Salt stress significantly contributes to major losses in agricultural productivity worldwide. The sustainable approach for salinity-accrued toxicity has been explored. The use of plant growth regulators/phytohormones, mineral nutrients and other signaling molecules is one of the major approaches for reversing salt-induced toxicity in plants. Application of the signaling molecules such as nitric oxide (NO) and ethylene (ETH) and major mineral nutrient such as nitrogen (N) and sulfur (S) play significant roles in combatting the major consequences of salt stress impacts in plants. However, the literature available on gaseous signaling molecules (NO/ETH) or/and mineral nutrients (N/S) stands alone, and major insights into the role of NO or/and ETH along with N and S in plant-tolerance to salt remained unclear. Thus, this review aimed to (a) briefly overview salt stress and highlight salt-induced toxicity, (b) appraise the literature reporting potential mechanisms underlying the role of gaseous signaling molecules and mineral nutrient in salt stress tolerance, and (c) discuss NO and ETH along with N and S in relation to salt stress tolerance. In addition, significant issues that have still to be investigated in this context have been mentioned.
- FORMAT CHANGE
1. Introduction
2. Salt Stress: An Overview
Contingent to the amounts and type of salts, also the amount of sodium present and soil alkalinity, salt-affected soils can be non-saline or sodic and saline (Table 1).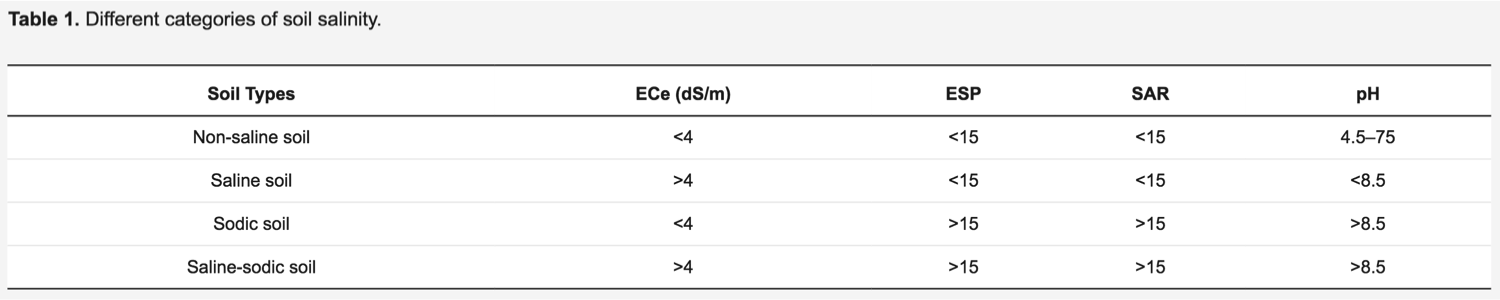 ECe, electrical conductivity; ESP, exchangeable sodium percentage (relative amount of the Na ion present on the soil surface; SAR, sodium adsorption ratio (a measure of the amount of Na relative to Ca and Mg.
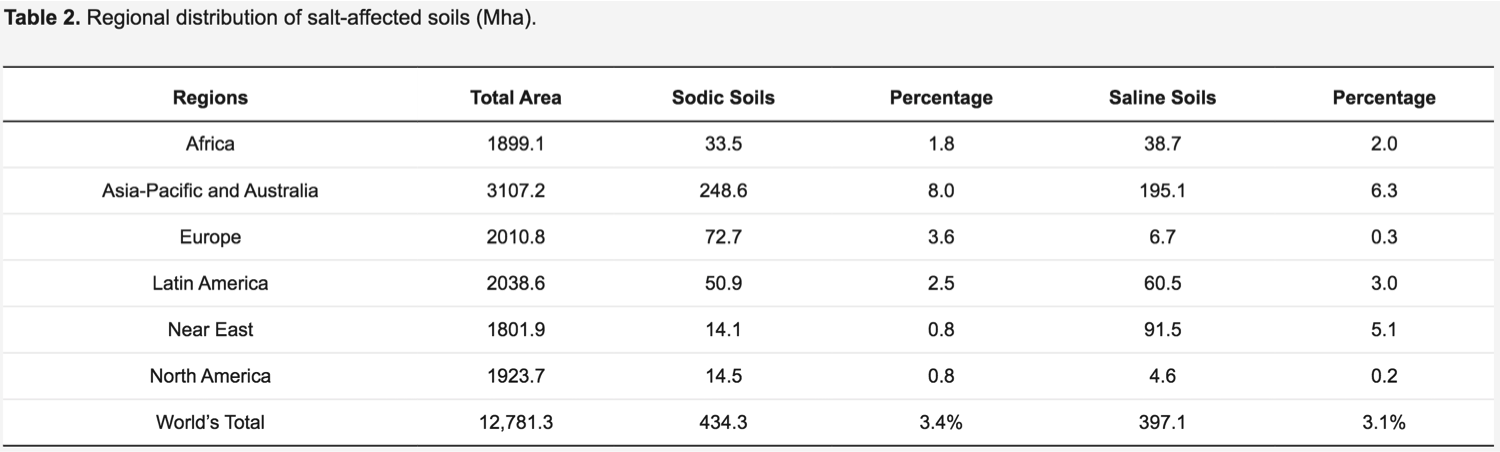
Salt stress is the issue of almost all the continents and under a wide range of climates. Conversely, this stress issue is more urgent in arid and semi-arid climates contrasted with the humid climate, where yearly precipitation is not as much as evapotranspiration in the world. At a global scale, about 12,781 Mha areas are affected by salinity and sodicity stresses. The regions with a preponderance of salt-affected soils are shown in Table 2.
ECe, electrical conductivity; ESP, exchangeable sodium percentage (relative amount of the Na ion present on the soil surface; SAR, sodium adsorption ratio (a measure of the amount of Na relative to Ca and Mg.
Salt stress is the issue of almost all the continents and under a wide range of climates. Conversely, this stress issue is more urgent in arid and semi-arid climates contrasted with the humid climate, where yearly precipitation is not as much as evapotranspiration in the world. At a global scale, about 12,781 Mha areas are affected by salinity and sodicity stresses. The regions with a preponderance of salt-affected soils are shown in Table 2.
 It is remarkable to note that ~85% of the total global area is only slightly to moderately affected by high salt concentrations; whereas, from harsh to extreme limitations for crop production occur in the remainder 15% [24]. The countries where significant salt-affected soils exist including but not limited to such as India (27%); Pakistan (28%), Israel (13%), Australia (20%), China (15%), Iraq (50%), and Egypt (30%) [25].
It is remarkable to note that ~85% of the total global area is only slightly to moderately affected by high salt concentrations; whereas, from harsh to extreme limitations for crop production occur in the remainder 15% [24]. The countries where significant salt-affected soils exist including but not limited to such as India (27%); Pakistan (28%), Israel (13%), Australia (20%), China (15%), Iraq (50%), and Egypt (30%) [25].
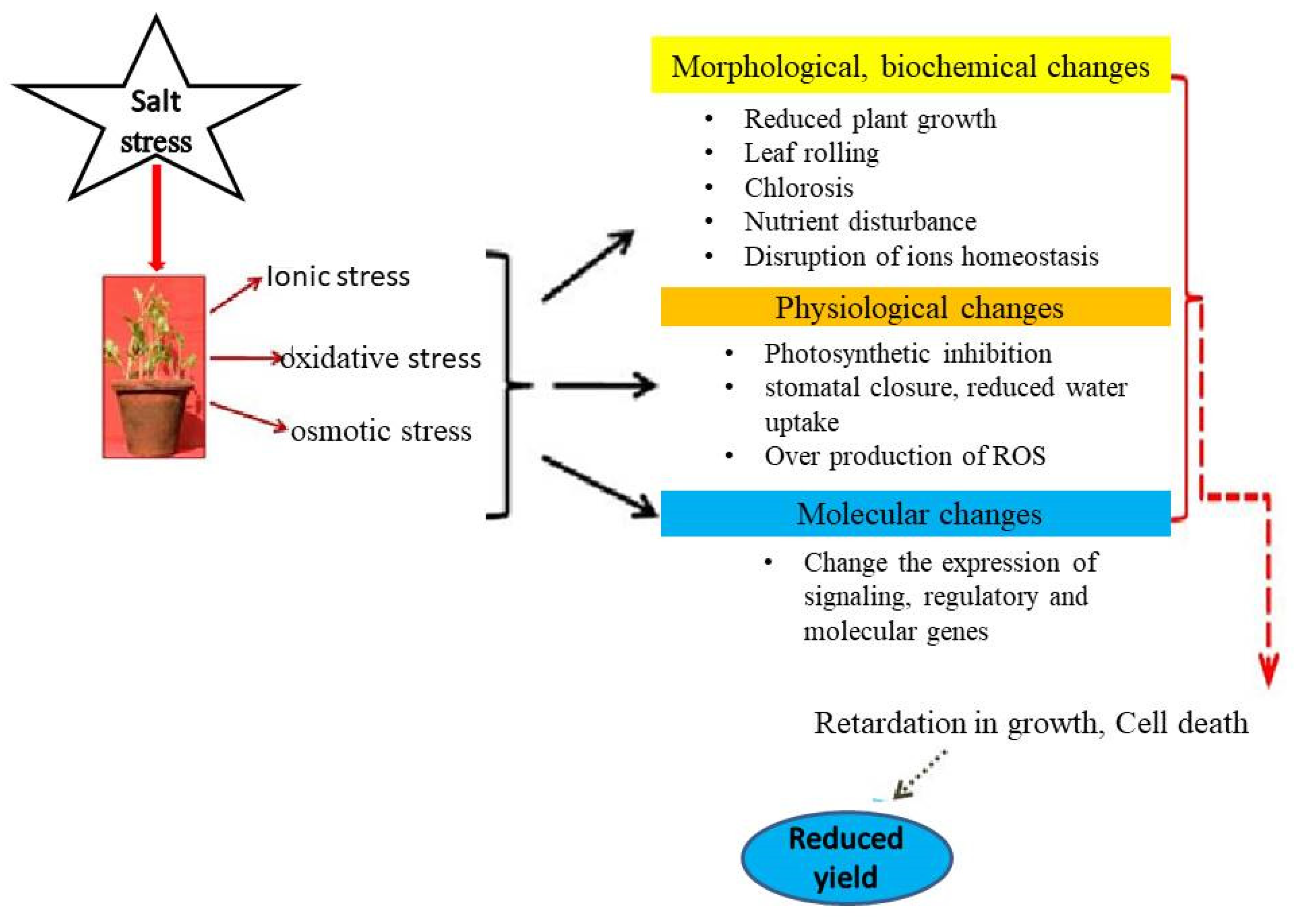
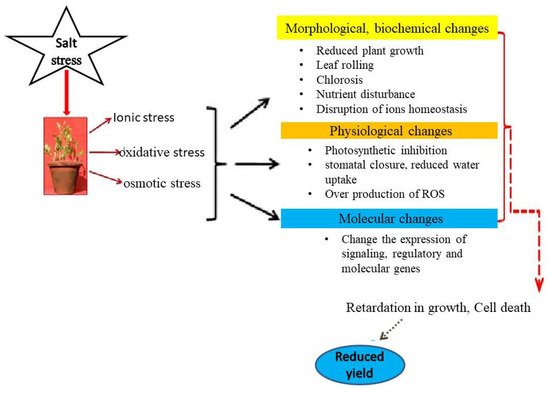
2.1. Salinity Stress Impacts in Plant
2.1. Salinity Stress Impacts in Plants
s
2.1.1. Growth and Development
2.1.2. Photosynthetic Functions
2.1.3. Oxidative Stress and Antioxidant Metabolism
2.1.4. Nutrients Uptake, Assimilation and Related Traits
2.1.5. Crop Yield
3. Nitrogen in Salt Tolerance
4. Sulfur in Salt Tolerance
5. Coordination of N and S in Salt Stress Tolerance
6. Nitric Oxide
6.1. NO Generation and Signaling
6.2. Nitric Oxide in Salt Tolerance
7. Ethylene
7.1. Ethylene Biosynthesis and Signaling
7.2. Ethylene in Salt Tolerance
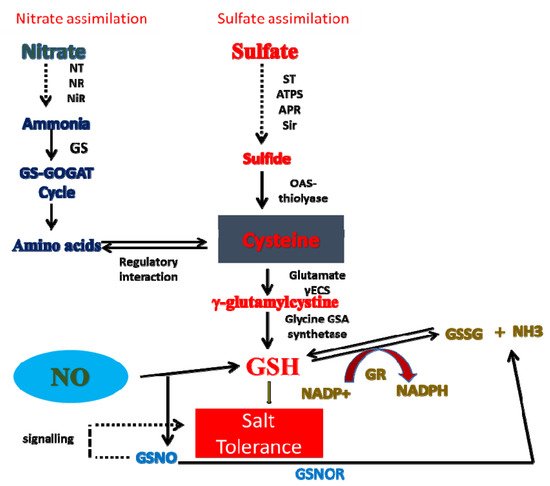
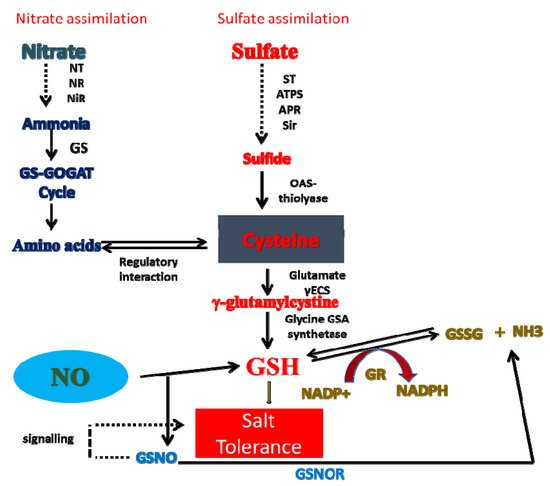
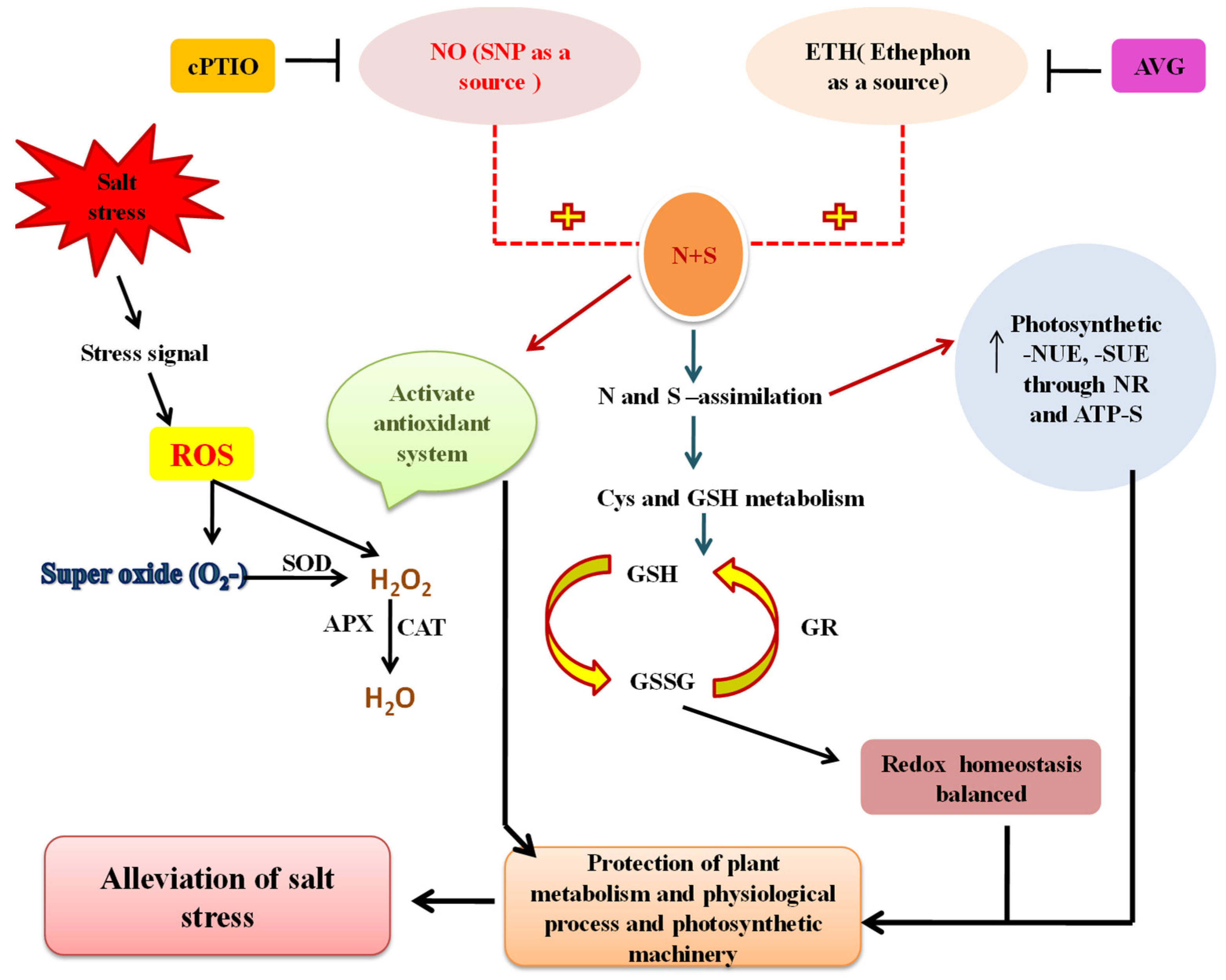
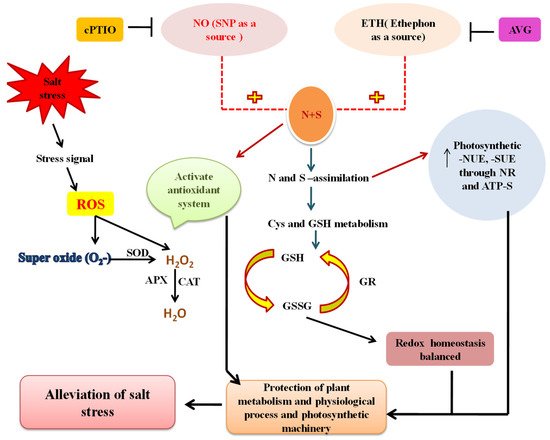
8. Crosstalk of NO with N and S in Salt Tolerance
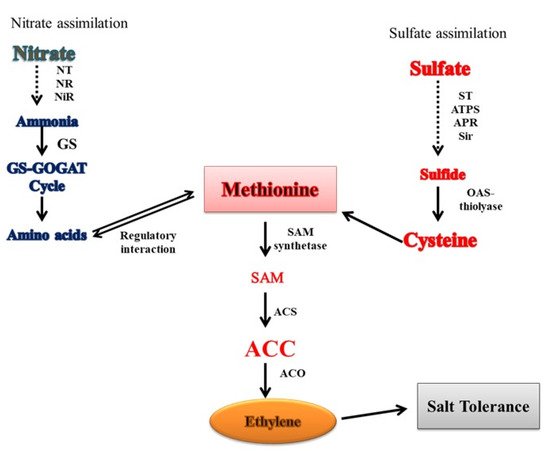
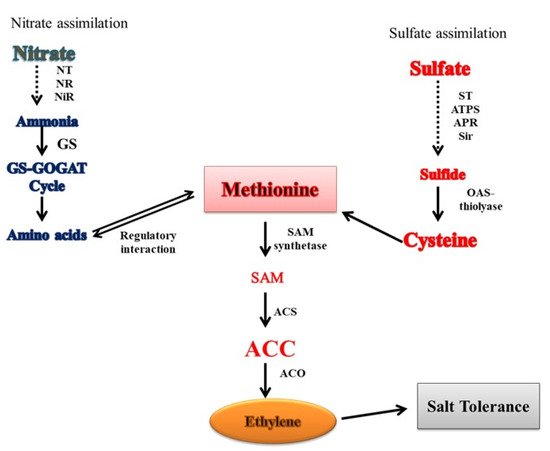
94. Crosstalk of Ethylene with N and S in Salt Tolerance
10. Conclusions: Bridging the Gaps in Understanding Salinity Tolerance
References
