteu (mg GAE/100 mL juice) |
Antioxidant activity shows a slight increase during shelf-life and overall antioxidant activity for both juices maintained high values along shelf-life. Ascorbic acid reducing around 70–80% of ascorbic acid levels in 90 days. TPC showed a slight decrease over time, and no significant difference between TP and HPP. | [119] |
| Organic Grape juice | HPP: 600 MPa, 3 min, nr Storage: 6 months, 4 °C in PLA and PET bottles |
ABTS (mmol Trolox/L) Phenol index (PI): Spectroscopy (μg gallic acid/mL) Flavonol index (FI): Spectroscopy (μg galangin /mL) Anthocyanins: Spectroscopy (μg delphinidin chloride/mL) |
HPP do not cause significant changes on antioxidant activity. The PLA samples showed lower PI (−10%) than juice in PET bottles, with a significant decrease in shelf-life. The absorbances for FI and for anthocyanins showed the same trend reported for PI. The HPP juices with PET packaging showed the highest values. PLA juices had a significant decrease during storage, whereas samples in PET bottles showed a strong decrease at the 6th month. |
[120] |
| Pitaya juice | HPP: 550 and 600 MPa, 16 and 15 min, 20 °C Storage: 60 days |
DPPH (mM Trolox/g in dry based) TPC: Folin-Ciocalteu (mg GAE/g in dry based) Betalains: Spectroscopy (mg/g in dry based) |
Antioxidant activity was not affected by HPP but had a 5% decrease after storage. Phenolic compounds were not affected by HPP but had a 10% decrease after storage. Betaxanthins showed no significant changes during storage (21.1 to 18.9 mg/g), while betacyanins showed a 28% reduction |
[121] |
| Red grapefruit juice | HPP: 550 MPa, 10 min, 25 °C TP: 110 °C, 8.6 s Storage: 30 days, 4 °C |
DPPH (mmoles Trolox/L) FRAP (mmoles Trolox/L) TPC: Folin-Ciocalteu (mg GAE/100 g) Ascorbic acid: HPLC-UV-vis (mg/100 mL) |
Antioxidant activity decreased by 1.4% (DPPH) and 8.5 % (FRAP) after TP; no change with unprocessed samples during storage. DPPH and FRAP was decreased by 5.0 and 14.3% in HPP-treated grapefruit juice and 5.3 and 12.8% in TP-treated grapefruit juice during total storage, respectively. No differences in TPC in HPP juices, but TP-treated samples decreased by 7.7% After processing HPP caused 8.82% loss of AA, while TP resulted in 27% loss. During 30 days storage, AA in HPP- and TP-treated samples showed a reduction by 21.1 and 22.4%, respectively. |
[122] |
| Strawberry, apple, lemon juice | HPP: 500 MPa, 15 min, 20 °C TP: 86 °C, 1 min US: 376 W, 10 min, 35 °C Storage: 12 days, 4 °C |
DPPH (% inhibition) TPC: Folin-Ciocalteu (mg GAE/L juice) Anthocyanins: Spectroscopy (mg/L) Ascorbic acid: Spectroscopy (mg/100 mL) |
Increased by 2.7% in HPP, maintained in TP. Increased by 18% and 7% after HPP and US, respectively, and maintained by the TP. Maintained by HPP but decreased by 16% and 12% after US and HT, respectively. Ascorbic acid content in the juice blends were decreased by 9%, 11%, and 23% after HPP, US, and TP, respectively. |
[123] |
| Tomato juice | HPP: 400 and 600 MPa, 15 min, 32–38 °C TP1: 74 °C, 2 min TP2: 90 °C, 1 min Storage: 14 days, 6 °C |
ABTS (μmol Trolox/g fruit in dry weight) FRAP (μmol Trolox/g fruit in dry weight) TPC: Folin-Ciocalteu (mg GAE/g juice, dry based) TPI: HPLC (μg/100 g dry based) |
Greatest decrease 13% for TP1 and during storage and retention of 95% for HPP samples. Almost unaffected in HPP samples and a decrease up to 14% in HT samples. At the end of the storage period HPP samples increased between 4.6 and 22.1% of their polyphenol content and heated samples lost between 15.2 and 30.6%. HPP samples increased up to 6.6% during storage and decreased up to 50.6% in TP heated samples. |
[124] |
| White grape juice | HPP: 300 and 600 MPa, 3 min, 20–29 °C TP: 90 °C, 1 min Storage: 20 days, 4 °C |
ABTS (mmol Trolox/L) TPC: HPLC-DAD (mg/mL) Anthocyanins: Spectroscopy (mg/mL) |
HPP significantly enhanced the ABTS value of grape juice from 8 to 20% during storage. TPC showed the most significant decrease, retaining only 71.4% of the ABTS value. Slight decrease during storage and for both treatments. Better retention in HPP (97.4%) compared to TP (92.2%). | [125] |
| White grape juice concentrate | HPP: 200, 300 and 400 MPa, 2 and 4 min, 20 °C; Storage: 35 days, 4 °C |
DPPH (µmol Trolox/100 mL) ORAC (µmol Trolox/100 mL) TPC: Spectroscopy (mg GAE/100 mL) Total Flavonoids: Folin-Ciocalteu (mg quercetin equivalent/100 mL) Individual phenols: HPLC-DAD (mg/L) |
HPP caused a slight but significant decrease of both ORAC and DPPH values. The retention of antioxidant activity significantly increased at HPP over 300 MPa/2 min, showing a positive effect of higher pressure. An increase of 8.9 and 37.1% in HPP samples at zero days and after 35 days, respectively. A decrease of 15.8% and 7.5% was observed in HPP samples at zero days and after 35 days, respectively. |
[126] |
4.3. Beverages and Other Fruit-Based Products
4.3.1. Syrup
4.3.2. Jam/Jelly
4.3.3. Beverages
4.3.4. Smoothies
| Fruit Product | Treatment Conditions | Antioxidant Method (Units) | Main Effects after Storage | References |
|---|---|---|---|---|
| Peach syrup | Pre-treated ohmic heating and HPP: 600 MPa, 3 min Storage: 4 °C |
TPC: Folin-Ciocalteu (mg catechin equivalents/kg) Ascorbic acid: HPLC-DAD (mg AA/kg) |
No significant increase of TPC (5%). Low reduction of ascorbic acid (6%). |
[136] |
| Strawberry jam | HPP: 200, 400 and 600 MPa, 30 min, 50 °C Storage: 3 months, room temperature |
Ascorbic acid: Spectroscopy (mg AA/100 g) Anthocyanin: Spectroscopy (mg anthocyanin/100 g) DPPH (%) TPC: Folin-Ciocalteu (mg gallic acid/100 g) Total flavonoids: Spectroscopy (mg catechin/100 g) |
Lower pressures: less reduction of the physico-chemical attributes but failed to yield proper gelling characteristics. Best sensory appeal: jam obtained at 600 MPa and was stable for a period of 3 months. |
