Fruit colour represents a genetic trait with ecological and nutritional value. Plants mainly use colour to attract animals and favour seed dispersion. Thus, in many species, fruit colour coevolved with frugivories and their preferences. Environmental factors, however, represented other adaptive forces and further diversification was driven by domestication. All these factors cooperated in the evolution of tomato fruit, one of the most important in human nutrition. Tomato phylogenetic history showed two main steps in colour evolution: the change from green-chlorophyll to red-carotenoid pericarp, and the loss of the anthocyanic pigmentation. These events likely occurred with the onset of domestication. Then spontaneous mutations repeatedly occurred in carotenoid and phenylpropanoid pathways, leading to colour variants which often were propagated. Introgression breeding further enriched the panel of pigmentation patterns.
- tomato
- colour
- carotenoids
- flavonoids
- lycopene
- naringenin chalcon
- anthocyanins
- mutations
- introgression breeding
- domestication
1. Fruit Colour and Its Evolution in Tomato Plants
1.1. Function and Evolution of Fruit Colour
Function and Evolution of Fruit Colour
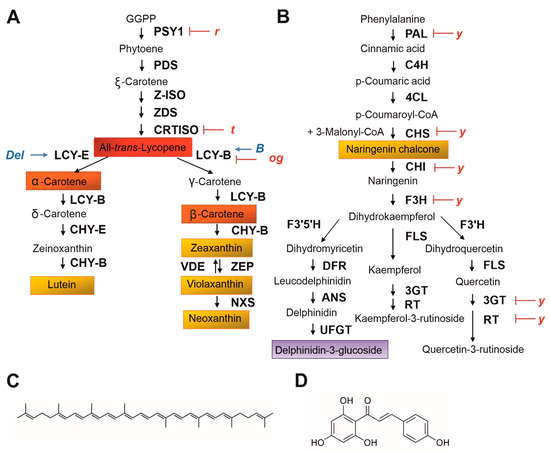
1.2. Carotenoid and Flavonoid Pigments as Determinants of Fruit Colour in Solanaceae
2. Carotenoid and Flavonoid Pigments as Determinants of Fruit Colour in Solanaceae
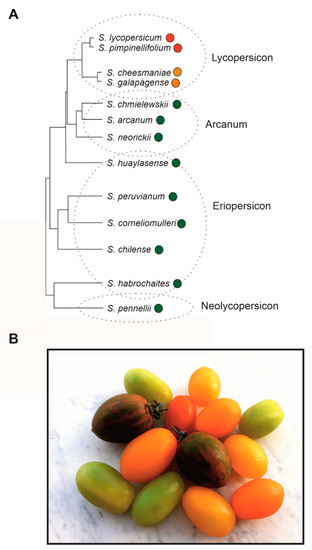
1.3. Phylogenetic Reconstruction and Human Selection of Fruit Colour in Tomato
3. Phylogenetic Reconstruction and Human Selection of Fruit Colour in Tomato
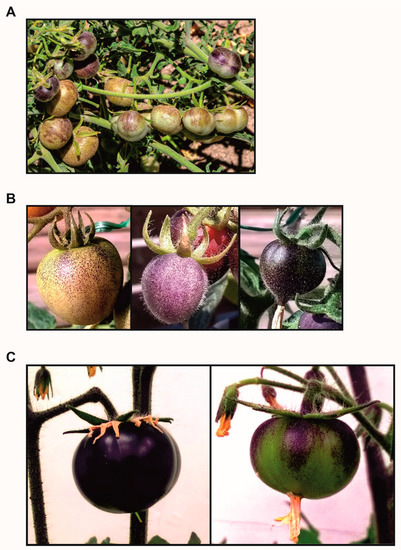
References
- Willson, M.F.; Whelan, C.J. The Evolution of Fruit Color in Fleshy-Fruited Plants. Am. Nat. 1990, 136, 790–809.
- Valenta, K.; Kalbitzer, U.; Razafimandimby, D.; Omeja, P.; Ayasse, M.; Chapman, C.A.; Nevo, O. The evolution of fruit colour: Phylogeny, abiotic factors and the role of mutualists. Sci. Rep. 2018, 8, 14302.
- Lu, L.; Fritsch, P.W.; Matzke, N.J.; Wang, H.; Kron, K.A.; Li, D.-Z.; Wiens, J.J. Why is fruit colour so variable? Phylogenetic analyses reveal relationships between fruit-colour evolution, biogeography and diversification. Glob. Ecol. Biogeogr. 2019, 28, 891–903.