- Sehar, Z.; Masood, A.; Khan, N.A.; Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environmental and Experimental Botany 2019, 161, 277-289, 10.1016/j.envexpbot.2019.01.010.Sehar, Z.; Masood, A.; Khan, N.A. Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environ. Exp. Bot. 2019, 161, 277–289.
- Jahan, B.; Al Ajmi, M.F.; Rehman, M.T.; Khan, N.A.; Nitric oxide regulates photosynthetic performance and stomatal behaviour supplemented with nitrogen and sulfur in mustard under salt stress. Physiologia Plantarum 2019, 168, 490-510, 10.1111/ppl.13056.Jahan, B.; Al Ajmi, M.F.; Rehman, M.T.; Khan, N.A. Treatment of nitric oxide supplemented with nitrogen and sulfur regulates photosynthetic performance and stomatal behavior in mustard under salt stress. Physiol. Plant. 2020, 168, 490–510.
- Khanna, R.R.; Jahan, B.; Iqbal, N.; Khan, N.A.; AlAjmi, M.F.; Rehman, M.T.; Khan, M.I.R.; GABA reverses salt‐inhibited photosynthetic and growth responses through its influence on NO‐mediated nitrogen‐sulfur assimilation and antioxidant system in wheat. Journal of Biotechology 2021, 325, 73-82, 10.1016/j.jbiotec.2020.11.015.Khanna, R.R.; Jahan, B.; Iqbal, N.; Khan, N.A.; AlAjmi, M.F.; Rehman, M.T.; Khan, M.I.R. GABA reverses salt-inhibited photosynthetic and growth responses through its influence on NO-mediated nitrogen-sulfur assimilation and antioxidant system in wheat. J. Biotech. 2021, 325, 73–82.
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud,W; High Salinity Induces Different Oxidative Stress and Antioxidant Responses in Maize Seedlings Organs. Frontiers in Plant Science 2016, 7, 276, 10.3389/fpls.2016.00276.Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30.
- Wawrzyńska, A.; Sirko, A; To control and to be controlled: understanding the Arabidopsis SLIM1 function in sulfur deficiency through comprehensive investigation of the EIL protein family. Frontiers in Plant Science 2014, 5, 575, 10.3389/fpls.2014.00575.Hasanuzzaman, M.; Nahar, K.; Alam, M.; Bhowmik, P.C.; Hossain, M.; Rahman, M.M.; Fujita, M. Potential use of halophytes to remediate saline soils. Biomed. Res. Int. 2014, 2014, 589341.
- Kumar, P.; Sharma, P.K.; Soil Salinity and Food Security in India. Frontiers in Sustainable Food Systems 2020, 4, 533781, 10.3389/fsufs.2020.533781.Kumar, P.; Sharma, P.K. Soil salinity and food security in India. Front. Sustain. Syst. 2020, 4, 533781.
- Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S.; Salinity induced oxidative stress alters the physiological responses and improves the biofuel potential of green microalgae Acutodesmus dimorphus. Bioresource Technology 2017, 244, 1376-1383, 10.1016/j.biortech.2017.05.003.Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S. Salinity induced oxidative stress alters the physiological responses and improves the biofuel potential of green microalgae Acutodesmus dimorphus. Bioresour. Technol. 2017, 244, 1376–1383.
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Chen, J.T.; An Overview of Hazardous Impacts of Soil Salinity in Crops, Tolerance Mechanisms, and Amelioration through Selenium Supplementation. International Journal of Molecular Sciences 2019, 21, 148, 10.3390/ijms21010148.Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Chen, J.T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int. J. Mol. Sci. 2020, 21, 148.
- Fatma, M.; Khan, M.I.R.; Masood, A.; Khan, N.A.; Coordinate changes in assimilatory sulfate reduction are correlated to salt tolerance: Involvement of phytohormones.. Annual Review & Research in Biology 2013, 3, 267-295.Fatma, M.; Khan, M.I.R.; Masood, A.; Khan, N.A. Coordinate changes in assimilatory sulfate reduction are correlated to salt tolerance: Involvement of phytohormones. Annu. Res. Rev. Biol. 2013, 3, 267–295.
- Jahan, B.; Iqbal, N.; Fatma, M.; Sehar, Z.; Masood, A.; Sofo, A.; D’Ippolito, I.; Khan, N.A; Ethylene supplementation combined with split application of nitrogen and sulfur protects salt-inhibited photosynthesis through optimization of proline metabolism and antioxidant system in mustard (Brassica juncea L.). Plants 2021, 10, 1303, 10.3390/plants10071303.Jahan, B.; Iqbal, N.; Fatma, M.; Sehar, Z.; Masood, A.; Sofo, A.; D’Ippolito, I.; Khan, N.A. Ethylene supplementation combined with split application of nitrogen and sulfur protects salt-inhibited photosynthesis through optimization of proline metabolism and antioxidant system in mustard (Brassica juncea L.). Plants 2021, 10, 1303.
- Cuiyu Liu; Xueqing Zhao; Junxin Yan; Zhaohe Yuan; Mengmeng Gu; Effects of Salt Stress on Growth, Photosynthesis, and Mineral Nutrients of 18 Pomegranate (Punica granatum) Cultivars. Agronomy 2019, 10, 27, 10.3390/agronomy10010027.Liu, C.; Zhao, X.; Yan, J.; Yuan, Z.; Gu, M. Effects of salt stress on growth, photosynthesis, and mineral nutrients of 18 pomegranate (Punica granatum) cultivars. Agronomy 2020, 10, 27.
