The tremendous increase in the production and consumption of titanium dioxide (TiO2) nanoparticles (NPs) in numerous industrial products and applications has augmented the need to understand their role in wastewater treatment technologies. The use of TiO2 NPs as the representative of photocatalytic technology for industrial wastewater treatment is coming to the horizon. As the use of industrial wastewater to feed agriculture land has been a common practice across the globe and the sewage sludge generated from wastewater treatment plants is also used as fertilizer in agricultural soils. Therefore, it is necessary to be aware of possible exposure pathways of these NPs, especially in the perspective of wastewater treatment and their impacts on the agro-environment.
- TiO2 NPs
- applications
- wastewater treatment
- agro-environment
1. Introduction
TiO2 NPs are one of the most extensively used NPs in different sectors [1]. For example, TiO2 NPs are widely used in the agriculture sector for different purposes such as nano-pesticides and nano-fertilizers to introduce sustainable agricultural practices [2]. The availability of these nano-based agrochemicals in the market is expected to rise in near future [3]. Similarly, the use of TiO2 NPs has also gained the utmost importance in other fields, and eventually from different sources, the inevitable release of these NPs into the environment is obvious either through a direct or indirect route. For example, in 2008, the first evidence of TiO2 NPs leaching (3.5 × 107 NPs per L) into the aquatic environment from facade paints was reported [4]. In 2011, TiO2 NPs were first detected in effluents of wastewater treatment plants, which were discharged into freshwater bodies where these NPs can cause unknown ecological risks [5]. TiO2 NPs have also been observed to detach from some textiles and paints due to washing or weathering and to run into wastewater treatment plants [6][7] and especially in sewage sludge reaching the approximate concentration of 2 g·kg−1 [8]. Sewage sludge is commonly employed as soil fertilizer in agriculture at the rate of approximately 3 tons per hectare (on a dry weight basis) annually [9][10][11] and become an ultimate source of TiO2 NPs dissemination in agricultural soils. However, the overall concentration of these NPs in the environment through direct exposure route will be much higher than the indirect release. Interestingly, in both soil and water medium, TiO2 NPs can be used for purification purposes due to their unique characteristics of photocatalysis in the presence of ultraviolet (UV) light [12][13]. Figure 1 below illustrates the brief overview of TiO2 NPs applications, their role in wastewater treatment and their impacts on agro-environment.