[137] |
| Sapodilla jam | HPP: 400 MPa, 10 min, 27 °C Storage: 3 months, ambient temperature |
TPC: Folin-Ciocalteu (mg GAE/100 g fruit) | Reduction of TPC after storage. | [138] |
| Tiger nuts’ milk | HPP: 400, 500 and 600 MPa, 90–120-180 s, 11 °C Storage: 8 days, 4 °C |
TPC: Folin-Ciocalteu (mg GAE/100 mL) DPPH (μmol Trolox/L) ORAC (mM Trolox) |
Loss in vitamin C content (8.22 to 5.93 mg/100 mL) of sample treated at 600 MPa/180 s. Decrease of TPC from 139.14 to 95.85 mg GAE/100 mL after 600 MPa/120 s. No modification of DPPH and ORAC. |
[139] |
| Aloe vera-litchi mixed beverage | HPP: 600 MPa, 15 min, 56 °C TP: 95 °C, 10 min Storage: 120 days, 4 °C; 60 days, 15 °C; 30 days, 25 °C in dark |
Ascorbic acid: Spectroscopy (mg/100 mL) TPC: Folin-Ciocalteu (mg GAE/100 mL) |
Ascorbic acid content decreased at all the temperatures. Minimal changes in phenolic content. At 4 °C, the self-life was 100 days (HPP) and 80 days (TP). |
[140] |
| Lemongrass-lime mixed beverage | HPP: 200, 250, 300 and 400 MPa, 1–2 min, 25 °C Storage: 8 weeks, 4 °C |
Ascorbic acid: Spectroscopy (mg/100 mL) TPC: Folin-Ciocalteu (μg GAE/mL) DPPH (μg Trolox/mL) |
HPP at 250 MPa ensured microbiological safety and no significant losses of vitamin C and phenolic compounds during the first 3–4 weeks. | [141] |
| Whey-based sweet lime beverage | HPP: 500 MPa, 10 min, 25 °C TP: 90 °C, 60 s Storage 120 days, 4 °C |
TPC: Folin-Ciocalteu (mg GAE/L) DPPH (%) |
Self-life: 120 days (HPP) and 75 days (TP). 60% (TPC) and 78% (DPPH) retention after storage. |
[142] |
| Papaya beverage | HPP: 350, 450, 550 and 650 MPa, 5 and 10 min, 20 °C TP: 110 °C, 8.6 s Storage: 40 days, 4 °C |
Total carotenoids: Spectroscopy (mg/100 mL) TPC: Folin-Ciocalteu (mg GAE/100 mL) DPPH (Trolox) FRAP (Trolox) |
Similar retention (70%) of carotenoids with HPP or TP. Better preservation of TPC with HPP (79%) than with TP (74%). FRAP and DPPH decreased in HPP-samples by 37.2% and 22.0%, respectively. |
[128] |
| Mango smoothie | HPP: 400, 500 and 600 MPa, 0–15 min, 20 °C TP: 90 °C, 20 min Storage: 15 days, 4 °C |
Total carotenoids: Spectroscopy (mg/100 mL) | Carotenoid content decreased significantly in all treated mango smoothies. | [143] |
| Mixed fruit and vegetable smoothie | HPP: 630 MPa, 6 min, 22 °C Storage: 28 days, 5 °C |
Ascorbic acid: HPLC-DAD (mg AA/kg smoothie) TPC: Folin-Ciocalteu (mg GAE/kg smoothie) DPPH (μmol Trolox/kg smoothie) FRAP (μmol Trolox/kg smoothie) |
Increase in the initial values of nutritional quality indicators and decrease during refrigerated storage. | [132] |
| Mixed fruit and vegetable smoothie | HPP: 630 MPa, 6 min, 22 °C Storage: 28 days, 5 °C |
TPC: Folin-Ciocalteu (mg GAE/100 g smoothie) DPPH (μmol Trolox/100 g smoothie) FRAP (μmol Trolox/100 g smoothie) |
Increase in the initial values of antioxidants indicators and decrease during room storage. | [144] |
| Orange vegetables smoothie | HPP: 0, 300, 400, 500 and 600 MPa, 5 min, 23 °C Storage: 7 days, 5 °C |
TPC: Folin-Ciocalteu (mg chlorogenic acid/kg) | TPC increased in all samples, mainly at 300–400 MPa. | [145] |
| Multifruit smoothie | HPP: 350 and 450 MPa, 5 min, <25 °C, and 600 MPa, 3 min, <25 °C TP: 85 °C, 7 min Storage: 48 h, 4 °C under darkness |
DPPH (% inhibition) FRAP (μmol Fe2+/100 mL) Vitamin C: HPLC-DAD (mg/100 mL) TPC: Folin-Ciocalteu (mg GAE/100 mL) Total flavonoids: Spectroscopy (mg quercetin/100 mL) |
Decrease of FRAP and DPPH. Vitamin C, phenols and flavonoids were retained during storage. |
[133] |
| Red fruit-based smoothie | HPP: 350 MPa, 5 min, 10 °C TP: 85 °C, 7 min Storage: 28 days, 4 °C alternately (12 h/12 h) kept under artificial lighting and darkness |
DPPH (% inhibition) FRAP (μmol Fe2+/L) Vitamin C: HPLC-UV (mg/100 mL) TPC: Folin-Ciocalteu (mg GAE/100 mL) |
Degradation rate of vitamin C, total phenols, and antioxidant capacities during storage. | [146][147] |
| Multi-vegetables smoothie with apple | HPP: 350 MPa, 5 min, 10 °C TP: 85 °C, 7 min Storage: 28 days, 4 °C |
FRAP (μmol Fe2+/100 mL) Vitamin C: HPLC-UV (mg/100 mL) TPC: Folin-Ciocalteu (mg GAE/100 mL) Total flavonoids: Spectroscopy (mg quercetin/100 mL) |
FRAP value and vitamin C content were very low and degraded during storage. Low levels of total phenols and flavonoids remained stable. |
[148][149] |
| Multi-fruit smoothie | HPP: 350 MPa, 5 min, 10 °C TP: 85 °C, 7 min Storage: 21 days, 4 °C |
DPPH (IC50) FRAP (μmol Fe2+/100 mL) Vitamin C: HPLC-UV (mg/100 mL) TPC: Folin-Ciocalteu (mg GAE/100 mL) Total flavonoids: Spectroscopy (mg quercetin/100 mL) |
Except for the flavonoids, which remained stable for up to 21 days of storage, all parameters decreased during storage. | [150] |
| Multi-fruit milk smoothie | HPP: 450 and 600 MPa, 3 min, 20 °C TP: 80 °C, 3 min Storage: 45 days, 4 °C |
Carotenoids: HPLC-DAD (mg/100 mL) Ascorbic acid: HPLC-DAD (mg/100 mL) TPC: Folin-Ciocalteu (mg GAE/100 mL) FRAP (μmol Trolox/100 mL) DPPH (IC50) |
Ascorbic acid retention (95% and 92%) and decrease of total phenolics (12% and 11%), FRAP (45% and 61%) and DPPH (19% and 34%) for HPP-550 and HPP-650, respectively. | [151] |
| Multi-fruit soymilk smoothie | HPP: 550 and 650 MPa, 3 min, 20 °C TP: 80 °C, 3 min Storage: 45 days, 4 °C |
Carotenoids: HPLC-DAD (mg/L) Ascorbic acid: HPLC-DAD (mg/L) TPC: Folin-Ciocalteu (mg GAE/L) FRAP (mg Trolox/L) DPPH (% scavenging) |
Decrease of total carotenoid content (4% for HPP-550 and 6% for HPP-650), ascorbic acid (43%), FRAP (26% at 550 MPa and 31% at 650 MPa) and DPPH. Increase (12%) in total phenolic content. |
[129] |
References
- Oms-Oliu, G.; Odriozola-Serrano, I.; Soliva-Fortuny, R.; Elez-Martínez, P.; Martín-Belloso, O. Stability of health-related compounds in plant foods through the application of non thermal processes. Trends Food Sci. Technol. 2012, 23, 111–123.