- Yujie Qiao; Lina Yin; Bomei Wang; Qingbo Ke; Xiping Deng; Shiwen Wang; Melatonin promotes plant growth by increasing nitrogen uptake and assimilation under nitrogen deficient condition in winter wheat. Plant Physiology and Biochemistry 2019, 139, 342-349, 10.1016/j.plaphy.2019.03.037.Qiao, Y.; Yin, L.; Wang, B.; Ke, Q.; Deng, X.; Wang, S. Melatonin promotes plant growth by increasing nitrogen uptake and assimilation under nitrogen deficient condition in winter wheat. Plant Physiol. Biochem. 2019, 139, 342–349.
- Xu, G.; Fan, X.; Miller, A.J.; Plant nitrogen assimilation and use efficiency. Annual Review of Plant Biology 2012, 63, 153-182, 10.1146/annurev-arplant-042811-105532.Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182.
- Wang, Y.; Wang, M.; Li, Y.; Wu, A.; Huang, J; Effects of arbuscular mycorrhizal fungi on growth and nitrogen uptake of Chrysanthemum morifolium under salt stress. PLoS ONE 2018, 13, e0196408, 10.1371/journal.pone.0196408.Wang, Y.; Wang, M.; Li, Y.; Wu, A.; Huang, J. Effects of arbuscular mycorrhizal fungi on growth and nitrogen uptake of Chrysanthemum morifolium under salt stress. PLoS ONE 2018, 13, e0196408.
- Hussain, S.J.; Khan, N.A.; Anjum, N.A.; Masood, A.; Khan, M.I.R.; Mechanistic Elucidation of Salicylic Acid and Sulphur-Induced Defence Systems, Nitrogen Metabolism, Photosynthetic, and Growth Potential of Mungbean (Vigna radiata) Under Salt Stress. Journal of Plant Growth Regulation 2020, 40, 1000-1016, 10.1007/s00344-020-10159-4.Hussain, S.J.; Khan, N.A.; Anjum, N.A.; Masood, A.; Khan, M.I.R. Mechanistic elucidation of salicylic acid and sulphur-induced defence systems, nitrogen metabolism, photosynthetic, and growth potential of mungbean (Vigna radiata) under salt stress. J. Plant Growth Regul. 2021, 40, 1000–1016.
- Fatma, M.; Iqbal, N.; Gautam, H.; Sehar, Z.; Sofo, A.; D’Ippolito, I.; Khan, N.A; Ethylene and Sulfur Coordinately Modulate the Antioxidant System and ABA Accumulation in Mustard Plants Under Salt Stress. Plants 2021, 10, 180, 10.3390/plants10010180.Fatma, M.; Iqbal, N.; Gautam, H.; Sehar, Z.; Sofo, A.; D’Ippolito, I.; Khan, N.A. Ethylene and sulfur coordinately modulate the antioxidant system and ABA accumulation in mustard plants under salt stress. Plants 2021, 10, 180.
- Fatma, M.; Masood, A.; Per, T.S.; Khan, N.A; Nitric Oxide Alleviates Salt Stress Inhibited Photosynthetic Performance by Interacting with Sulfur Assimilation in Mustard. Frontiers in Plant Science 2016, 7, 521-521, 10.3389/fpls.2016.00521.Fatma, M.; Masood, A.; Per, T.S.; Khan, N.A. Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front. Plant Sci. 2016, 7, 521.
- Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Freschi, L.; Corpas, F.J.; Khan, N.A; Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environmental Science and Pollution Research 2016, 24, 2273-2285, 10.1007/s11356-016-7947-8.Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Freschi, L.; Corpas, F.J.; Khan, N.A. Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ. Sci. Pollut. Res. 2017, 24, 2273–2285.
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A; Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiology and Biochemistry 2017, 115, 126-140, 10.1016/j.plaphy.2017.03.018.Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance, Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140.
- Hasanuzzaman, M.; Oku, H.; Nahar, K.; Bhuyan, M.B.; Al Mahmud, J.; Baluska, F.; Fujita, M; Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnology Reports 2018, 12, 77-92, 10.1007/s11816-018-0480-0.Hasanuzzaman, M.; Oku, H.; Nahar, K.; Bhuyan, M.B.; Al Mahmud, J.; Baluska, F.; Fujita, M. Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnol. Rep. 2018, 12, 77–92.
- Borbély, P.; Poór, P.; Tari, I; Changes in physiological and photosynthetic parameters in tomato of different ethylene status under salt stress: Effects of exogenous 1-aminocyclopropane-1-carboxylic acid treatment and the inhibition of ethylene signalling. Plant Physiology and Biochemistry 2020, 156, 345-356, 10.1016/j.plaphy.2020.09.019.Borbély, P.; Poór, P.; Tari, I. Changes in physiological and photosynthetic parameters in tomato of different ethylene status under salt stress: Effects of exogenous 1-aminocyclopropane-1-carboxylic acid treatment and the inhibition of ethylene signalling. Plant Physiol. Biochem. 2020, 156, 345–356.
- Sehar, Z.; Iqbal, N.; Khan, M.I.R.; Masood, A.; Rehman, M.T.; Hussain, A.; AlAjmi, M.F.; Ahmad, A.; Khan, N.A; Ethylene reduces glucose sensitivity and reverses photosynthetic repression through optimization of glutathione production in salt-stressed wheat (Triticum aestivum L.). Scientific Reports 2021, 11, 1-12, 10.1038/s41598-021-92086-2.Sehar, Z.; Iqbal, N.; Khan, M.I.R.; Masood, A.; Rehman, M.T.; Hussain, A.; AlAjmi, M.F.; Ahmad, A.; Khan, N.A. Ethylene reduces glucose sensitivity and reverses photosynthetic repression through optimization of glutathione production in salt-stressed wheat (Triticum aestivum L.). Sci. Rep. 2021, 11, 1–12.
- Iqbal, N.; Umar, S.; Khan, N.A.; Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). Journal of Plant Physiology 2015, 178, 84-91, 10.1016/j.jplph.2015.02.006.Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91.
- Wicke, B.; Smeets, E.; Dornburg, V.; Vashev, B.; Gaiser, T.; Turkenburg, W.; Faaij, A; The global technical and economic potential of bioenergy from salt-affected soils. Energy & Environmental Science 2011, 4, 2669-2681, 10.1039/c1ee01029h.Xu, G.Y.; Rocha, P.S.; Wang, M.L.; Xu, M.L.; Cui, Y.C.; Li, L.Y.; Zhu, Y.X.; Xia, X. A novel rice calmodulin-like gene, OsMSR2, enhances drought and salt tolerance and increases ABA sensitivity in Arabidopsis. Planta 2011, 234, 47–59.
- Rasool, S.; Hameed, A.; Azooz, M.M.; Siddiqi, T.O.; Ahmad, P; Salt stress: Causes, types and responses of plants. In Ecophysiology and Responses of Plants under Salt Stress; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer Science & Business Media: New York, NY, USA 2012, 0, 1-24.Akbarimoghaddam, H.; Galavi, M.; Ghanbari, A.; Panjehkeh, N. Salinity effects on seed germination and seedling growth of bread wheat cultivars. Trakia J. Sci. 2011, 9, 43–50.
- Xu, G.Y.; Rocha, P.S.; Wang, M.L.; Xu, M.L.; Cui, Y.C.; Li, L.Y.; Zhu, Y.X.; Xia, X; A novel rice calmodulin-like gene, OsMSR2, enhances drought and salt tolerance and increases ABA sensitivity in Arabidopsis. Planta 2011, 234, 47-59, 10.1007/s00425-011-1386-z.Sharma, P.; Sardana, V.; Banga, S.S. Salt tolerance of Indian mustard (Brassicajuncea L.) at germination and early seedling growth. Environ. Exp. Biol. 2013, 11, 39–46.
- Akbarimoghaddam, H.; Galavi, M.; Ghanbari, A.; Panjehkeh, N; Salinity effects on seed germination and seedling growth of bread wheat cultivars. Trakia Journal of Sciences 2011, 9, 43-50.Chowdhury, F.T.; Halim, M.A.; Hossain, F.; Akhtar, N. Effects of sodium chloride on germination and seedling growth of Sunflower (Helianthus annuus L.). Jahangirnagar Uni. J. Biol Sci. 2018, 7, 35–44.
- Sharma, P.; Sardana, V.; Banga, S.S; Salt tolerance of Indian mustard (Brassicajuncea L.) at germination and early seedling growth. Environmental and Experimental Biology 2013, 11, 39-46.Rahneshan, Z.; Nasibi, F.; Moghadam, A.A. Effects of salinity stress on some growth, physiological, biochemical parameters and nutrients in two pistachio (Pistaciavera L.) rootstocks. J. Plant Interact. 2018, 13, 73–82.
- Chowdhury, F.T.; Halim, M.A.; Hossain, F.; Akhtar, N; Effects of sodium chloride on germination and seedling growth of Sunflower (Helianthus annuus L.). Jahangirnagar University Journal of Biological Sciences 2018, 7, 35-44, 10.3329/jujbs.v7i1.37972.Jaarsma, R.; de Vries, R.S.; de Boer, A.H. Effect of salt stress on growth, Na+ accumulation and proline metabolism in potato (Solanum tuberosum) cultivars. PLoS ONE 2013, 8, e60183.
- Rahneshan, Z.; Nasibi, F.; Moghadam, A.A; Effects of salinity stress on some growth, physiological, biochemical parameters and nutrients in two pistachio (Pistacia vera L.) rootstocks. Journal of Plant Interactions 2018, 13, 73-82, 10.1080/17429145.2018.1424355.Faghire, M.; Mohamed, F.; Taoufiq, K.; Fghire, R.; Bargaz, A.; Mandri, B.; Oufdou, K.; Laury, A.; Drevon, J.J.; Ghoulam, C. Genotypic variation of nodules’ enzymatic activities in symbiotic nitrogen fixation among common bean (Phaseolus vulgaris L.) genotypes grown under salinity constraint. Symbiosis 2013, 60, 115–122.
- Jaarsma, R.; de Vries, R.S.; de Boer, A.H; Effect of Salt Stress on Growth, Na+ Accumulation and Proline Metabolism in Potato (Solanum tuberosum) Cultivars. PLOS ONE 2013, 8, e60183, 10.1371/journal.pone.0060183.Latrach, L.; Farissi, M.; Mouradi, M.; Makoudi, B.; Bouizgaren, A.; Ghoulam, C. Growth and nodulation of alfalfa-rhizobia symbiosis under salinity: Electrolyte leakage, stomatal conductance, and chlorophyll fluorescence. Turk. J. Agric. For. 2014, 38, 320–326.
- Faghire, M.; Mohamed, F.; Taoufiq, K.; Fghire, R.; Bargaz, A.; Mandri, B.; Oufdou, K.; Laury, A.; Drevon, J.J.; Ghoulam, C; et al. Genotypic variation of nodules’ enzymatic activities in symbiotic nitrogen fixation among common bean (Phaseolus vulgaris L.) genotypes grown under salinity constraint. Symbiosis 2013, 60, 115-122, 10.1007/s13199-013-0247-x.Javid, M.; Ford, R.; Norton, R.; Nicolas, M. Sodium and boron exclusion in two Brassica juncea cultivars exposed to the combined treatments of salinity and boron at moderate alkalinity. Biologia 2014, 69, 1157–1163.
- Latrach, L.; Farissi, M.; Mouradi, M.; Makoudi, B.; Bouizgaren, A.; Ghoulam, C; Growth and nodulation of alfalfa-rhizobia symbiosis under salinity: electrolyte leakage, stomatal conductance, and chlorophyll fluorescence. Turkish Journal of Agriculture and Forestry 2014, 38, 320-326, 10.3906/tar-1305-52.Ghanem, A.E.; Mohamed, E.; Kasem, A.M.; El-Ghamery, A.A. Differential salt tolerance strategies in three halophytes from the same ecological habitat: Augmentation of antioxidant enzymes and compounds. Plants 2021, 10, 1100.
- Javid, M.; Ford, R.; Norton, R.; Nicolas, M; Sodium and boron exclusion in two Brassica juncea cultivars exposed to the combined treatments of salinity and boron at moderate alkalinity. Biologia 2014, 69, 1157-1163, 10.2478/s11756-014-0412-6.Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 2017, 8, 295.
- Ghanem, A.E.; Mohamed, E.; Kasem, A.M.; El-Ghamery, A.A; Differential Salt Tolerance Strategies in Three Halophytes from the Same Ecological Habitat: Augmentation of Antioxidant Enzymes and Compounds. Plants 2021, 10, 1100, 10.3390/plants10061100.Mahdieh, M.; Habibollahi, N.; Amirjani, M.R.; Abnosi, M.H.; Ghorbanpour, M. Exogenous silicon nutrition ameliorates salt-induced stress by improving growth and efficiency of PSII in Oryza sativa L. cultivars. J. Soil Sci. Plant Nutr. 2015, 15, 1050–1060.