- Sinnott-Armstrong, M.A.; Donoghue, M.J.; Jetz, W.J. Dispersers and environment drive global variation in fruit colour syndromes. Ecol. Lett. 2021, 24, 1387–1399.
- Fischer, K.E.; Chapman, C.A. Frugivores and fruit syndromes–Differences in patterns at the genus and species level. Oikos 1993, 66, 472–482.
- Jordano, P. Angiosperm fleshy fruits and seed dispersers: A comparative analysis of adaptation and constraints in plant-animal interactions. Am. Nat. 1995, 145, 163–191.
- Stournaras, K.E.; Lo, E.; Böhning-Gaese, K.; Cazetta, E.; Dehling, D.M.; Schleuning, M.; Caswell Stoddard, M.; Donoghue, M.J.; Prum, R.O.; Schaefer, H.M. How colorful are fruits? Limited color diversity in fleshy fruits on local and global scales. New Phytol. 2013, 198, 617–629.
- Lomascolo, S.B.; Levey, D.; Kimball, R.T.; Bolker, B.M.; Alborn, H.T. Dispersers shape fruit diversity in Ficus (Moraceae). Proc. Natl. Acad. Sci. USA 2010, 107, 14668–14672.
- Galetti, M.; Guevara, R.; Côrtes, M.C.; Fadini, R.; Von Matter, S.; Leite, A.B.; Labecca, F.; Ribeiro, T.; Carvalho, C.S.; Collevatti, R.G.; et al. Functional extinction of birds drives rapid evolutionary changes in seed size. Science 2013, 340, 1086–1090.
- Nevo, O.; Valenta, K.; Kleiner, A.; Razafimandimby, D.; Jeffrey, J.A.J.; Chapman, C.A.; Ayasse, M. The evolution of fruit scent: Phylogenetic and developmental constraints. BMC Evol. Biol. 2020, 20, 138.
- Paran, I.; van der Knaap, E. Genetic and molecular regulation of fruit and plant domestication traits in tomato and pepper. J. Exp. Bot. 2007, 58, 3841–3852.
- Zeller, U.; Göttert, T. The relations between evolution and domestication reconsidered–Implications for systematics, ecology, and nature conservation. Glob. Ecol. Conserv. 2019, 20, e00756.
- Lin, T.; Zhu, G.; Zhang, J.; Xu, X.; Yu, Q.; Zheng, Z.; Zhang, Z.; Lun, Y.; Li, S.; Wang, X.; et al. Genomic analyses provide insights into the history of tomato breeding. Nat. Genet. 2014, 46, 1220–1226.
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19.
- Wang, L.; Li, J.; Zhao, J.; He, C. Evolutionary developmental genetics of fruit morphological variation within the Solanaceae. Front. Plant. Sci. 2015, 6, 248.
- Rosati, C.; Diretto, G.; Giuliano, G. Biosynthesis and engineering of carotenoids and apocarotenoids in plants: State of the art and future prospects. Biotechnol. Genet. Eng. Rev. 2010, 26, 139–162.
- Dhar, M.K.; Sharma, R.; Koul, A.; Kaul, S. Development of fruit color in Solanaceae: A story of two biosynthetic pathways. Brief. Funct. Genom. 2015, 14, 199–212.
- Tohge, T.; Perez de Souza, L.; Fernie, A.R. Current understanding of the pathways of flavonoid biosynthesis in model and crop plants. J. Exp. Bot. 2017, 68, 4013–4028.
- Li, Z.; Vickrey, T.L.; McNally, M.G.; Sato, S.J.; Clemente, T.E.; Mower, J.P. Assessing Anthocyanin Biosynthesis in Solanaceae as a Model Pathway for Secondary Metabolism. Genes 2019, 10, 559.
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 2015, 20, 176–185.
- Egea, I.; Barsan, C.; Bian, W.; Purgatto, E.; Latché, A.; Chervin, C.; Bouzayen, M.; Pech, J.C. Chromoplast differentiation: Current status and perspectives. Plant Cell Physiol. 2010, 51, 1601–1611.
- Alba, R.; Payton, P.; Fei, Z.; McQuinn, R.; Debbie, P.; Martin, G.B.; Tanksley, S.D.; Giovannoni, J.J. Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. Plant Cell 2005, 17, 2954–2965.
- Martel, C.; Vrebalov, J.; Tafelmeyer, P.; Giovannoni, J.J. The tomato MADS-box transcription factor RIPENING INHIBITOR interacts with promoters involved in numerous ripening processes in a COLORLESS NONRIPENING-dependent manner. Plant Physiol. 2011, 157, 1568–1579.