- Yamaguchi, T.; Katsuda, M.; Oda, Y.; Terao, J.; Kanazawa, K.; Oshima, S.; Inakuma, T.; Ishiguro, Y.; Takamura, H.; Matoba, T. Influence of polyphenol and ascorbate oxidases during cooking process on the radical-scavenging activity of vegetables. Food Sci. Technol. Res. 2003, 9, 79–83.
- Leong, S.Y.; Oey, I. Effect of endogenous ascorbic acid oxidase activity and stability on vitamin C in carrots (Daucus carota subsp. sativus) during thermal treatment. Food Chem. 2012, 134, 2075–2085.
- Rodríguez Leyton, M. Desafíos para el consumo de frutas y verduras. Rev. Fac. Med. Hum. 2019, 19, 105–112.
- Gómez Salas, G.; Quesada Quesada, D.; Chinnock, A. Consumo de frutas y vegetales en la población urbana costarricense: Resultados del Estudio Latino Americano de Nutrición y Salud (ELANS)-Costa Rica. Población Salud Mesoamérica 2020, 18, 450–470.
- Garnett, T.; Mathewson, S.; Angelides, P.; Borthwick, F. Policies and actions to shift eating patterns: What works? In A Review of the Evidence of the Effectiveness of Interventions Aimed at Shifting Diets in More Sustainable and Healthy Directions; Food Climate Research Network; University of Oxford: Oxford, UK, 2015.
- World Health Organization (WHO). Diet, Nutrition and the Prevention of Chronic Diseases (Report of a Joint WHO/FAO Expert Consultation 916); World Health Organization: Geneva, Switzerland, 2003.
- Lock, K.; Pomerleau, J.; Causer, L.; Altmann, D.R.; McKee, M. The global burden of disease attributable to low consumption of fruit and vegetables: Implications for the global strategy on diet. Bull. WHO Health Organ. 2005, 83, 100–108.
- World Health Organization (WHO). Assessing National Capacity for the Prevention and Control of Noncommunicable Diseases (Report of the 2019 Global Survey); World Health Organization: Geneva, Switzerland, 2020.
- Johnson, S.; Tittenbrun, Z.; Romero, Y.; Torode, J.; Frech, S.; Abdel-Wahab, M.; Juric, A.; Stevens, L.; Bray, F.; Piñeros, M.; et al. The World Cancer Declaration: Time to consolidate wins and work towards 2025. Lancet 2021, 22, 296–298.
- Balaji, S.M.; Seeberger, G.K.; Hennedige, O. Burden of oral diseases and noncommunicable diseases: An Asia-Pacific perspective. Indian J. Dent. Res. 2018, 29, 820–829.
- Raphaelli, C.O.; Azevedo, J.G.; Dalmazo, G.O.; Vinholes, J.R.; Braganhol, E.; Vizzotto, M.; Nora, L. Effect of fruit secondary metabolites on melanoma: A systematic review of in vitro studies. Curr. Bioact. Compd. 2020, 16, 1009–1035.
- Choi, A.; Ha, K.; Joung, H.; Song, Y. Frequency of consumption of whole fruit, not fruit juice, is associated with reduced prevalence of obesity in Korean adults. J. Acad. Nutr. Diet. 2019, 119, 1842–1851.
- Werneck, A.O.; Oyeyemi, A.L.; Szwarcwald, C.L.; Sardinha, L.B.; Silva, D.R. Body mass index trajectories and noncommunicable diseases in women: The role of leisure time physical activity. Am. J. Hum. Biol. 2020, 33, e23492.
- Shah, S.; Munyuzangabo, M.; Gaffey, M.F.; Kamali, M.; Jain, R.P.; Als, D.; Meteke, S.; Radhakrishnan, A.; Siddiqui, F.J.; Ataullahjan, A.; et al. Delivering non-communicable disease interventions to women and children in conflict settings: A systematic review. BMJ Glob. Health 2020, 5, e002047.
- Aminde, L.N.; Dzudie, A.; Mapoure, Y.N.; Tantchou, J.C.; Veerman, J.L. Estimation and determinants of direct medical costs of ischaemic heart disease, stroke and hypertensive heart disease: Evidence from two major hospitals in Cameroon. BMC Health Serv. Res. 2021, 21, 140.
- Bassatne, A.; Harb, H.; Jaafar, B.; Romanos, J.; Ammar, W.; El-Hajj Fuleihan, G. Disease burden of osteoporosis and other non-communicable diseases in Lebanon. Osteoporos. Int. 2020, 31, 1769–1777.
- Yahaya, I.; Wright, T.; Babatunde, O.O.; Corp, N.; Helliwell, T.; Dikomitis, L.; Mallen, C.D. Prevalence of osteoarthritis in lower middle- and low-income countries: A systematic review and meta-analysis. Rheumatol. Int. 2021, 41, 1221–1231.