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X; et al. Exogenous Melatonin Confers Salt Stress Tolerance to Watermelon by Improving Photosynthesis and Redox Homeostasis. Frontiers in Plant Science 2017, 8, 295, 10.3389/fpls.2017.00295.Jiang, C.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Maruo, T. A correlation analysis on chlorophyll content and SPAD value in tomato leaves. Hortic. Res. 2017, 71, 37–42.
- Mahdieh, M.; Habibollahi, N.; Amirjani, M.R.; Abnosi, M.H.; Ghorbanpour, M; Exogenous silicon nutrition ameliorates salt-induced stress by improving growth and efficiency of PSII in Oryza sativa L. cultivars. Journal of Soil Science and Plant Nutrition 2015, 15, 1050-1060, 10.4067/s0718-95162015005000073.Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S. Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol. Biochem. 2010, 48, 16–20.
- Jiang, C.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Maruo, T; A correlation analysis on chlorophyll content and SPAD value in tomato leaves. Horticulture Research 2017, 71, 37-42, 10.20776/S18808824-71-P37.Kalaji, H.M.; Bosa, K.; Kościelniak, J.; Żuk-Gołaszewska, K. Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ. Exp. Bot. 2011, 73, 64–72.
- Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S; Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiology and Biochemistry 2010, 48, 16-20, 10.1016/j.plaphy.2009.10.006.Fatma, M.; Iqbal, N.; Sehar, Z.; Alyemeni, M.N.; Kaushik, P.; Khan, N.A.; Ahmad, P. Methyl jasmonate protects the ps ii system by maintaining the stability of chloroplast d1 protein and accelerating enzymatic antioxidants in heat-stressed wheat plants. Antioxidants 2021, 10, 1216.
- Kalaji, H.M.; Bosa, K.; Kos´cielniak, J.; Z˙ uk-Gołaszewska, K; Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environmental and Experimental Botany 2011, 73, 64-72, 10.1016/j.envexpbot.2010.10.009.Singh, M.; Singh, V.P.; Prasad, S.M. Responses of photosynthesis, nitrogen and proline metabolism to salinity stress in Solanum lycopersicum under different levels of nitrogen supplementation. Plant Physiol. Biochem. 2016, 109, 72–83.
- Fatma, M.; Iqbal, N.; Sehar, Z.; Alyemeni, M.N.; Kaushik, P.; Khan, N.A.; Ahmad, P; Methyl Jasmonate Protects the PS II System by Maintaining the Stability of Chloroplast D1 Protein and Accelerating Enzymatic Antioxidants in Heat-Stressed Wheat Plants. Antioxidants 2021, 10, 1216, 10.3390/antiox10081216.Nxele, X.; Klein, A.; Ndimba, B.K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S. Afr. J. Bot. 2017, 108, 261–266.
- Singh, M.; Singh, V.P.; Prasad, S.M; Responses of photosynthesis, nitrogen and proline metabolism to salinity stress in Solanum lycopersicum under different levels of nitrogen supplementation. Plant Physiology and Biochemistry 2016, 109, 72-83, 10.1016/j.plaphy.2016.08.021.Aldesuquy, H.; Baka, Z.; Mickky, B. Kinetin and spermine mediated induction of salt tolerance in wheat plants: Leaf area, photosynthesis and chloroplast ultrastructure of flag leaf at ear emergence. Egypt. J. Basic Appl. Sci. 2014, 1, 77–87.
- Nxele, X.; Klein, A.; Ndimba, B.K; Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. South African Journal of Botany 2017, 108, 261-266, 10.1016/j.sajb.2016.11.003.Wu, X.; Zhu, Z.; Li, X.; Zha, D. Effects of cytokinin on photosynthetic gas exchange, chlorophyll fluorescence parameters and antioxidative system in seedlings of eggplant (Solanum melongena L.) under salinity stress. Acta Physiol. Plant. 2012, 34, 2105–2114.
- Aldesuquy, H.; Baka, Z.; Mickky, B; Kinetin and spermine mediated induction of salt tolerance in wheat plants: Leaf area, photosynthesis and chloroplast ultrastructure of flag leaf at ear emergence. Egyptian Journal of Basic and Applied Sciences 2014, 1, 77-87, 10.1016/j.ejbas.2014.03.002.Hussain, S.; Bai, Z.; Huang, J.; Cao, X.; Zhu, L.; Zhu, C.; Zhang, J. 1-Methylcyclopropene modulates physiological, biochemical, and antioxidant responses of rice to different salt stress levels. Front. Plant Sci. 2019, 10, 124.
- Wu, X.; Zhu, Z.; Li, X.; Zha, D; Effects of cytokinin on photosynthetic gas exchange, chlorophyll fluorescence parameters and antioxidative system in seedlings of eggplant (Solanum melongena L.) under salinity stress. Acta Physiologiae Plantarum 2012, 34, 2105-2114, 10.1007/s11738-012-1010-2.Khalid, A.; Athar, H.U.R.; Zafar, Z.U.; Akram, A.; Hussain, K.; Manzoor, H.; Al-Qurainy, F.; Ashraf, M. Photosynthetic capacity of canola (Brassica napus L.) plants as affected by glycinebetaine under salt stress. J. Appl. Bot. Food Qual. 2015, 88, 78–86.
- Hussain, S.; Bai, Z.; Huang, J.; Cao, X.; Zhu, L.; Zhu, C.; Zhang, J; 1-Methylcyclopropene Modulates Physiological, Biochemical, and Antioxidant Responses of Rice to Different Salt Stress Levels. Frontiers in Plant Science 2019, 10, 124, 10.3389/fpls.2019.00124.Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Ahmad, I.; Lukatkin, A.S.; Pereira, E.; Durate, A.C.; et al. Lipids and proteins-major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Pollut. Res. 2015, 22, 4099–4121.
- Khalid, A.; Athar, H.U.R.; Zafar, Z.U.; Akram, A.; Hussain, K.; Manzoor, H.; Al-Qurainy, F.; Ashraf, M; Photosynthetic capacity of canola (Brassica napus L.) plants as affected by glycinebetaine under the salt stress. Journal of Applied Botany and Food Quality 2015, 88, 78-86, 10.5073/jabfq.2015.088.011.Tammam, A.A.; Fakhry, E.M.; El-Sheekh, M. Effect of salt stress on antioxidant system and the metabolism of the reactive oxygen species in Dunaliellasalina and Dunaliellatertiolecta. Afr. J. Biotechnol. 2011, 10, 3795–3808.
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Ahmad, I.; Lukatkin, A.S.; Pereira, E.; Durate, A.C.; et al.et al Lipids and proteins—major targets of oxidative modifications in abiotic stressed plants. Environmental Science and Pollution Research 2014, 22, 4099-4121, 10.1007/s11356-014-3917-1.AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front. Plant Sci. 2016, 7, 276.
- Tammam, A.A.; Fakhry, E.M.; El-Sheekh, M; Effect of salt stress on antioxidant system and the metabolism of the reactive oxygen species in Dunaliellasalina and Dunaliella tertiolecta. African Journal of Biotechnology 2011, 10, 3795-3808, 10.5897/AJB10.2392.Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578.
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud,W; High Salinity Induces Different Oxidative Stress and Antioxidant Responses in Maize Seedlings Organs. Frontiers in Plant Science 2016, 7, 276, 10.3389/fpls.2016.00276.Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; Ghassemi-Golezani, K. Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (‘Glycine max’ L.). Plant Omics 2012, 5, 60–67.
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A; Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses. International Journal of Molecular Sciences 2015, 16, 13561-13578, 10.3390/ijms160613561.Patel, A.D.; Jadeja, H.; Pandey, A.M. Effect of salinization of soil on growth, water status and nutrient accumulation in seedlings of Acacia auriculiformis (Fabaceae). J. Plant Nutr. 2010, 33, 914–932.
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; Ghassemi‐Golezani, K; Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (‘Glycine max’ L.). Plant Omics 2012, 5, 60-67.Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies, a review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075.
- Patel, A.D.; Jadeja, H.; Pandey, A.M; Effect of salinization of soil on growth, water status and nutrient accumulation in seedlings of Acacia auriculiformis (Fabaceae). Journal of Plant Nutrition 2010, 33, 914-932, 10.1080/01904161003669939.Ashraf, M.; Shahzad, S.M.; Imtiaz, M.; Rizwan, M.S.; Arif, M.S.; Kausar, R. Nitrogen nutrition and adaptation of glycophytes to saline environment: A review. Arch. Agron. Soil Sci. 2018, 64, 1181–1206.
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M; Effect of salinity stress on plants and its tolerance strategies: a review. Environmental Science and Pollution Research 2014, 22, 4056-4075, 10.1007/s11356-014-3739-1.Rasheed, F.; Anjum, N.A.; Masood, A.; Sofo, A.; Khan, N.A. The key roles of salicylic acid and sulfur in plant salinity stress tolerance. J. Plant Growth Regul. 2020, 1–14.
- Ashraf, M.; Shahzad, S.M.; Imtiaz, M.; Rizwan, M.S.; Arif, M.S.; Kausar, R; Nitrogen nutrition and adaptation of glycophytes to saline environment: A review. Archives of Agronomy and Soil Science 2018, 64, 1181-1206, 10.1080/03650340.2017.1419571.Wu, H.; Zhang, X.; Giraldo, J.P.; Shabala, S. It is not all about sodium: Revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant Soil 2018, 431, 1–17.
- Rasheed, F.; Anjum, N.A.; Masood, A.; Sofo, A.; Khan, N.A; The key roles of salicylic acid and sulfur in plant salinity stress tolerance. Journal of Plant Growth Regulation 2020, 1, 1-14, 10.1007/s00344-020-10257-3.Wu, H.; Zhu, M.; Shabala, L.; Zhou, M.; Shabala, S. K+ retention in leaf mesophyll, an overlooked component of salinity tolerance mechanism: A case study for barley. J. Integr. Plant Biol. 2015, 57, 171–185.
- Wu, H.; Zhang, X.; Giraldo, J.P.; Shabala, S; It is not all about sodium: revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant and Soil 2018, 431, 1-17, 10.1007/s11104-018-3770-y.Tabassum, T.; Farooq, M.; Ahmad, R.; Zohaib, A.; Wahid, A. Seed priming and transgenerational drought memory improves tolerance against salt stress in bread wheat. Plant Physiol. Biochem. 2017, 118, 362–369.
- Wu, H.; Zhu, M.; Shabala, L.; Zhou, M.; Shabala, S; K+ retention in leaf mesophyll, an overlooked component of salinity tolerance mechanism: A case study for barley. Journal of Integrative Plant Biology 2014, 57, 171-185, 10.1111/jipb.12238.Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Tanveer, M.; Ihsan, M.Z.; Shah, A.N.; Ullah, A.; Khan, F.; Ullah, S.; et al. A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiol. Biochem. 2016, 103, 191–198.
- Tabassum, T.; Farooq, M.; Ahmad, R.; Zohaib, A.; Wahid, A; Seed priming and transgenerational drought memory improves tolerance against salt stress in bread wheat. Plant Physiology and Biochemistry 2017, 118, 362-369, 10.1016/j.plaphy.2017.07.007.Tang, H.; Niu, L.; Wei, J.; Chen, X.; Chen, Y. Phosphorus limitation improved salt tolerance in maize through tissue mass density increase, osmolytes accumulation, and Na+ uptake inhibition. Front. Plant Sci. 2019, 10, 856.
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Tanveer, M.; Ihsan, M.Z.; Shah, A.N.; Ullah, A.; Khan, F.; Ullah, S.; et al.et al A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiology and Biochemistry 2016, 103, 191-198, 10.1016/j.plaphy.2016.03.001.Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38.
- Tang, H.; Niu, L.; Wei, J.; Chen, X.; Chen, Y; Phosphorus Limitation Improved Salt Tolerance in Maize Through Tissue Mass Density Increase, Osmolytes Accumulation, and Na+ Uptake Inhibition. Frontiers in Plant Science 2019, 10, 856, 10.3389/fpls.2019.00856.Wani, A.S.; Ahmad, A.; Hayat, S.; Tahir, I. Epibrassinolide and proline alleviate the photosynthetic and yield inhibition under salt stress by acting on antioxidant system in mustard. Plant Physiol. Biochem. 2019, 135, 385–394.
- Zörb, C.; Geilfus, C.M.; Dietz, K.J; Salinity and crop yield. Plant Biology 2019, 21, 31-38.Hasanuzzaman, M.; Nahar, K.; Fujita, M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In Ecophysiology and Responses of Plants under Salt Stress; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer Science & Business Media: New York, NY, USA, 2012; pp. 25–87.