- Adato, A.; Mandel, T.; Mintz-Oron, S.; Venger, I.; Levy, D.; Yativ, M.; Domínguez, E.; Wang, Z.; De Vos, R.C.H.; Jetter, R.; et al. Fruit-surface flavonoid accumulation in tomato is controlled by a SlMYB12-regulated transcriptional network. PLoS Genet. 2009, 5, e1000777.
- Razifard, H.; Ramos, A.; Della Valle, A.L.; Bodary, C.; Goetz, E.; Manser, E.J.; Li, X.; Zhang, L.; Visa, S.; Tieman, D.; et al. Genomic Evidence for Complex Domestication History of the Cultivated Tomato in Latin America. Mol. Biol. Evol. 2020, 37, 1118–1132.
- Welty, N. The Early History of Tomato Fruit Morphology. Available online: https://vanderknaaplab.uga.edu/documents/The-early-history-of-tomato-fruit-morphology-characteristics.pdf (accessed on 23 July 2021).
- Peralta, I.E.; Spooner, D.M. Granule-bound starch synthase (GBSSI) gene phylogeny of wild tomatoes (Solanum L. section Lycopersicon Wettst. Subsection Lycopersicon). Am. J. Bot. 2001, 88, 1888–1902.
- 100 Tomato Genome Sequencing Consortium. Exploring genetic variation in the tomato (Solanum section Lycopersicon) clade by whole-genome sequencing. Plant J. 2014, 80, 136–148.
- Rodriguez, F.; Wu, F.; Ané, C.; Tanksley, S.; Spooner, D.M. Do potatoes and tomatoes have a single evolutionary history, and what proportion of the genome supports this history? BMC Evol. Biol. 2009, 9, 191.
- Bedinger, P.A.; Chetelat, R.T.; McClure, B.; Moyle, L.C.; Rose, J.K.C.; Stack, S.M.; van der Knaap, E.; Baek, Y.S.; Lopez-Casado, G.; Covey, P.A.; et al. Interspecific reproductive barriers in the tomato clade: Opportunities to decipher mechanisms of reproductive isolation. Sex. Plant Reprod. 2011, 24, 171–187.
- Ronen, G.; Carmel-Goren, L.; Zamir, D.; Hirschberg, J. An alternative pathway to beta-carotene formation in plant chromoplasts discovered by map-based cloning of beta and old-gold color mutations in tomato. Proc. Natl. Acad. Sci. USA 2000, 97, 11102–11107.
- Yoo, H.J.; Park, W.J.; Lee, G.-M.; Oh, C.-S.; Yeam, I.; Won, D.-C.; Kim, C.K.; Lee, J.M. Inferring the Genetic Determinants of Fruit Colors in Tomato by Carotenoid Profiling. Molecules 2017, 22, 764.
- Isaacson, T.; Ronen, G.; Zamir, D.; Hirschberg, J. Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of beta-carotene and xanthophylls in plants. Plant Cell 2002, 14, 333–342.
- Tanambell, H.; Bishop, K.S.; Quek, S.Y. Tangerine tomatoes: Origin, biochemistry, potential health benefits and future prospects. Crit. Rev. Food Sci. Nutr. 2020, 12, 1–12.
- Fray, R.G.; Grierson, D. Identification and genetic analysis of normal and mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppression. Plant Mol. Biol. 1993, 22, 589–602.
- Barry, C.S.; McQuinn, R.P.; Chung, M.Y.; Besuden, A.; Giovannoni, J.J. Amino acid substitutions in homologs of the STAY-GREEN protein are responsible for the green-flesh and chlorophyll retainer mutations of tomato and pepper. Plant Physiol. 2008, 147, 179–187.
- Barry, C.S.; Pandey, P. A survey of cultivated heirloom tomato varieties identifies four new mutant alleles at the green-flesh locus. Mol. Breed. 2009, 24, 269–276.
- Ballester, A.R.; Molthoff, J.; de Vos, R.; Hekkert, B.T.; Orzaez, D.; Fernández-Moreno, J.P.; Tripodi, P.; Grandillo, S.; Martin, C.; Heldens, J.; et al. Biochemical and molecular analysis of pink tomatoes: Deregulated expression of the gene encoding transcription factor SlMYB12 leads to pink tomato fruit color. Plant Physiol. 2010, 152, 71–84.
- Kang, S.-I.; Hwang, I.; Goswami, G.; Jung, H.-J.; Nath, U.K.; Yoo, H.-J.; Lee, J.M.; Nou, I.S. Molecular Insights Reveal Psy1, SGR, and SlMYB12 Genes are Associated with Diverse Fruit Color Pigments in Tomato (Solanum lycopersicum L.). Molecules 2017, 22, 2180.