- Hwalla, N.; Jaafar, Z.; Sawaya, S. Dietary management of type 2 diabetes in the MENA region: A review of the evidence. Nutrients 2021, 13, 1060.
- Filippov, M.; Tatarnikova, O.; Pozdnyakova, N.; Vorobyov, V. Inflammation /bioenergetics-associated neurodegenerative pathologies and concomitant diseases: A role of mitochondria targeted catalase and xanthophylls. Neural Regen. Res. 2021, 16, 223–233.
- Shahidi, F.; Yeo, J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573.
- Kakkar, S.; Tandon, R.; Tandon, N. Utilizing fruits and vegetables waste as functional food: A review. Plant Cell. Biotechnol. Mol. Biol. 2021, 22, 41–58.
- Ramírez-Pulido, B.; Bas-Bellver, C.; Betoret, N.; Barrera, C.; Seguí, L. Valorization of vegetable fresh-processing residues as functional powdered ingredients. A review on the potential impact of pretreatments and drying methods on bioactive compounds and their bioaccessibility. Front. Sustain. Food Syst. 2021, 5, 654313.
- Food and Agriculture Organization of the United Nations (FAO). Food Loss and Waste Database; FAO: Rome, Italy, 2019; Available online: http://www.fao.org/platform-food-loss-waste/flw-data/en/ (accessed on 31 July 2021).
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary fiber from underutilized plant resources-A positive approach for valorization of fruit and vegetable wastes. Sustainability 2020, 12, 5401.
- Yadav, S.K.; Kauldhar, B.S.; Sandhu, P.P.; Thakur, K.; Sucheta; Sharma, T.R. Retrospect and prospects of secondary agriculture and bioprocessing. J. Plant Biochem. Biotechnol. 2020, 29, 1–14.
- Rodríguez-García, S.L.; Raghavan, V. Green extraction techniques from fruit and vegetable waste to obtain bioactive compounds—A review. Crit. Rev. Food Sci. Nutr. 2021, 1, 1–21.
- Fernández-Sestelo, A.B.; Sendra de Saa, R.; Pérez-Lamela, C.; Torrado-Agrasar, A.; Rúa, M.L.; Pastrana-Castro, L. Overall quality properties in pressurized kiwi purée: Microbial, physicochemical, nutritive and sensory test during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2013, 20, 64–72.
- Panwar, D.; Saini, A.; Panesar, P.S.; Chopra, H.K. Unraveling the scientific perspectives of citrus by-products utilization: Progress towards circular economy. Trends Food Sci. Technol. 2021, 111, 549–562.
- Khouryieh, H.A. Novel and emerging technologies used by the U.S. food processing industry. Innov. Food Sci. Emerg. Technol. 2021, 67, 102559.
- Wootton-Beard, P.C.; Ryan, L. Improving public health? The role of antioxidant-rich fruit and vegetable beverages. Food Res. Int. 2011, 44, 3135–3148.
- Baranda, A.B.; Montes, P. HPP for improving preservation of vitamin and antioxidant contents in vegetable matrices. In Present and Future of High Pressure Processing: A Tool for Developing Innovative, Sustainable, Safe and Healthy Foods; Barba, F.J., Tonello-Samson, C., Puértolas, E., Lavilla, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 2; pp. 15–70.
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3.
- Capanoglu, E.; Kamiloglu, S.; Ozkan, G.; Apak, R. Evaluation of antioxidant activity/capacity measurement methods for food products. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications, 1st ed.; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2018; Chapter 13; pp. 273–286.
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715.
- Jideani, A.I.O.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyasi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67.
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant activity and healthy benefits of natural pigments in fruits: A Review. Int. J. Mol. Sci. 2021, 22, 4945.
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential health benefits of plant food-derived bioactive components: An overview. Foods 2021, 10, 839.
- Apak, R. Current issues in antioxidant measurement. J. Agric. Food Chem. 2019, 67, 9187–9202.
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10.
- Yang, C.S.; Ho, C.T.; Zhang, J.; Wan, X.; Zhang, K.; Lim, J. Antioxidants: Differing meanings in food science and health science. J. Agric. Food Chem. 2018, 66, 3063–3068.
- Prior, R.L.; Wu, X.L.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302.
- Sun, Y.; Yang, C.; Tsao, R. Nomenclature and general classification of antioxidant activity/capacity assays. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications, 1st ed.; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2018; Chapter 1; pp. 1–19.
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781.
- Pisoschi, A.M.; Negulescu, G.P. Methods for total antioxidant activity determination: A review. Biochem. Anal. Biochem. 2011, 1, 106.
- Włodarska, K.; Pawlak-Lemańska, K.; Khmelinskii, I.; Sikorska, E. Explorative study of apple juice fluorescence in relation to antioxidant properties. Food Chem. 2016, 210, 593–599.
- Juárez-Gómez, J.; Ramírez-Silva, M.T.; Guzmán-Hernández, D.S.; Romero-Romo, M.; Palomar-Pardavé, M. Novel electrochemical method to evaluate the antioxidant capacity of infusions and beverages, based on in situ formation of free superoxide radicals. Food Chem. 2020, 332, 127409.
- Yeo, J.; Shahidi, F. Critical re-evaluation of DPPH assay: Presence of pigments affects the results. J. Agric. Food Chem. 2019, 67, 7526–7529.
- Aguilera, Y.; Martin-Cabrejas, M.A.; González de Mejía, E. Phenolic compounds in fruits and beverages consumed as part of the Mediterranean diet: Their role in prevention of chronic diseases. Phytochem. Rev. 2016, 15, 405–423.
- Barba, F.J.; Esteve, M.J.; Frígola, A. Bioactive Components from Leaf Vegetable Products. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 11; Volume 41, pp. 321–346.
- Shashirekha, M.N.; Mallikarjuna, S.E.; Rajarathnam, S. Status of bioactive compounds in foods, with focus on fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2015, 55, 1324–1339.
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy effects of plant polyphenols: Molecular mechanisms. Int. J. Mol. Sci. 2020, 21, 1250.
- Schreiner, M.; Huyskens-Keil, S. Phytochemicals in fruit and vegetables: Health promotion and postharvest elicitors. Crit. Rev. Plant Sci. 2006, 25, 267–278.
- Singh, R.; Kumari, N. Comparative determination of phytochemicals and antioxidant activity from leaf and fruit of Sapindus mukorrossi Gaertn.—A valuable medicinal tree. Ind. Crops Prod. 2015, 73, 1–8.
- Mozos, I.; Flangea, C.; Vlad, D.C.; Gug, C.; Mozos, C.; Stoian, D.; Luca, C.T.; Horbańczuk, J.O.; Horbańczuk, O.K.; Atanasov, A.G. Effects of anthocyanins on vascular health. Biomolecules 2021, 11, 811.