- Wani, A.S.; Ahmad, A.; Hayat, S.; Tahir, I; Epibrassinolide and proline alleviate the photosynthetic and yield inhibition under salt stress by acting on antioxidant system in mustard. Plant Physiology and Biochemistry 2019, 135, 385-394, 10.1016/j.plaphy.2019.01.002.Breseghello, F.; Coelho, A.S.G. Traditional and modern plant breeding methods with examples in rice (Oryza sativa L.). J. Agric. Food Chem. 2013, 61, 8277–8286.
- Hasanuzzaman, M.; Nahar, K.; Fujita, M.; Plant Response to Salt Stress and Role of Exogenous Protectants to Mitigate Salt-Induced Damages. In Ecophysiology and responses of plants under salt stress (pp. 25-87). Springer, New York, NY 2012, ., pp. 25-87, 10.1007/978-1-4614-4747-4_2.Ashfaque, F.; Khan, M.I.R.; Khan, N.A. Exogenously applied H2O2 promotes proline accumulation, water relations, photosynthetic efficiency and growth of wheat (Triticumaestivum L.) under salt stress. Annu. Res. Rev. Biol. 2014, 4, 105–120.
- Breseghello, F.; Coelho, A.S.G; Traditional and Modern Plant Breeding Methods with Examples in Rice (Oryza sativa L.). Journal of Agricultural and Food Chemistry 2013, 61, 8277-8286, 10.1021/jf305531j.Nazar, R.; Iqbal, N.; Syeed, S.; Khan, N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2011, 168, 807–815.
- Ashfaque, F.; Khan, M.I.R.; Khan, N.A; Exogenously applied H2O2 promotes proline accumulation, water relations, photosynthetic efficiency and growth of wheat (Triticum aestivum L.) under salt stress. Annual Research & Review in Biology 2014, 4, 105-120.Astolfi, S.; Zuchi, S. Adequate S supply protects barley plants from adverse effects of salinity stress by increasing thiol contents. Acta Physiol. Plant. 2013, 35, 175–181.
- Nazar, R.; Iqbal, N.; Syeed, S.; Khan, N.A; Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. Journal of Plant Physiology 2011, 168, 807-815, 10.1016/j.jplph.2010.11.001.The, C.Y.; Shaharuddin, N.A.; Ho, C.L.; Mahmood, M. Exogenous proline significantly affects the plant growth and nitrogen assimilation enzymes activities in rice (Oryza sativa) under salt stress. Acta Physiol. Plant. 2016, 38, 151.
- Astolfi, S.; Zuchi, S; Adequate S supply protects barley plants from adverse effects of salinity stress by increasing thiol contents. Acta Physiologiae Plantarum 2012, 35, 175-181, 10.1007/s11738-012-1060-5.Fuertes-Mendizábal, T.; Bastías, E.I.; González-Murua, C.; González-Moro, M. Nitrogen assimilation in the highly salt and boron-tolerant ecotype Zea mays L. Amylacea. Plants 2020, 9, 322.
- The, C.Y.; Shaharuddin, N.A.; Ho, C.L.; Mahmood, M; Exogenous proline significantly affects the plant growth and nitrogen assimilation enzymes activities in rice (Oryza sativa) under salt stress. Acta Physiologiae Plantarum 2016, 38, 1-10, 10.1007/s11738-016-2163-1.Goel, P.; Singh, A.K. Abiotic stresses downregulate key genes involved in nitrogen uptake and assimilation in Brassica juncea L. PLoS ONE 2015, 10, e0143645.
- Fuertes‐Mendizábal, T.; Bastías, E.I.; González‐Murua, C.; González‐Moro, M; Nitrogen Assimilation in the Highly Salt- and Boron-Tolerant Ecotype Zea mays L. Amylacea. Plants 2020, 9, 322, 10.3390/plants9030322.Ahanger, M.A.; Qin, C.; Begum, N.; Maodong, Q.; Dong, X.X.; El-Esawi, M.; El-Sheikh, M.A.; Alatar, A.A.; Zhang, L. Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism.; and secondary metabolite accumulation. BMC Plant Biol. 2019, 19, 479.
- Goel, P.; Singh, A.K; Abiotic Stresses Downregulate Key Genes Involved in Nitrogen Uptake and Assimilation in Brassica juncea L.. PLOS ONE 2015, 10, e0143645-e0143645, 10.1371/journal.pone.0143645.Singh, A.; Hussain, I.; Singh, N.B.; Singh, H. Uptake, translocation and impact of green synthesized nanoceria on growth and antioxidant enzymes activity of Solanum lycopersicum L. Ecotoxicol. Environ. Saf. 2019, 182, 109410.
- Ahanger, M.A.; Qin, C.; Begum, N.; Maodong, Q.; Dong, X.X.; El‐Esawi, M.; El‐Sheikh, M.A.; Alatar, A.A.; Zhang, L; Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation.. BMC Plant Biology 2019, 19, 479-12, 10.1186/s12870-019-2085-3.Coelho, D.G.; Miranda, R.D.S.; Paula-Marinho, S.D.O.; de Carvalho, H.H.; Prisco, J.T.; Gomes-Filho, E. Ammonium nutrition modulates K+ and N uptake, transport and accumulation during salt stress acclimation of sorghum plants. Arch. Agron. Soil Sci. 2020, 66, 1991–2004.
- Singh, A.; Hussain, I.; Singh, N.B.; Singh, H; Uptake, translocation and impact of green synthesized nanoceria on growth and antioxidant enzymes activity of Solanum lycopersicum L.. Ecotoxicology and Environmental Safety 2019, 182, 109410, 10.1016/j.ecoenv.2019.109410.Soliman, M.; Elkelish, A.; Souad, T.; Alhaithloul, H.; Farooq, M. Brassinosteroid seed priming with nitrogen supplementation improves salt tolerance in soybean. Physiol. Mol. Biol. Plants 2020, 26, 501–511.
- Coelho, D.G.; Miranda, R.D.S.; Paula‐Marinho, S.D.O.; de Carvalho, H.H.; Prisco, J.T.; Gomes‐Filho, E; Ammonium nutrition modulates K+ and N uptake, transport and accumulation during salt stress acclimation of sorghum plants. Archives of Agronomy and Soil Science 2020, 66, 1991-2004, 10.1080/03650340.2019.1704736.Per, T.S.; Khan, N.A.; Masood, A.; Fatma, M. Methyl jasmonate alleviates cadmium-induced photosynthetic damages through increased S-assimilation and glutathione production in mustard. Front. Plant Sci. 2016, 7, 1933.
- Soliman, M.; Elkelish, A.; Souad, T.; Alhaithloul, H.; Farooq, M; Brassinosteroid seed priming with nitrogen supplementation improves salt tolerance in soybean. Physiology and Molecular Biology of Plants 2020, 26, 501-511, 10.1007/s12298-020-00765-7.Fatma, M.; Masood, A.; Per, T.S.; Rasheed, F.; Khan, N.A. Interplay between nitric oxide and sulfur assimilation in salt tolerance in plants. Crop J. 2016, 4, 153–161.
- Per, T.S.; Khan, N.A.; Masood, A.; Fatma, M; Methyl Jasmonate Alleviates Cadmium-Induced Photosynthetic Damages through Increased S-Assimilation and Glutathione Production in Mustard. Frontiers in Plant Science 2016, 7, 1933, 10.3389/fpls.2016.01933.Fatma, M.; Asgher, M.; Masood, A.; Khan, N.A. Excess sulfur supplementation improves photosynthesis and growth in mustard under salt stress through increased production of glutathione. Environ. Exp. Bot. 2014, 107, 55–63.
- Fatma, M.; Masood, A.; Per, T.S.; Rasheed, F.; Khan, N.A; Interplay between nitric oxide and sulfur assimilation in salt tolerance in plants. The Crop Journal 2016, 4, 153-161, 10.1016/j.cj.2016.01.009.Seth, C.S.; Remans, T.; Keunen, E.; Jozefczak, M.; Gielen, H.; Opdenakker, K.; Cuypers, A. Phytoextraction of toxic metals: A central role for glutathione. Plant Cell Environ. 2012, 35, 334–346.
- Fatma, M.; Asgher, M.; Masood, A.; Khan, N.A; Excess sulfur supplementation improves photosynthesis and growth in mustard under salt stress through increased production of glutathione. Environmental and Experimental Botany 2014, 107, 55-63, 10.1016/j.envexpbot.2014.05.008.Kumar, D.; Chattopadhyay, S. Glutathione modulates the expression of heat shock proteins via the transcription factors BZIP10 and MYB21 in Arabidopsis. J. Exp. Bot. 2018, 69, 3729–3743.
- Seth, C.S.; Remans, T.; Keunen, E.; Jozefczak, M.; Gielen, H.; Opdenakker, K.; Cuypers, A; Phytoextraction of toxic metals: A central role for glutathione. Plant Cell Environ 2012, 35, 334-346.Iqbal, N.; Umar, S.; Khan, N.A.; Corpas, F.J. Nitric oxide and hydrogen sulfide coordinately reduce glucose sensitivity and decrease oxidative stress via ascorbate-glutathione cycle in heat-stressed wheat (Triticum aestivum L.) plants. Antioxidants 2021, 10, 108.
- Kumar, D.; Chattopadhyay, S; Glutathione modulates the expression of heat shock proteins via the transcription factors BZIP10 and MYB21 in Arabidopsis. Journal of Experimental Botany 2018, 69, 3729-3743, 10.1093/jxb/ery166.Mera, R.; Torres, E.; Abalde, J. Sulphate.; more than a nutrient; protects the microalga Chlamydomonas moewusii from cadmium toxicity. Aquat. Toxicol. 2014, 148, 92–103.
- Iqbal, N.; Umar, S.; Khan, N.A.; Corpas, F.J; Nitric Oxide and Hydrogen Sulfide Coordinately Reduce Glucose Sensitivity and Decrease Oxidative Stress via Ascorbate-Glutathione Cycle in Heat-Stressed Wheat (Triticum aestivum L.) Plants. Antioxidants 2021, 10, 108, 10.3390/antiox10010108.Romero, T.C.; Nortes, T.P.A.; Pedrero, S.F.; Mounzer, O.; Alarcón, C.J.J.; Bayona, G.J.M.; Nicolás, N.E. Assessment of the viability of using saline reclaimed water in grapefruit in medium to long term. Span. J. Agric. Res. 2014, 12, 1137–1148.
- Mera, R.; Torres, E.; Abalde, J; Sulphate, more than a nutrient, protects the microalga Chlamydomonas moewusii from cadmium toxicity. Aquatic Toxicology 2014, 148, 92-103, 10.1016/j.aquatox.2013.12.034.Riffat, A.L.I.A.; Sajid, M.; Ahmad, A. Alleviation of adverse effects of salt stress on growth og maize Zea mays L. by sulfur supplementation. Pak. J. Bot. 2020, 523, 763–773.
- Romero, T.C.; Nortes, T.P.A.; Pedrero, S.F.; Mounzer, O.; Alarcón, C.J.J.; Bayona, G.J.M.; Nicolás, N.E; Assessment of the viability of using saline reclaimed water in grapefruit in medium to long term. Spanish Journal of Agricultural Research 2014, 12, 1137-1148, 10.5424/sjar/2014124-5495.de Souza Freitas, W.E.; de Oliveira, A.B.; Mesquita, R.O.; de Carvalho, H.H.; Prisco, J.T.; Gomes-Filho, E. Sulfur-induced salinity tolerance in lettuce is due to a better P and K uptake.; lower Na/K ratio and an efficient antioxidative defense system. Sci. Hortic. 2019, 257, 108764.
- Riffat, A.L.I.A.; Sajid, M.; Ahmad, A; Alleviation of adverse effects of salt stress on growth og maize Zea mays L. by sulfur supplementation. Pakistan Journal of Botany 2020, 523, 763-773.Aziz, A.; Ashraf, M.; Sikandar, S.; Asif, M.; Akhtar, N.; Shahzad, S.M.; Babar, B.H. Optimizing sulfur for improving salt tolerance of sunflower Helianthus annuus L. Soil Environ. 2019, 38, 222–233.
- de Souza Freitas, W.E.; de Oliveira, A.B.; Mesquita, R.O.; de Carvalho, H.H.; Prisco, J.T.; Gomes‐Filho, E; Sulfur-induced salinity tolerance in lettuce is due to a better P and K uptake, lower Na/K ratio and an efficient antioxidative defense system. Scientia Horticulturae 2019, 257, 108764, 10.1016/j.scienta.2019.108764.Coleto, I.; de la Peña, M.; Rodríguez-Escalante, J.; Bejarano, I.; Glauser, G.; Aparicio-Tejo, P.M.; Marino, D. Leaves play a central role in the adaptation of nitrogen and sulfur metabolism to ammonium nutrition in oilseed rape Brassica napus. BMC Plant Biol. 2017, 17, 1–13.