- Gonzali, S.; Mazzucato, A.; Perata, P. Purple as a tomato: Towards high anthocyanin tomatoes. Trends Plant Sci. 2009, 14, 237–241.
- Jones, C.M.; Mes, P.; Myers, J.R. Characterization and inheritance of the Anthocyanin fruit (Aft) tomato. J. Hered. 2003, 94, 449–456.
- Mes, P.J.; Boches, P.; Myers, J.R. Characterization of tomatoes expressing anthocyanin in the fruit. J. Am. Soc. Hortic. Sci. 2008, 33, 262–269.
- Mustilli, A.C.; Fenzi, F.; Ciliento, R.; Alfano, F.; Bowler, C. Phenotype of the tomato high pigment-2 mutant is caused by a mutation in the tomato homolog of DEETIOLATED1. Plant Cell 1999, 11, 145–157.
- Lieberman, M.; Segev, O.; Gilboa, N.; Lalazar, A.; Levin, I. The tomato homolog of the gene encoding UV-damaged DNA binding protein 1 (DDB1) underlined as the gene that causes the high pigment-1 mutant phenotype. Theor. Appl. Genet. 2004, 108, 1574–1581.
- Vrebalov, J.; Ruezinsky, D.; Padmanabhan, V.; White, R.; Medrano, D.; Drake, R.; Schuch, W.; Giovannoni, J. A MADS-box gene necessary for fruit ripening at the tomato Ripening-Inhibitor (Rin) locus. Science 2002, 296, 343–346.
- Manning, K.; Tör, M.; Poole, M.; Hong, Y.; Thompson, A.J.; King, G.J.; Giovannoni, J.J.; Seymour, G.B. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat. Genet. 2006, 38, 948–952.
- Zhu, M.; Chen, G.; Zhou, S.; Tu, Y.; Wang, Y.; Dong, T.; Hu, Z. A new tomato NAC (NAM/ATAF1/2/CUC2) transcription factor, SlNAC4, functions as a positive regulator of fruit ripening and carotenoid accumulation. Plant Cell Physiol. 2014, 55, 119–135.
- Lanahan, M.B.; Yen, H.C.; Giovannoni, J.J.; Klee, H.J. The never ripe mutation blocks ethylene perception in tomato. Plant Cell 1994, 6, 521–530.
- Zhu, H.L.; Zhu, B.Z.; Shao, Y.; Wang, X.G.; Lin, X.J.; Xie, Y.H.; Li, Y.C.; Gao, H.Y.; Luo, Y.B. Tomato fruit development and ripening are altered by the silencing of LeEIN2 gene. J. Integr. Plant Biol. 2006, 48, 1478–1485.
- Hu, Z.; Deng, L.; Chen, X.; Wang, P.; Chen, G. Co-suppression of the EIN2-homology gene LeEIN2 inhibits fruit ripening and reduces ethylene sensitivity in tomato. Russ. J. Plant Physiol. 2010, 57, 554–559.
- Zhao, W.; Gao, L.; Li, Y.; Wang, M.; Zhang, L.; Zhao, L. Yellow-fruited phenotype is caused by 573 bp insertion at 5’ UTR of YFT1 allele in yft1 mutant tomato. Plant Sci. 2020, 300, 110637.
- Ranc, N.; Muños, S.; Santoni, S.; Causse, M. A clarified position for Solanum lycopersicum var. cerasiforme in the evolutionary history of tomatoes (solanaceae). BMC Plant Biol. 2008, 8, 130.
- Jenkins, J.A.; Mackinney, G. Inheritance of Carotenoid Differences in the Tomato Hybrid Yellow x Tangerine. Genetics 1953, 38, 107–116.
- Wang, X.; Gao, L.; Jiao, C.; Stravoravdis, S.; Hosmani, P.S.; Saha, S.; Zhang, J.; Mainiero, S.; Strickler, S.R.; Catala, C.; et al. Genome of Solanum pimpinellifolium provides insights into structural variants during tomato breeding. Nat. Commun. 2020, 11, 5817.
- Efremov, G.I.; Slugina, M.A.; Shchennikova, A.V.; Kochieva, E.Z. Differential Regulation of Phytoene Synthase PSY1 During Fruit Carotenogenesis in Cultivated and Wild Tomato Species (Solanum section Lycopersicon). Plants 2020, 9, 1169.
- Verhoeyen, M.E.; Bovy, A.; Collins, G.; Muir, S.; Robinson, S.; de Vos, C.H.R.; Colliver, S. Increasing antioxidant levels in tomatoes through modification of the flavonoid biosynthetic pathway. J. Exp. Bot. 2002, 53, 2099–2106.