- Wang, Y.; Lin, J.; Tian, J.; Si, X.; Jiao, X.; Zhang, W.; Gong, E.; Li, B. Blueberry malvidin-3-galactoside suppresses hepatocellular carcinoma by regulating apoptosis, proliferation, and metastasis pathways in vivo and in vitro. J. Agric. Food Chem. 2019, 67, 625–636.
- Cefali, L.C.; Franco, J.G.; Nicolini, G.F.; Ataide, J.A.; Mazzola, P.G. In vitro antioxidant activity and solar protection factor of blackberry and raspberry extracts in topical formulation. J. Cosmet. Dermatol. 2019, 18, 539–544.
- Concha-Meyer, A.A.; Sepúlveda, G.; Pérez-Díaz, R.; Torres, C.A. Effect of preservation processing on quality attributes and phenolic profile of maqui (Aristotelia chilensis mol. Stuntz) fruit. LWT Food Sci. Technol. 2021, 149, 111920.
- Marszałek, K.; Woźniak, Ł.; Kruszewski, B.; Skapska, S. The effect of high pressure techniques on the stability of anthocyanins in fruit and vegetables. Int. J. Mol. Sci. 2017, 18, 277.
- Yi, Y.-S. Flavonoids: Nutraceuticals for rheumatic diseases via targeting of inflammasome activation. Int. J. Mol. Sci. 2021, 22, 488.
- Khazaei, H.; Pesce, M.; Patruno, A.; Aneva, I.Y.; Farzaei, M.H. Medicinal plants for diabetes associated neurodegenerative diseases: A systematic review of preclinical studies. Phytother. Res. 2021, 35, 1697–1718.
- Liu, S.; Xiao, P.; Kuang, Y.; Hao, J.; Huang, T.; Liu, E. Flavonoids from sea buckthorn: A review on phytochemistry, pharmacokinetics and role in metabolic diseases. J. Food Biochem. 2021, 45, e13724.
- Kaulmann, A.; Jonville, M.-C.; Schneider, Y.-J.; Hoffmann, L.; Bohn, T. Carotenoids, polyphenols and micronutrient profiles of Brassica oleraceae and plum varieties and their contribution to measures of total antioxidant capacity. Food Chem. 2014, 155, 240–250.
- Khoo, H.E.; Azlan, A.; Ismail, A.; Abas, F. Influence of different extraction media on phenolic contents and antioxidant capacity of defatted Dabai (Canarium odontophyllum) fruit. Food Anal. Methods 2012, 5, 339–350.
- Biehler, E.; Kaulmann, A.; Hoffmann, L.; Krause, E.; Bohn, T. Dietary and host-related factors influencing carotenoid bioaccessibility from spinach (Spinacia oleracea). Food Chem. 2011, 125, 1328–1334.
- Tewari, S.; Sehrawat, R.; Nema, P.K.; Kaur, B.P. Preservation effect of high pressure processing on ascorbic acid of fruits and vegetables: A review. J. Food Biochem. 2017, 41, e12319.
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741.
- Morris, M.C.; Schneider, J.A.; Tangney, C.C.; Nag, S.; Bennett, D.A.; Honer, W.G.; Barnes, L.L. Brain tocopherols related to Alzheimer disease neuropathology in humans. Alzheimer’s Dement. 2015, 11, 32–39.
- López-Gámez, G.; Élez-Martínez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Recent advances toward the application of non-thermal technologies in food processing: An insight on the bioaccessibility of health-related constituents in plant-based products. Foods 2021, 10, 1538.
- Thakur, N.; Raigond, P.; Singh, Y.; Mishra, T.; Singh, B.; Lal, M.K.; Dutt, S. Recent updates on bioaccessibility of phytonutrients. Trends Food Sci. Technol. 2020, 97, 366–380.
- Mieszczakowska-Frąc, M.; Celejewska, K.; Płocharski, W. Impact of innovative technologies on the content of vitamin C and its bioavailability from processed fruit and vegetable products. Antioxidants 2021, 10, 54.
- Desmarchelier, C.; Borel, P. Overview of carotenoid bioavailability determinants: From dietary factors to host genetic variations. Trends Food Sci. Technol. 2017, 69, 270–280.
- Schweiggert, R.M.; Carle, R. Carotenoid deposition in plant and animal foods and its impact on bioavailability. Crit. Rev. Food Sci. Nutr. 2017, 57, 1807–1830.
- Melse-Boonstra, A. Bioavailability of micronutrients from nutrient-dense whole foods: Zooming in on dairy, vegetables, and fruits. Front. Nutr. 2020, 7, 101.
- Golovinskaia, O.; Wang, C.-K. Review of functional and pharmacological activities of berries. Molecules 2021, 26, 3904.
- Starowicz, M.; Achrem-Achremowicz, B.; Piskuła, M.K.; Zieliński, H. Phenolic compounds from apples: Reviewing their occurrence, absorption, bioavailability, processing, and antioxidant activity—A review. Pol. J. Food Nutr. Sci. 2020, 70, 321–336.
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849.
- Cilla, A.; Bosch, L.; Barberá, R.; Alegría, A. Effect of processing on the bioaccessibility of bioactive compounds-A review focusing on carotenoids, minerals, ascorbic acid, tocopherols and polyphenols. J. Food Comp. Anal. 2018, 68, 3–15.
- Ghazzawi, H.A.; Al-Sayyed, H.F.; Al-Kurd, R.A.; Mwalla, M.M.; Arafat, T.A.; AbdelQader, S.M. Effect of different extraction solvents on the antioxidant content and capacity of nine seasonal fruits. Clin. Nutr. Open Sci. 2021, 38, 33–42.
- Hosen, M.Z.; Biswas, A.; Islam, M.R.; Nazrul, M.; Bhuiyan, I.; Hossain, S.J. Comparison of physicochemical and antioxidant properties of edible fruits in the sundarbans’ mangrove forest, Bangladesh. Bangladesh J. Bot. 2020, 49, 671–678.
- Kolla, M.C.; Laya, A.; Bayang, J.P.; Koubala, B.B. Effect of different drying methods and storage conditions on physical, nutritional, bioactive compounds and antioxidant properties of doum (Hyphaene thebaica) fruits. Heliyon 2021, 7, e06678.
- Suwanwong, Y.; Boonpangrak, S. Phytochemical contents, antioxidant activity, and anticancer activity of three common guava cultivars in Thailand. Eur. J. Integr. Med. 2021, 42, 101290.