- Aziz, A.; Ashraf, M.; Sikandar, S.; Asif, M.; Akhtar, N.; Shahzad, S.M.; Babar, B.H; Optimizing sulfur for improving salt tolerance of sunflower (Helianthus annuus L.). Soil & Environment 2019, 38, 222-233, 10.25252/se/19/71647.Jobe, T.O.; Zenzen, I.; Rahimzadeh, K.P.; Kopriva, S. Integration of sulfate assimilation with carbon and nitrogen metabolism in transition from C3 to C4 photosynthesis. J. Exp. Bot. 2019, 70, 4211–4221.
- Coleto, I.; de la Peña, M.; Rodríguez‐Escalante, J.; Bejarano, I.; Glauser, G.; Aparicio‐Tejo, P.M.; Marino, D; Leaves play a central role in the adaptation of nitrogen and sulfur metabolism to ammonium nutrition in oilseed rape (Brassica napus). BMC Plant Biology 2017, 17, 1-13, 10.1186/s12870-017-1100-9.Prodhan, M.A.; Finnegan, P.M.; Lambers, H. How does evolution in phosphorus-impoverished landscapes impact plant nitrogen and sulfur assimilation? Trends Plant Sci. 2019, 24, 69–82.
- Jobe, T.O.; Zenzen, I.; Rahimzadeh, K.P.; Kopriva, S; Integration of sulfate assimilation with carbon and nitrogen metabolism in transition from C3 to C4 photosynthesis. Journal of Experimental Botany 2019, 70, 4211-4221, 10.1093/jxb/erz250.Rais, L.; Masood, A.; Inam, A.; Khan, N. Sulfur and nitrogen co-ordinately improve photosynthetic efficiency, growth and proline accumulation in two cultivars of mustard under salt stress. J. Plant Biochem. Physiol. 2013, 1.
- Prodhan, M.A.; Finnegan, P.M.; Lambers, H; How does evolution in phosphorus‐impoverished landscapes impact plant nitrogen and sulfur assimilation?. Trends of Plant Sciences 2019, 24, 69-82.Zhang, N.N.; Zou, H.; Lin, X.Y.; Pan, Q.; Zhang, W.Q.; Zhang, J.H.; Chen, J. Hydrogen sulfide and rhizobia synergistically regulate nitrogen N assimilation and remobilization during N deficiency-induced senescence in soybean. Plant Cell Environ. 2020, 43, 1130–1147.
- Rais, L.; Masood, A.; Inam, A.; Khan, N; Sulfur and Nitrogen Co-ordinately Improve Photosynthetic Efficiency, Growth and Proline Accumulation in Two Cultivars of Mustard Under Salt Stress. Journal of Plant Biochemistry & Physiology 2013, 1, 1-6, 10.4172/2329-9029.1000101.Lancaster, J.R., Jr. Nitric oxide: A brief overview of chemical and physical properties relevant to therapeutic applications. Future Sci. OA 2015, 1.
- Zhang, N.N.; Zou, H.; Lin, X.Y.; Pan, Q.; Zhang, W.Q.; Zhang, J.H.; Chen, J; Hydrogen sulfide and rhizobia synergistically regulate nitrogen (N) assimilation and remobilization during N deficiency‐induced senescence in soybean. Plant, Cell & Environment 2020, 43, 1130-1147, 10.1111/pce.13736.Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.G.; Yun, B.W. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ. Exp. Bot. 2019, 161, 120–133.
- Lancaster, J.R., Jr; Nitric oxide: a brief overview of chemical and physical properties relevant to therapeutic applications. Future Science OA 2015, 1, FSO.15.59, 10.4155/fso.15.59.Fancy, N.N.; Bahlmann, A.K.; Loake, G.J. Nitric oxide functions in plant abiotic stress. Plant Cell Environ. 2017, 40, 462–472.
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.G.; Yun, B.W; Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environmental and Experimental Botany 2019, 161, 120-133, 10.1016/j.envexpbot.2019.02.003.Akram, N.A.; Iqbal, M.; Muhammad, A.; Ashraf, M.; Al-Qurainy, F.; Shafiq, S. Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. Protoplasma 2018, 255, 163–174.
- Fancy, N.N.; Bahlmann, A.K.; Loake, G.J; Nitric oxide functions in plant abiotic stress. Plant Cell and Environment 2017, 40, 462-472.Ahmad, P.; Abass, A.M.; Nasser, A.M.; Wijaya, L.; Alam, P.; Ashraf, M. Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J. Plant Interact. 2018, 13, 64–72.
- Akram, N.A.; Iqbal, M.; Muhammad, A.; Ashraf, M.; Al‐Qurainy, F.; Shafiq, S; Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. Protoplasma 2017, 255, 163-174, 10.1007/s00709-017-1140-x.Tian, X.; He, M.; Wang, Z.; Zhang, J.; Song, Y.; He, Z.; Dong, Y. Application of nitric oxide and calcium nitrate enhances tolerance of wheat seedlings to salt stress. Plant Growth Regul. 2015, 77, 343–356.
- Ahmad, P.; Abass, A.M.; Nasser, A.M.; Wijaya, L.; Alam, P.; Ashraf, M; Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. Journal of Plant Interactions 2018, 13, 64-72, 10.1080/17429145.2017.1420830.Gaupels, F.; Kuruthukulangarakoola, G.T.; Durner, J. Upstream and downstream signals of nitric oxide in pathogen defence. Curr. Opin. Plant Biol. 2011, 14, 707–714.
- Tian, X.; He, M.; Wang, Z.; Zhang, J.; Song, Y.; He, Z.; Dong, Y; Application of nitric oxide and calcium nitrate enhances tolerance of wheat seedlings to salt stress. Plant Growth Regulation 2015, 77, 343-356, 10.1007/s10725-015-0069-3.Martínez-Ruiz, A.; Cadenas, S.; Lamas, S. Nitric oxide signaling: Classical, less classical, and nonclassical mechanisms. Free Radic. Biol. Med. 2011, 51, 17–29.
- Gaupels, F.; Kuruthukulangarakoola, G.T.; Durner, J; Upstream and downstream signals of nitric oxide in pathogen defence. Current Opinion in Plant Biology 2011, 14, 707-714, 10.1016/j.pbi.2011.07.005.Corpas, F.J.; González-Gordo, S.; Cañas, A.; Palma, J.M. Nitric oxide and hydrogen sulfide in plants, which comes first? J. Exp. Bot. 2019, 70, 4391–4404.
- Martínez‐Ruiz, A.; Cadenas, S.; Lamas, S; Nitric oxide signaling: Classical, less classical, and nonclassical mechanisms. Free Radical Biology and Medicine 2011, 51, 17-29, 10.1016/j.freeradbiomed.2011.04.010.Prochazkova, D.; Haisel, D.; Pavlikova, D. Nitric oxide biosynthesis in plants-the short overview. Plant Soil Environ. 2014, 60, 129–134.
- Corpas, F.J.; González‐Gordo, S.; Cañas, A.; Palma, J.M; Nitric oxide and hydrogen sulfide in plants: which comes first?. Journal of Experimental Botany 2019, 70, 4391-4404, 10.1093/jxb/erz031.Planchet, E.; Kaiser, W.M. Nitric oxide NO detection by DAF fluorescence and chemiluminescence: A comparison using abiotic and biotic NO sources. J. Exp. Bot. 2006, 57, 3043–3055.
- Prochazkova, D.; Haisel, D.; Pavlikova, D; Nitric oxide biosynthesis in plants‐the short overview. Plant Soil and Environment 2014, 60, 129-134.Leterrier, M.; Airaki, M.; Palma, J.M.; Chaki, M.; Barroso, J.B.; Corpas, F.J. Arsenic triggers the nitric oxide (NO) and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis. Environ. Pollut. 2012, 166, 136–143.
- Planchet, E.; Kaiser, W.M; Nitric oxide NO detection by DAF fluorescence and chemiluminescence: A comparison using abiotic and biotic NO sources.. Journal of Experimental Botany 2006, 57, 3043-3055.Sharma, A.; Kapoor, D.; Wang, J.; Landi, M.; Zheng, B.; Yan, D.; Yuan, H. Nitric oxide mediated mechanisms adopted by plants to cope with salinity. Biol. Plant. 2020, 64, 512–518.
- Leterrier, M.; Airaki, M.; Palma, J.M.; Chaki, M.; Barroso, J.B.; Corpas, F.J; Arsenic triggers the nitric oxide (NO) and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis. Environmental Pollution 2012, 166, 136-143, 10.1016/j.envpol.2012.03.012.Astier, J.; Gross, I.; Durner, J. Nitric oxide production in plants, an update. J. Exp. Bot. 2018, 69, 3401–3411.
- Sharma, A.; Kapoor, D.; Wang, J.; Landi, M.; Zheng, B.; Yan, D.; Yuan, H; Nitric oxide mediated mechanisms adopted by plants to cope with salinity. Biologia plantarum 2020, 64, 512-518, 10.32615/bp.2020.070.Gupta, K.J.; Fernie, A.R.; Kaiser, W.M.; van Dongen, J.T. On the origins of nitric oxide. Trends Plant Sci. 2011, 16, 160–168.
- Astier, J.; Gross, I.; Durner, J; Nitric oxide production in plants: an update. Journal of Experimental Botany 2017, 69, 3401-3411, 10.1093/jxb/erx420.Baudouin, E. The language of nitric oxide signalling. Plant Biol. 2011, 13, 233–242.
- Gupta, K.J.; Fernie, A.R.; Kaiser, W.M.; van Dongen, J.T; On the origins of nitric oxide. Trends of Plant Sciences 2011, 13, 233-242.Wang, Y.; Lin, A.; Loake, G.J.; Chu, C. H2O2-induced leaf cell death and the crosstalk of reactive nitric/oxygen species F. J. Integr. Plant Biol. 2013, 55, 202–208.
- Baudouin E; The language of nitric oxide signalling. Plant Biology 2011, 13, 233-242, 10.1111/j.1438-8677.2010.00403.x.Yu, S.; Wang, W.; Wang, B. Recent progress of salinity tolerance research in plants. Russ. J. Genet. 2012, 48, 497–505.
- Wang, Y.; Lin, A.; Loake, G.J.; Chu, C; H2 O2 -induced Leaf Cell Death and the Crosstalk of Reactive Nitric/Oxygen SpeciesF. Journal of Integrative Plant Biology 2013, 55, 202-208, 10.1111/jipb.12032.Vishwakarma, A.; Wany, A.; Pandey, S.; Bulle, M.; Kumari, A.; Kishorekumar, R.; Gupta, K.J. Current approaches to measure nitric oxide in plants. J. Exp. Bot. 2019, 70, 4333–4343.
- Sh Yu; W. Wang; B. Wang; Recent progress of salinity tolerance research in plants.. Russian Journal of Genetics 2012, 48, 497-505, 10.1134/s1022795412050225.Gould, K.S.; Lamotte, O.; Klinguer, A.; Pugin, A.; Wendehenne, D. Nitric oxide production in tobacco leaf cells, a generalized stress response? Plant Cell Environ. 2003, 26, 1851–1862.
- Vishwakarma, A.; Wany, A.; Pandey, S.; Bulle, M.; Kumari, A.; Kishorekumar, R.; Gupta, K.J; Current approaches to measure nitric oxide in plants. Journal of Experimental Botany 2019, 70, 4333-4343, 10.1093/jxb/erz242.Mur, L.A.; Prats, E.; Pierre, S.; Hall, M.A.; Hebelstrup, K.H. Integrating nitric oxide into salicylic acid and jasmonic acid/ethylene plant defense pathways. Front. Plant Sci. 2013, 4, 215.
- Gould, K.S.; Lamotte, O.; Klinguer, A.; Pugin, A.; Wendehenne, D; Nitric oxide production in tobacco leaf cells: a generalized stress response?. Plant, Cell & Environment 2003, 26, 1851-1862, 10.1046/j.1365-3040.2003.01101.x.Mata-Pérez, C.; Sánchez-Calvo, B.; Padilla, M.N.; Begara-Morales, J.C.; Valderrama, R.; Corpas, F.J.; Barroso, J.B. Nitro-fatty acids in plant signaling: New key mediators of nitric oxide metabolism. Redox Biol. 2017, 11, 554–561.
- Mur, L.A.; Prats, E.; Pierre, S.; Hall, M.A.; Hebelstrup, K.H; Integrating nitric oxide into salicylic acid and jasmonic acid/ ethylene plant defense pathways. Frontiers in Plant Science 2013, 4, 215, 10.3389/fpls.2013.00215.Jagodzik, P.; Tajdel-Zielinska, M.; Ciesla, A.; Marczak, M.; Ludwikow, A. Mitogen-activated protein kinase cascades in plant hormone signaling. Front. Plant Sci. 2018, 9, 1387.
- Mata‐Pérez, C.; Sánchez‐Calvo, B.; Padilla, M.N.; Begara‐Morales, J.C.; Valderrama, R.; Corpas, F.J.; Barroso, J.B; Nitro-fatty acids in plant signaling: New key mediators of nitric oxide metabolism. Redox Biology 2017, 11, 554-561, 10.1016/j.redox.2017.01.002.Asai, S.; Ohta, K.; Yoshioka, H. MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Ncotianabenthamiana. Plant Cell 2008, 20, 1390–11406.
- Jagodzik, P.; Tajdel‐Zielinska, M.; Ciesla, A.; Marczak, M.; Ludwikow, A; Mitogen-Activated Protein Kinase Cascades in Plant Hormone Signaling. Frontiers in Plant Science 2018, 9, 1387, 10.3389/fpls.2018.01387.Gadelha, C.G.; de Souza Miranda, R.; Alencar, N.L.M.; Costa, J.H.; Prisco, J.T.; Gomes-Filho, E. Exogenous nitric oxide improves salt tolerance during establishment of Jatropha curcas seedlings by ameliorating oxidative damage and toxic ion accumulation. J. Plant Physiol. 2017, 212, 69–79.
- Asai, S.; Ohta, K.; Yoshioka, H; MAPK Signaling Regulates Nitric Oxide and NADPH Oxidase-Dependent Oxidative Bursts inNicotiana benthamiana. The Plant Cell 2008, 20, 1390-1406, 10.1105/tpc.107.055855.Liu, S.; Yang, R.; Pan, Y.; Ma, M.; Pan, J.; Zhao, Y.; Zhang, L. Nitric oxide contributes to minerals absorption.; proton pumps and hormone equilibrium under cadmium excess in Trifoliumrepens L. plants. Ecotoxicol. Environ. Saf. 2015, 119, 35–46.
- Gadelha, C.G.; de Souza Miranda, R.; Alencar, N.L.M.; Costa, J.H.; Prisco, J.T.; Gomes‐Filho, E; Exogenous nitric oxide improves salt tolerance during establishment of Jatropha curcas seedlings by ameliorating oxidative damage and toxic ion accumulation. Journal of Plant Physiology 2017, 212, 69-79, 10.1016/j.jplph.2017.02.005.Arora, D.; Bhatla, S.C. Melatonin and nitric oxide regulate sunflower seedling growth under salt stress accompanying differential expression of Cu/Zn SOD and Mn SOD. Free Radic. Biol. Med. 2017, 106, 315–328.
- Liu, S.; Yang, R.; Pan, Y.; Ma, M.; Pan, J.; Zhao, Y.; Zhang, L; Nitric oxide contributes to minerals absorption, proton pumps and hormone equilibrium under cadmium excess in Trifolium repens L. plants. Ecotoxicology and Environmental Safety 2015, 119, 35-46, 10.1016/j.ecoenv.2015.04.053.Babaei, S.; Niknam, V.; Behmanesh, M. Comparative effects of nitric oxide and salicylic acid on salinity tolerance in saffron Crocus sativus. Plant Biosyst. Int. J. Plant Biol. 2021, 155, 73–82.
- Arora, D.; Bhatla, S.C; Melatonin and nitric oxide regulate sunflower seedling growth under salt stress accompanying differential expression of Cu/Zn SOD and Mn SOD. Free Radical Biology and Medicine 2017, 106, 315-328, 10.1016/j.freeradbiomed.2017.02.042.Huang, J.; Wei, H.; Li, L.; Yu, S. Transcriptome analysis of nitric oxide-responsive genes in upland cotton Gossypiumhirsutum. PLoS ONE 2018, 13, e0192367.
- Babaei, S.; Niknam, V.; Behmanesh, M; Comparative effects of nitric oxide and salicylic acid on salinity tolerance in saffron (Crocus sativus). Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology 2020, 155, 73-82, 10.1080/11263504.2020.1727975.Maslennikova, D.R.; Allagulova, C.R.; Fedorova, K.A.; Plotnikov, A.A.; Avalbaev, A.M.; Shakirova, F.M. Cytokinins contribute to realization of nitric oxide growth-stimulating and protective effects on wheat plants. Russ. J. Plant Physiol. 2017, 64, 665–671.
- Huang, J.; Wei, H.; Li, L.; Yu, S; Transcriptome analysis of nitric oxide-responsive genes in upland cotton (Gossypium hirsutum). PLOS ONE 2018, 13, e0192367, 10.1371/journal.pone.0192367.León, J.; Castillo, M.C.; Coego, A.; Lozano-Juste, J.; Mir, R. Diverse functional interactions between nitric oxide and abscisic acid in plant development and responses to stress. J. Exp. Bot. 2014, 65, 907–921.
- Maslennikova, D.R.; Allagulova, C.R.; Fedorova, K.A.; Plotnikov, A.A.; Avalbaev, A.M.; Shakirova, F.M; Cytokinins contribute to realization of nitric oxide growth-stimulating and protective effects on wheat plants. Russian Journal of Plant Physiology 2017, 64, 665-671, 10.1134/s1021443717040094.Neljubov, D.N. Pflanzen. Beih. Bot. Zentralbl. 1901, 10, 128–139.
- León, J.; Castillo, M.C.; Coego, A.; Lozano‐Juste, J.; Mir, R; Diverse functional interactions between nitric oxide and abscisic acid in plant development and responses to stress. Journal of Experimental Botany 2014, 65, 907-921, 10.1093/jxb/ert454.Hua, J. Isolation of components involved in ethylene signaling. In Ethylene in Plants, 1st ed.; Wen, C.K., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 27–44.
- Neljubov, D.N; Pflanzen. Beih. Bot. Zentralbl 1901, 10, 128-139.Abeles, F.B.; Morgan, P.W.; Saltveit, M.E., Jr. Ethylene in Plant Biology, 2nd ed.; Academic Press: San Diego, CA, USA, 2012.
- Hua, J.; Isolation of Components Involved in Ethylene Signaling. In Ethylene in Plants, 1st ed.; Wen, C.K., Ed.; Springer: Dordrecht, The Netherlands 2015, ., pp. 27-44, 10.1007/978-94-017-9484-8_2.Abiri, R.; Shaharuddin, N.A.; Maziah, M.; Yusof, Z.N.B.; Atabaki, N.; Sahebi, M.; Hanafi, M.M. Role of ethylene and the APETALA 2/ethylene response factor superfamily in rice under various abiotic and biotic stress conditions. Environ. Exp. Bot. 2017, 134, 33–44.
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E.; Ethylene in Plant Biology. 2nd ed.; Academic Press: San Diego, CA 2012, ., ., 10.1016/C2009-0-03226-7.Iqbal, N.; Nazar, R.; Syeed, S.; Masood, A.; Khan, N.A. Exogenously-sourced ethylene increases stomatal conductance, photosynthesis, and growth under optimal and deficient nitrogen fertilization in mustard. J. Exp. Bot. 2011, 62, 4955–4963.
- Abiri, R.; Shaharuddin, N.A.; Maziah, M.; Yusof, Z.N.B.; Atabaki, N.; Sahebi, M.; Hanafi, M.M; Role of ethylene and the APETALA 2/ethylene response factor superfamily in rice under various abiotic and biotic stress conditions. Environmental and Experimental Botany 2017, 134, 33-44, 10.1016/j.envexpbot.2016.10.015.García, M.J.; Romera, F.J.; Lucena, C.; Alcántara, E.; Pérez-Vicente, R. Ethylene and the regulation of physiological and morphological responses to nutrient deficiencies. Plant Physiol. 2015, 169, 51–60.
- Iqbal, N.; Nazar, R.; Syeed, S.; Masood, A.; Khan, N.A; Exogenously-sourced ethylene increases stomatal conductance, photosynthesis, and growth under optimal and deficient nitrogen fertilization in mustard. Journal of Experimental Botany 2011, 62, 4955-4963, 10.1093/jxb/err204.Wang, H.; Liang, X.; Wan, Q.; Wang, X.; Bi, Y. Ethylene and nitric oxide are involved in maintaining ion homeostasis in Arabidopsis callus under salt stress. Planta 2009, 230, 293–307.
- García, M.J.; Romera, F.J.; Lucena, C.; Alcántara, E.; Pérez‐Vicente, R; Ethylene and the Regulation of Physiological and Morphological Responses to Nutrient Deficiencies. Plant Physiology 2015, 169, 51-60, 10.1104/pp.15.00708.Xu, J.; Zhang, S. Ethylene biosynthesis and regulation in plants. In Ethylene in Plants, 1st ed.; Wen, C.K., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 1–25.
- Wang, H.; Liang, X.; Wan, Q.; Wang, X.; Bi, Y; Ethylene and nitric oxide are involved in maintaining ion homeostasis in Arabidopsis callus under salt stress. Planta 2009, 230, 293-307, 10.1007/s00425-009-0946-y.Yang, S.F.; Hoffman, N.E. Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189.
- Xu, J.; Zhang, S.; Ethylene Biosynthesis and Regulation in Plants. In Ethylene in Plants, 1st ed.; Wen, C.K., Ed.; Springer: Dordrecht, The Netherlands 2015, ., pp. 1-25, 10.1007/978-94-017-9484-8_1.Chang, C. Q&A: How do plants respond to ethylene and what is its importance? BMC Plant Biol. 2016, 14, 1–7.
- Yang, S.F.; Hoffman, N.E; Ethylene Biosynthesis and its Regulation in Higher Plants. Annual Review of Plant Biology 1984, 35, 155-189, 10.1146/annurev.arplant.35.1.155.Lin, Z.; Zhong, S.; Grierson, D. Recent advances in ethylene research. J. Exp. Bot. 2009, 60, 3311–3336.
- Chang C; Q&A: How do plants respond to ethylene and what is its importance?. BMC Biology 2016, 14, 1-7, 10.1186/s12915-016-0230-0.Tsuchisaka, A.; Theologis, A. Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol. 2004, 136, 2982–3000.
- Lin, Z.; Zhong, S.; Grierson, D; Recent advances in ethylene research. Journal of Experimental Botany 2009, 60, 3311-3336, 10.1093/jxb/erp204.Lyzenga, W.J.; Booth, J.K.; Stone, S.L. The Arabidopsis RING-type E3 ligase XBAT32 mediates the proteasomal degradation of the ethylene biosynthetic enzyme.; 1-aminocyclopropane-1-carboxylate synthase 7. Plant J. 2012, 71, 23–34.
- Tsuchisaka, A.; Theologis, A; Unique and Overlapping Expression Patterns among the Arabidopsis 1-Amino-Cyclopropane-1-Carboxylate Synthase Gene Family Members. Plant Physiology 2004, 136, 2982-3000, 10.1104/pp.104.049999.Ji, Y.; Guo, H. From endoplasmic reticulum (ER) to nucleus: EIN2 bridges the gap in ethylene signaling. Mol. Plant 2013, 6, 11–14.
- Lyzenga, W.J.; Booth, J.K.; Stone, S.L; The Arabidopsis RING-type E3 ligase XBAT32 mediates the proteasomal degradation of the ethylene biosynthetic enzyme, 1-aminocyclopropane-1-carboxylate synthase 7. The Plant Journal 2012, 71, 23-34, 10.1111/j.1365-313x.2012.04965.x.Dolgikh, V.A.; Pukhovaya, E.M.; Zemlyanskaya, E.V. Shaping ethylene response: The role of EIN3/EIL1 transcription factors. Front. Plant Sci. 2019, 10, 1030.
- Ji, Y.; Guo, H; From Endoplasmic Reticulum (ER) to Nucleus: EIN2 Bridges the Gap in Ethylene Signaling. Molecular Plant 2013, 6, 11-14, 10.1093/mp/sss150.Riyazuddin, R.; Verma, R.; Singh, K.; Nisha, N.; Keisham, M.; Bhati, K.K.; Gupta, R. Ethylene: A master regulator of salinity stress tolerance in plants. Biomolecules 2020, 10, 959.
- Dolgikh, V.A.; Pukhovaya, E.M.; Zemlyanskaya, E.V; Shaping Ethylene Response: The Role of EIN3/EIL1 Transcription Factors. Frontiers in Plant Science 2019, 10, 1030, 10.3389/fpls.2019.01030.Khan, M.I.R.; Jahan, B.; AlAjmi, M.F.; Rehman, M.T.; Khan, N.A. Ethephon mitigates nickel stress by modulating antioxidant system.; glyoxalase system and proline metabolism in Indian mustard. Physiol. Mol. Biol. Plants 2020, 26, 1201–1213.
- Riyazuddin, R.; Verma, R.; Singh, K.; Nisha, N.; Keisham, M.; Bhati, K.K.; Gupta, R; Ethylene: A Master Regulator of Salinity Stress Tolerance in Plants. Biomolecules 2020, 10, 959, 10.3390/biom10060959.Cui, M.; Lin, Y.; Zu, Y.; Efferth, T.; Li, D.; Tang, Z. Ethylene increases accumulation of compatible solutes and decreases oxidative stress to improve plant tolerance to water stress in Arabidopsis. J. Plant Biol. 2015, 58, 193–201.