- Liu, Z.; Liu, B.; Wen, H.; Tao, Y.; Shao, Y. Phytochemical profiles, nutritional constituents and antioxidant activity of black wolfberry (Lycium ruthenicum Murr.). Ind. Crops Prod. 2020, 154, 112692.
- Żebrowska, J.; Dyduch-Siemińska, M.; Gawroński, J.; Jackowska, I.; Pabich, M. Genetic estimates of antioxidant properties in the conventionally and in vitro propagated strawberry (Fragaria ananassa Duch.). Food Chem. 2019, 299, 125110.
- da Franca, L.G.; Alves Filho, E.; Ribeiro, L.B.; Evangelista, J.S.B.; Silva, L.M.; de Souza, P.A.; Moura, C.F.H.; Canuto, K.M.; de Aragão, F.A.S. Metabolomic profiling of acerola clones according to the ripening stage. J. Food Meas. Charact. 2021, 15, 416–424.
- Xu, M.; Shen, C.; Zheng, H.; Xu, Y.; Xue, C.; Zhu, B.; Hu, J. Metabolomic analysis of acerola cherry (Malpighia emarginata) fruit during ripening development via UPLC-Q-TOF and contribution to the antioxidant activity. Food Res Int. 2020, 130, 108915.
- Mansour, R. Determination of nutritional composition in citrus fruits (C. aurantium) during maturity. Nutr. Food Sci. 2019, 49, 299–317.
- Zacarías-García, J.; Rey, F.; Gil, J.-V.; Rodrigo, M.J.; Zacarías, L. Antioxidant capacity in fruit of citrus cultivars with marked differences in pulp coloration: Contribution of carotenoids and vitamin C. Food Sci. Tecnol. Int. 2021, 27, 210–222.
- Mertoglu, K.; Eskimez, I.; Polat, M.; Okatan, V.; Korkmaz, N.; Gulbandilar, A.; Bulduk, I. Determination of anti-microbial and phyto-chemical characteristics of some blackberry cultivars. Fresen. Environ. Bull. 2021, 30, 1789–1795.
- Barba, F.J.; Terefe, N.S.; Buckow, R.; Knorr, D.; Orlien, V. New opportunities and perspectives of high pressure treatment to improve health and safety attributes of foods. A review. Food Res. Int. 2015, 77, 725–742.
- Jung, S.; Tonello-Samson, C. High Hydrostatic Pressure food processing: Potential and limitations. In Alternatives to Conventional Food Processing, 2nd ed.; Proctor, A., Ed.; RSC Publishing: Cambridge, UK, 2018; Chapter 7; Volume 53, pp. 251–315.
- Roobab, U.; Shabbir, M.A.; Khan, A.W.; Arshad, R.N.; Bekhit, A.E.; Zeng, X.A.; Inam-Ur-Raheem, M.; Aadil, R.M. High-pressure treatments for better quality clean-label juices and beverages: Overview and advances. LWT Food Sci. Technol. 2021, 149, 111828.
- Serment-Moreno, V. Microbial modeling needs for the Nonthermal Processing of Foods. Food Eng. Rev. 2020, 11, 1–25.
- Daher, D.; Le Gourrierec, S.; Pérez-Lamela, C. Effect of High Pressure Processing on the microbial inactivation in fruit preparations and other vegetable based beverages. Agriculture 2017, 7, 72.
- Huang, H.W.; Wu, S.J.; Lu, J.K.; Shyu, Y.T.; Wang, C.Y. Current status and future trends of high-pressure processing in food industry. Food Control 2017, 72 Pt A, 1–8.
- Nielsen, H.B.; Sonne, A.-M.; Grunert, K.G.; Banati, D.; Pollak-Toth, A.; Lakner, Z.; Olsen, N.V.; Zontar, T.P.; Peterman, M. Consumer perception of the use of high-pressure processing and pulsed electric field technologies in food production. Appetite 2009, 52, 115–126.
- Plaza, L.; Colina, C.; de Ancos, B.; Sánchez-Moreno, C.; Cano, M.P. Influence of ripening and astringency on carotenoid content of high-pressure treated persimmon fruit (Diospyros kaki L.). Food Chem. 2012, 130, 591–597.
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220.
- Mukhopadhyay, S.; Sokorai, K.; Ukuku, D.; Fan, X.; Juneja, V. Effect of high hydrostatic pressure processing on the background microbial loads and quality of cantaloupe purée. Food Res. Int. 2017, 91, 55–62.
- Shen, J.; Gou, Q.; Zhang, Z.; Wang, M. Effects of high hydrostatic pressure on the quality and shelf-life of jujube (Ziziphus jujuba Mill.) pulp. Innov. Food Sci. Emerg. Technol. 2016, 36, 166–172.
- Hu, K.; Peng, D.; Wang, L.; Liu, H.; Xie, B.; Sun, Z. Effect of mild high hydrostatic pressure treatments on physiological and physicochemical characteristics and carotenoid bio-synthesis in postharvest mango. Postharvest Biol. Technol. 2021, 172, 111381.
- Camiro-Cabrera, M.; Escobedo-Avellaneda, Z.; Salinas-Roca, B.; Martín-Belloso, O.; Welti-Chanes, J. High hydrostatic pressure and temperature applied to preserve the antioxidant compounds of mango pulp (Mangifera indica L.). Food Bioprocess Technol. 2017, 10, 639–649.
- García-Cayuela, T.; Quiles, A.; Hernando, I.; Welti-Chanes, J.; Cano, M.P. Changes in bioactive compounds and microstructure in persimmon (Diospyros kaki L.) treated by high hydrostatic pressures during cold storage. J. Food Process. Preserv. 2018, 42, e13738.
- García-Parra, J.; González-Cebrino, F.; Delgado-Adámez, J.; Cava, R.; Martín-Belloso, O.; Élez-Martínez, P.; Ramírez, R. Effect of high-hydrostatic pressure and moderate-intensity pulsed electric field on plum. Food Sci. Technol. Int. 2018, 24, 145–160.
- Gao, G.; Rena, P.; Cao, X.; Yana, B.; Liao, X.; Suna, Z.; Wang, Y. Comparing quality changes of cupped strawberry treated by high hydrostatic pressure and thermal processing during storage. Food Bioprod. Process. 2016, 100, 221–229.
- Marszałek, K.; Woźniak, Ł.; Skąpska, S.; Mitek, M. High pressure processing and thermal pasteurization of strawberry purée: Quality parameters and shelf-life evaluation during cold storage. J. Food Sci. Technol. 2017, 54, 832–841.