- Khan, M.I.R.; Jahan, B.; AlAjmi, M.F.; Rehman, M.T.; Khan, N.A; Ethephon mitigates nickel stress by modulating antioxidant system, glyoxalase system and proline metabolism in Indian mustard. Physiology and Molecular Biology of Plants 2020, 26, 1201-1213, 10.1007/s12298-020-00806-1.Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene role in plant growth, development and senescence, interaction with other phytohormones. Front. Plant Sci. 2017, 8, 475.
- Cui, M.; Lin, Y.; Zu, Y.; Efferth, T.; Li, D.; Tang, Z; Ethylene increases accumulation of compatible solutes and decreases oxidative stress to improve plant tolerance to water stress in Arabidopsis. Journal of Plant Biology 2015, 58, 193-201, 10.1007/s12374-014-0302-z.Al Murad, M.; Razi, K.; Benjamin, L.K.; Lee, J.H.; Kim, T.H.; Muneer, S. Ethylene regulates sulfur acquisition by regulating the expression of sulfate transporter genes in oilseed rape. Physiol. Plant. 2020, 171, 533–545.
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R; Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Frontiers in Plant Science 2017, 8, 475, 10.3389/fpls.2017.00475.Shakar, M.; Yaseen, M.; Mahmood, R.; Ahmad, I. Calcium carbide induced ethylene modulate biochemical profile of Cucumis sativus at seed germination stage to alleviate salt stress. Sci. Hortic. 2016, 213, 179–185.
- Al Murad, M.; Razi, K.; Benjamin, L.K.; Lee, J.H.; Kim, T.H.; Muneer, S; Ethylene regulates sulfur acquisition by regulating the expression of sulfate transporter genes in oilseed rape. Physiologia Plantarum 2020, 171, 533-545, 10.1111/ppl.13157.Zhang, X.; Shi, Z.; Tian, Y.; Zhou, Q.; Cai, J.; Dai, T.; Jiang, D. Salt stress increases content and size of glutenin macropolymers in wheat grain. Food Chem. 2016, 197, 516–521.
- Shakar, M.; Yaseen, M.; Mahmood, R.; Ahmad, I; Calcium carbide induced ethylene modulate biochemical profile of Cucumis sativus at seed germination stage to alleviate salt stress. Scientia Horticulturae 2016, 213, 179-185, 10.1016/j.scienta.2016.10.037.Liu, C.; Li, J.; Zhu, P.; Yu, J.; Hou, J.; Wang, C.; Zhao, A. Mulberry EIL3 confers salt and drought tolerances and modulates ethylene biosynthetic gene expression. Peer J. 2019, 7, e6391.
- Zhang, X.; Shi, Z.; Tian, Y.; Zhou, Q.; Cai, J.; Dai, T.; Jiang, D; Salt stress increases content and size of glutenin macropolymers in wheat grain. Food Chemistry 2016, 197, 516-521, 10.1016/j.foodchem.2015.11.008.Shi, S.; Li, S.; Asim, M.; Mao, J.; Xu, D.; Ullah, Z.; Liu, G.; Wang, Q.; Liu, H. The Arabidopsis calcium-dependent protein kinases CDPKs and their roles in Plant growth regulation and abiotic stress responses. Int. J. Mol. Sci. 2018, 19, 1900.
- Liu, C.; Li, J.; Zhu, P.; Yu, J.; Hou, J.; Wang, C.; Zhao, A; Mulberry EIL3 confers salt and drought tolerances and modulates ethylene biosynthetic gene expression. Peer J 2019, 7, e6391, 10.7717/peerj.6391.Peng, J.; Li, Z.; Wen, X.; Li, W.; Shi, H.; Yang, L.; Zhu, H.; Guo, H. Salt-induced stabilization of EIN3/EIL1 confers salinity tolerance by deterring ROS accumulation in Arabidopsis. PLoS Gen. 2014, 10, e1004664.
- Shi, S.; Li, S.; Asim, M.; Mao, J.; Xu, D.; Ullah, Z.; Liu, G.; Wang, Q.; Liu, H; The Arabidopsis Calcium-Dependent Protein Kinases (CDPKs) and Their Roles in Plant Growth Regulation and Abiotic Stress Responses. International Journal of Molecular Sciences 2018, 19, 1900, 10.3390/ijms19071900.Xu, L.; Xiang, G.; Sun, Q.; Ni, Y.; Jin, Z.; Gao, S.; Yao, Y. Melatonin enhances salt tolerance by promoting MYB108A-mediated ethylene biosynthesis in grapevines. Hortic. Res. 2019, 6, 1–14.
- Peng, J.; Li, Z.; Wen, X.; Li, W.; Shi, H.; Yang, L.; Zhu, H.; Guo, H; Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in Arabidopsis. PLoS Genetics 2014, 10, e1004664, 10.1371/journal.pgen.1004664.Husain, T.; Fatima, A.; Suhel, M.; Singh, S.; Sharma, A.; Prasad, S.M.; Singh, V.P. A brief appraisal of ethylene signaling under abiotic stress in plants. Plant Signal. Behav. 2020, 15, 1782051.
- Xu, L.; Xiang, G.; Sun, Q.; Ni, Y.; Jin, Z.; Gao, S.; Yao, Y; Melatonin enhances salt tolerance by promoting MYB108A-mediated ethylene biosynthesis in grapevines. Horticulture Research 2019, 6, 1-14, 10.1038/s41438-019-0197-4.Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404.
- Husain, T.; Fatima, A.; Suhel, M.; Singh, S.; Sharma, A.; Prasad, S.M.; Singh, V.P; A brief appraisal of ethylene signaling under abiotic stress in plants. Plant Signaling & Behavior 2020, 15, 1782051, 10.1080/15592324.2020.1782051.Xu, H.; Liu, Q.; Yao, T.; Fu, X. Shedding light on integrative GA signaling. Curr. Opin. Plant Biol. 2014, 21, 89–95.
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al.et al Phytohormones and plant responses to salinity stress: a review. Plant Growth Regulation 2015, 75, 391-404, 10.1007/s10725-014-0013-y.Yang, R.; Hong, Y.; Ren, Z.; Tang, K.; Zhang, H.; Zhu, J.K.; Zhao, C. A role for PICKLE in the regulation of cold and salt stress tolerance in Arabidopsis. Front. Plant Sci. 2019, 10, 900.
- Xu, H.; Liu, Q.; Yao, T.; Fu, X; Shedding light on integrative GA signaling. Current Opinion in Plant Biology 2014, 21, 89-95, 10.1016/j.pbi.2014.06.010.Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.J. Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: Do we understand what they are whispering? Int. J. Mol. Sci. 2019, 20, 671.
- Yang, R.; Hong, Y.; Ren, Z.; Tang, K.; Zhang, H.; Zhu, J.K.; Zhao, C; A Role for PICKLE in the Regulation of Cold and Salt Stress Tolerance in Arabidopsis. Frontiers in Plant Science 2019, 10, 900, 10.3389/fpls.2019.00900.Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80, 67–74.
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.J; Signaling Crosstalk between Salicylic Acid and Ethylene/Jasmonate in Plant Defense: Do We Understand What They Are Whispering?. International Journal of Molecular Sciences 2019, 20, 671, 10.3390/ijms20030671.Nazar, R.; Umar, S.; Khan, N.A.; Sareer, O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S. Afr. J. Bot. 2015, 98, 84–94.
- Khan, M.I.R.; Asgher, M.; Khan, N.A; Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiology and Biochemistry 2014, 80, 67-74, 10.1016/j.plaphy.2014.03.026.Zhu, T.; Deng, X.; Zhou, X.; Zhu, L.; Zou, L.; Li, P.; Zhang, D.; Lin, H. Ethylene and hydrogen peroxide are involved in brassinosteroid-induced salt tolerance in tomato. Sci. Rep. 2016, 6, 35392.
- Nazar, R.; Umar, S.; Khan, N.A.; Sareer, O; Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. South African Journal of Botany 2015, 98, 84-94, 10.1016/j.sajb.2015.02.005.Buet, A.; Galatro, A.; Ramos-Artuso, F.; Simontacchi, M. Nitric oxide and plant mineral nutrition, current knowledge. J. Exp. Bot. 2019, 70, 4461–4476.
- Zhu, T.; Deng, X.; Zhou, X.; Zhu, L.; Zou, L.; Li, P.; Zhang, D.; Lin, H; Ethylene and hydrogen peroxide are involved in brassinosteroid-induced salt tolerance in tomato. Scientific Reports 2016, 6, 35392, 10.1038/srep35392.Santolini, J.; André, F.; Jeandroz, S.; Wendehenne, D. Nitric oxide synthase in plants: Where do we stand? Nitric Oxide 2017, 63, 30–38.
- Buet, A.; Galatro, A.; Ramos‐Artuso, F.; Simontacchi, M; Nitric oxide and plant mineral nutrition: current knowledge. Journal of Experimental Botany 2019, 70, 4461-4476, 10.1093/jxb/erz129.Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E. Nitrate reductase regulates plant nitricoxide homeostasis. Trends Plant Sci. 2017, 22, 163–174.
- Santolini, J.; André, F.; Jeandroz, S.; Wendehenne, D; Nitric oxide synthase in plants: Where do we stand?. Nitric Oxide 2017, 63, 30-38, 10.1016/j.niox.2016.09.005.Dong, F.; Simon, J.; Rienks, M.; Lindermayr, C.; Rennenberg, H. Effects of rhizopheric nitric oxide NO on N uptake in Fagus sylvatica seedlings depend on soil CO2 concentration.; soil N availability and N source. Tree Physiol. 2015, 35, 910–920.
- Chamizo‐Ampudia, A.; Sanz‐Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E; Nitrate Reductase Regulates Plant Nitric Oxide Homeostasis. Trends in Plant Science 2017, 22, 163-174, 10.1016/j.tplants.2016.12.001.Sun, J.; Zheng, N. Molecular mechanism underlying the plant NRT1. 1 dual-affinity nitrate transporter. Front. Physiol. 2015, 18, 6–386.
- Dong, F.; Simon, J.; Rienks, M.; Lindermayr, C.; Rennenberg, H; Effects of rhizopheric nitric oxide (NO) on N uptake in Fagus sylvatica seedlings depend on soil CO 2 concentration, soil N availability and N source. Tree Physiology 2015, 35, 910-920, 10.1093/treephys/tpv051.Wang, J.; Yu, S.X.; Zhang, M.; Cui, X.M. Exogenous nitric oxide-mediated GSH-PC synthesis pathway in tomato under copper stress. Russ. J. Plant Physiol. 2015, 62, 349–359.
- Sun, J.; Zheng, N; Molecular Mechanism Underlying the Plant NRT1.1 Dual-Affinity Nitrate Transporter. Frontiers in Physiology 2015, 6, 386, 10.3389/fphys.2015.00386.Masood, A.; Iqbal, N.; Khan, M.I.R.; Khan, N.A. The coordinated role of ethylene and glucose in sulfur-mediated protection of photosynthetic inhibition by cadmium. Plant Signal Behav. 2012, 7, 1420–1422.
- Wang, J.; Yu, S.X.; Zhang, M.; Cui, X.M; Exogenous Nitric Oxide-Mediated GSH–PC Synthesis Pathway in Tomato under Copper Stress. Russian Journal of Plant Physiology 2015, 62, 378-388, 10.7868/s0015330315030185.Saithong, T.; Saerue, S.; Kalapanulak, S.; Sojikul, P.; Narangajavana, J.; Bhumiratana, S. Gene co-expression analysis inferring the crosstalk of ethylene and gibberellin in modulating the transcriptional acclimation of cassava root growth in different seasons. PLoS ONE 2015, 10, e0137602.
- Masood, A.; Iqbal, N.; Khan, M.I.R.; Khan, N.A; The coordinated role of ethylene and glucose in sulfur-mediated protection of photosynthetic inhibition by cadmium. Plant Signaling & Behavior 2012, 7, 1420-1422, 10.4161/psb.22079.Wawrzyńska, A.; Sirko, A. To control and to be controlled: Understanding the Arabidopsis SLIM1 function in sulfur deficiency through comprehensive investigation of the EIL protein family. Front. Plant Sci. 2014, 5, 575.
- Saithong, T.; Saerue, S.; Kalapanulak, S.; Sojikul, P.; Narangajavana, J.; Bhumiratana, S; Gene Co-Expression Analysis Inferring the Crosstalk of Ethylene and Gibberellin in Modulating the Transcriptional Acclimation of Cassava Root Growth in Different Seasons. PLOS ONE 2015, 10, e0137602, 10.1371/journal.pone.0137602.
- Wawrzyńska, A.; Sirko, A; To control and to be controlled: understanding the Arabidopsis SLIM1 function in sulfur deficiency through comprehensive investigation of the EIL protein family. Frontiers in Plant Science 2014, 5, 575, 10.3389/fpls.2014.00575.