- Marszałek, K.; Mitek, M.; Skąpska, S. The effect of thermal pasteurization and high pressure processing at cold and mild temperatures on the chemical composition, microbial and enzyme activity in strawberry purée. Innov. Food Sci. Emerg. Technol. 2015, 27, 48–56.
- Deng, H.; Lei, J.; Yang, T.; Liu, M.; Meng, Y.; Guo, Y.; Xue, J. Effect of ultra-high pressure and high temperature short-time sterilization on the quality of NFC apple juice during storage. Sci. Agric. Sin. 2019, 52, 3903–3923.
- Szczepanska, J.; Pinto, C.A.; Skąpska, S.; Saraiva, J.A.; Marszałek, K. Effect of static and multi-pulsed high pressure processing on the rheological properties, microbial and physicochemical quality, and antioxidant potential of apple juice during refrigerated storage. LWT Food Sci. Technol. 2021, 150, 112038.
- Hasni, H.N.; Koh, P.C.; Noranizan, M.A.; Tahir, P.N.F.M.M.; Mohamad, A.; Limpot, N.; Hamid, N.; Aadil, R.M. High-pressure processing treatment for ready-to-drink Sabah Snake Grass juice. J. Food Process. Preserv. 2020, 44, e14508.
- Błaszczak, W.; Amarowicz, R.; Górecki, A.R. Antioxidant capacity, phenolic composition and microbial stability of aronia juice subjected to high hydrostatic pressure processing. Innov. Food Sci. Emerg. Technol. 2017, 39, 141–147.
- Queirós, R.P.; Rainho, D.; Santos, M.D.; Fidalgo, L.G.; Delgadillo, I.; Saraiva, J.A. High pressure and thermal pasteurization effects on sweet cherry juice microbiological stability and physicochemical properties. High Press. Res. 2015, 35, 69–77.
- Nayak, P.K.; Rayaguru, K.; Radha, K. Quality comparison of elephant apple juices after high-pressure processing and thermal treatment. J. Sci. Food Agric. 2017, 97, 1404–1411.
- Hu, Y.H.; Wang, C.Y.; Chen, B.Y. Effects of high-pressure processing and thermal pasteurization on quality and microbiological safety of jabuticaba (Myrciaria cauliflora) juice during cold storage. J. Food Sci. Technol. 2020, 57, 3334–3344.
- Zhao, L.; Wang, Y.; Hu, X.; Sun, Z.; Liao, X. Korla pear juice treated by ultrafiltration followed by high pressure processing or high temperature short time. LWT-Food Sci. Technol. 2016, 65, 283–289.
- Chaikham, P. Comparison of high hydrostatic pressure and thermal processing on physicochemical and antioxidant properties of Maoberry (Antidesma thwaitesianum Müell. Arg.) juice. Int. Food Res. J. 2015, 22, 1993–2001.
- Zou, H.; Lin, T.; Bi, X.; Zhao, L.; Wang, Y.; Liao, X. Comparison of High Hydrostatic Pressure, High-Pressure Carbon Dioxide and High-Temperature Short-Time Processing on quality of Mulberry Juice. Food Bioprocess Technol. 2016, 9, 217–231.
- Vieira, F.N.; Lourenço, S.; Fidalgo, L.G.; Santos, S.A.O.; Silvestre, A.J.D.; Jerónimo, E.; Saraiva, J.A. Long-term effect on bioactive components and antioxidant activity of thermal and high-pressure pasteurization of orange juice. Molecules 2018, 23, 2706.
- Spira, P.; Bisconsin-Junior, A.; Rosenthal, A.; Monteiro, M. Effects of high hydrostatic pressure on the overall quality of Pêra-Rio orange juice during shelf-life. Food Sci. Technol. Int. 2018, 24, 507–518.
- Pasini, F.; Riciputi, Y.; Fiorini, M.; Caboni, M.F. Effect of the storage time and packaging material on the antioxidant capacity and phenols content of organic grape juice stabilized by high hydrostatic pressure. Chem. Eng. Trans. 2019, 75, 235–240.
- Quiroz-González, B.; Rodríguez-Martínez, V.; Welti-Chanes, J.; del García-Mateos, M.R.; Corrales-García, J.; Ybarra-Moncada, M.C.; Leyva-Ruelas, G.; Torres, J.A. Refrigerated storage of high hydrostatic pressure (HHP) treated pitaya (Stenocereus pruinosus) juice. Rev. Mex. Ing. Quim. 2020, 19, 387–399.
- Gao, G.; Zhao, L.; Ma, Y.; Wang, Y.; Sun, Z.; Liao, X. Microorganisms and some quality of red grapefruit juice affected by High Pressure Processing and High Temperature Short Time. Food Bioprocess Technol. 2015, 8, 2096–2108.
- Feng, X.; Zhou, Z.; Wang, X.; Bi, X.; Ma, Y.; Xing, Y. Comparison of high hydrostatic pressure, ultrasound, and heat treatments on the quality of strawberry–apple–lemon juice blend. Foods 2020, 9, 218.
- Jez, M.; Blaszczak, W.; Penkacik, K.; Amarowicz, R. Quality parameters of juice obtained from hydroponically grown tomato processed with high hydrostatic pressure or heat pasteurization. Int. J. Food Sci. 2020, 2020, 4350461.
- Chang, Y.H.; Wu, S.J.; Chen, B.Y.; Huang, H.W.; Wang, C.Y. Effect of high-pressure processing and thermal pasteurization on overall quality parameters of white grape juice. J. Sci. Food Agric. 2017, 97, 3166–3172.
- Torres-Ossandón, M.J.; Castillo, L.; Ah-Hen, K.S.; Vega-Gálvez, A. Effect of high hydrostatic pressure processing on phytochemicals, antioxidant activity, and behavior of Botrytis cinerea in white grape juice concentrate. J. Food Process. Preserv. 2020, 44, e14864.
- Sánchez-Moreno, C.; De Ancos, B. High-pressure processing effect on nutrients and their stability. In High-Pressure Processing of Fruit and Vegetable Products; Houska, M., Ed.; CRC Press: New York, NY, USA, 2017; pp. 84–104.
- Chen, D.; Pang, X.; Zhao, J.; Gao, L.; Liao, X.; Wu, J.; Li, Q. Comparing the effects of high hydrostatic pressure and high temperature short time on papaya beverage. Innov. Food Sci. Emerg. Technol. 2015, 32, 16–28.
- Andrés, V.; Mateo, L.; Guillamón, E.; Villanueva, M.J.; Tenorio, M.D. High hydrostatic pressure treatment and storage of soy-smoothies: Colour, bioactive compounds and antioxidant capacity. LWT Food Sci. Technol. 2016, 69, 123–130.
- Elizondo-Montemayor, L.; Ramos-Parra, P.A.; Jacobo-Velázquez, D.A.; Treviño-Saldaña, N.; Marín-Obispo, L.M.; Ibarra-Garza, I.P.; Garcia-Amezquita, L.E.; del Follo-Martínez, A.; Welti-Chanes, J.; Hernández-Brenes, C. High hydrostatic pressure stabilized micronutrients and shifted dietary fibers, from insoluble to soluble, producing a low-glycemic index mango pulp. CYTA J. Food 2020, 18, 203–215.
- Gopal, K.R.; Kalla, A.M.; Srikanth, K. High Pressure Processing of fruits and vegetable products: A review. Int. J. Pure Appl. Biosci. 2017, 5, 680–692.
- Fernández, M.V.; Denoya, G.I.; Agueero, M.V.; Vaudagna, S.R.; Jagus, R.J. Quality preservation and safety ensurement of a vegetable smoothie by high-pressure processing. J. Food Process. Preserv. 2019, 44, e14326.
- Hurtado, A.; Picouet, P.; Jofré, A.; Guàrdia, M.D.; Ros, J.M.; Bañón, S. Application of High Pressure Processing for obtaining “fresh-like” fruit smoothies. Food Bioprocess Technol. 2015, 8, 2470–2482.
- da Silveira, T.F.F.; Cristianini, M.; Kuhnle, G.G.; Ribeiro, A.B.; Filho, J.T.; Godoy, H.T. Anthocyanins, non-anthocyanin phenolics, tocopherols and antioxidant capacity of açaí juice (Euterpe oleracea) as affected by high pressure processing and thermal pasteurization. Innov. Food Sci. Emerg. Technol. 2019, 55, 88–96.
- Andrés, V.; Villanueva, M.J.; Tenorio, M.D. Influence of high pressure processing on microbial shelf-life, sensory profile, soluble sugars, organic acid, and mineral content of milk- and soy-smoothies. LWT Food Sci. Tecnol. 2016, 65, 98–105.
- Rinaldi, M.; Littardi, P.; Paciulli, M.; Ganino, T.; Cocconi, E.; Barbanti, D.; Rodolfi, M.; Aldini, A.; Chiavaro, E. Impact of ohmic heating and high pressure processing on qualitative attributes of ohmic treated peach cubes in syrup. Foods 2020, 9, 1093.
- Sravani, V.J.; Ravi, N.; Roopa, N.; Kumar, S.; Pandey, A.K.; Chauhan, O.P. Use of high pressure technology for the development of novel jam and its quality evaluation during storage. J. Food Sci. Technol. 2017, 54, 3562–3568.
- Shinwari, K.J.; Rao, P.S. Rheological and physico-chemical properties of a reduced-sugar sapodilla (Manilkara zapota L.) jam processed under high-hydrostatic pressure. J. Food Process. Eng. 2020, 43, e13388.
- Elbrhami, A.A. A Comparative Study of the Effects of High Hydrostatic Pressure and Ultraviolet Light on Stability, Health Related Constituents and Quality Parameters of Tiger Nut Milk. Ph.D. Thesis, The University of Guelph, Guelph, ON, Canada, December 2016.
- Swami-Hulle, N.R.; Srinivasa-Rao, P. Effect of high pressure and thermal processing on quality changes of aloe vera-litchi mixed beverage (ALMB) during storage. J. Food Sci. Technol. 2016, 53, 359–369.
- Kieling, D.D.; Barbosa-Canovas, G.V.; Prudencio, S.H. Effects of high pressure processing on the physicochemical and microbiological parameters, bioactive compounds, and antioxidant activity of a lemongrass-lime mixed beverage. J. Food Sci. Technol. 2019, 56, 409–419.
- Bansal, V.; Jabeen, K.; Rao, P.S.; Prasad, P.; Yadav, S.K. Effect of high pressure processing (HPP) on microbial safety, physicochemical properties, and bioactive compounds of whey-based sweet lime (whey-lime) beverage. J. Food Meas. Charact. 2019, 13, 454–465.
- Bi, X.; Zhou, Z.; Qin, T.; Wang, X.; Ma, Y.; Xing, Y.; Che, Z. Effects of high pressure processing (HPP) on microorganisms and the quality of mango smoothies during storage. RSC Adv. 2020, 10, 31333–31341.
- Fernández, M.V.; Denoya, G.I.; Jagus, R.J.; Vaudagna, S.R.; Aguero, M.V. Microbiological, antioxidant and physicochemical stability of a fruit and vegetable smoothie treated by high pressure processing and stored at room temperature. LWT Food Sci Technol. 2019, 105, 206–210.
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Otón, M.; Artés, F.; Artés-Hernández, F. High hydrostatic pressure treatments for keeping quality of orange vegetables smoothies. Acta Hortic. 2018, 11, 575–580.
- Hurtado, A.; Guàrdia, M.D.; Picouet, P.; Jofré, A.; Ros, J.M.; Bañón, S. Stabilization of red fruit-based smoothies by high-pressure processing. Part A. Effects on microbial growth, enzyme activity, antioxidant capacity and physical stability. J. Sci. Food Agric. 2016, 97, 770–776.
- Hurtado, A.; Guàrdia, M.D.; Picouet, P.; Jofré, A.; Ros, J.M.; Bañón, S. Stabilisation of red fruit-based smoothies by high-pressure processing. Part II: Effects on sensory quality and selected nutrients. J. Sci. Food Agric. 2016, 97, 777–783.
- Hurtado, A.; Guàrdia, M.D.; Picouet, P.; Jofré, A.; Bañón, S.; Ros, J.M. Shelf-life extension of multi-vegetables smoothies by high-pressure processing compared with thermal treatment. Part I: Microbial and enzyme inhibition, antioxidant status and physical stability. J. Food Process Preserv. 2019, 43, e14139.
- Hurtado, A.; Guàrdia, M.D.; Picouet, P.; Jofré, A.; Bañón, S.; Ros, J.M. Thermal treatment. Part II: Retention of selected nutrients and sensory quality. J. Food Process. Preserv. 2019, 43, e14210.
- Picouet, P.A.; Hurtado, A.; Jofré, A.; Bañón, S.; Ros, J.M.; Guàrdia, M.D. Effects of thermal and high-pressure treatments on the microbiological, nutritional and sensory quality of a multi-fruit smoothie. Food Bioprocess Technol. 2016, 9, 1219–1232.
- Andrés, V.; Villanueva, M.J.; Tenorio, M.D. The effect of high-pressure processing on colour, bioactive compounds, and antioxidant activity in smoothies during refrigerated storage. Food Chem. 2016, 192, 328–335.