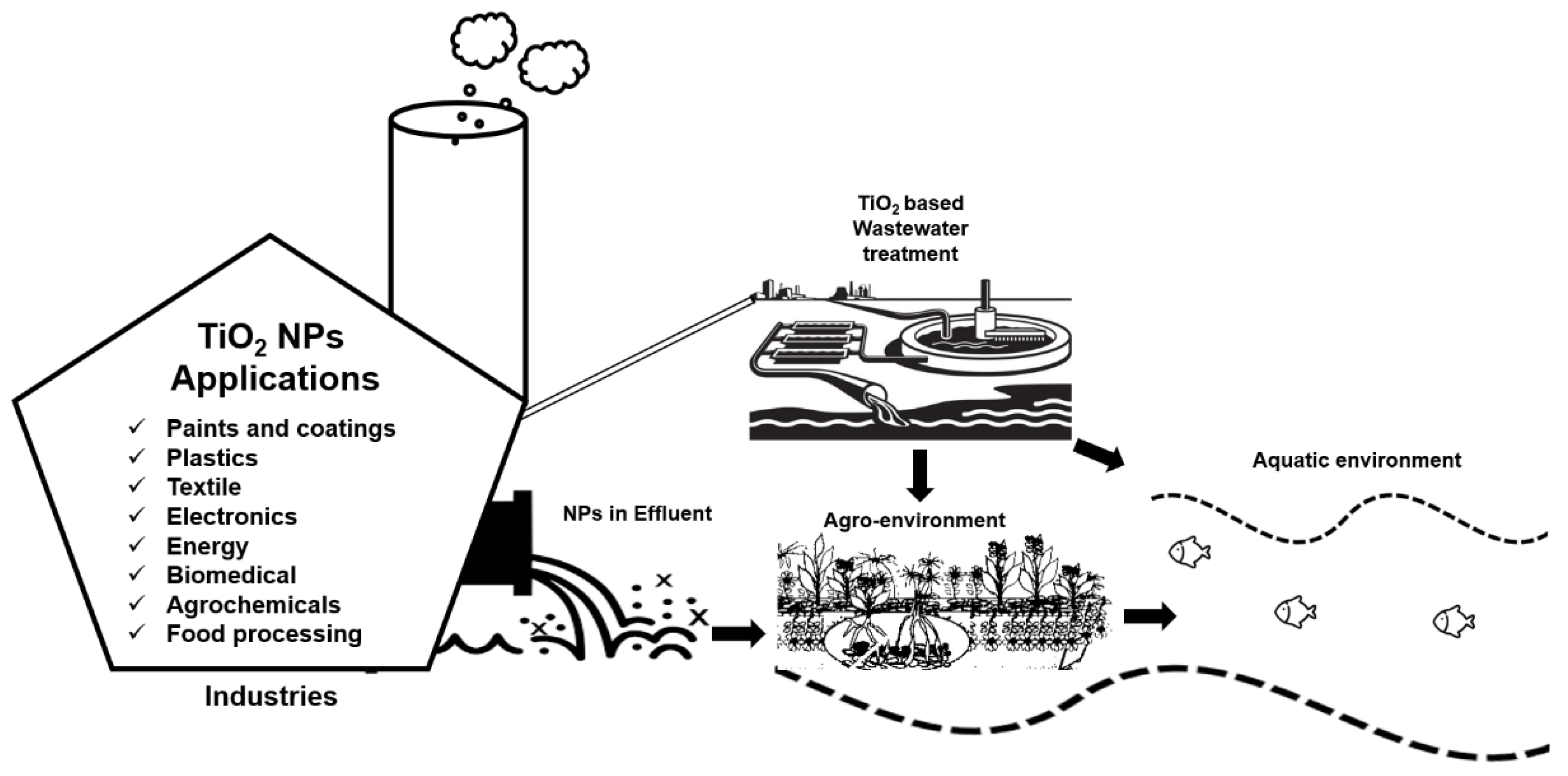
Figure 1. Illustration of the wide range of TiO2 NPs applications from industries, their release into the wastewater, and their possible exposure routes towards the agro-environment.
2. Wastewater Treatments
With the onset of industrialization, there has been a steady increase in the types and amount of pollutants released in the environment. These environmental problems have garnered much attention on the global scale, especially water scarcity. Global water scarcity is a temporal and graphical mismatch between freshwater resources and the world’s water demand. The increasing world population and urban industrialization have made water scarcity more alarming as shown in Figure 2, predicting the gap between supply (4200 billion m3) and demand (6900 billion m3) of freshwater in 2030. A major proportion of this water is used for the agriculture sector and then for the industrial sector.
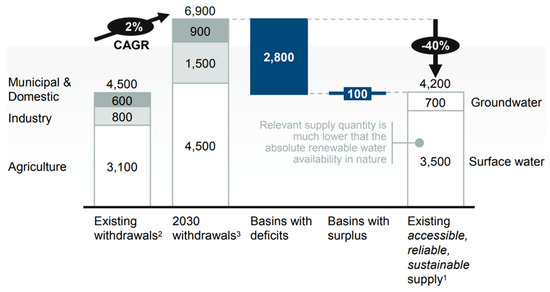
Figure 2. Comparison of current and future water demand, Reproduced with permission from [14], published by McKinsey & Company, New York, NY, USA, 2009.
With a growing world population, an ever-increasing demand for food production and potable water is questionable. The agriculture sector requires a surplus amount of water for irrigation. To avoid water scarcity issues, the reuse of wastewater is tremendously increasing across the planet. Reusing wastewater is a sustainable strategy to manage natural water resources [15] . However, the use of untreated wastewater for irrigation is a usual practice in developing countries causing serious threats to the ecosystem as well as human health. Specifically, carcinogenic pollutants pose a solemn threat to agricultural land, irrigated with industrial effluent without any treatment [16].
The whole world stands as a witness to unintended repercussions caused by rapid industrialization. The wastewater generated from industrial sectors has pronounced effects on humans as well as landmass fertility. Some industrial estates have operational wastewater treatment plants but unfortunately, they cannot handle a large proportion of industrial effluent. To meet the international standards of wastewater discharge, suitable technologies are required for wastewater treatment before discharging to streams. It could help to reduce the burden on freshwater resources by reusing treated water in various industrial processes. Due to the widely used application of nanotechnology, challenges, and opportunities of using engineered nanomaterials (ENMs) in wastewater treatment is a matter of endless concern. Based on the wastewater standards, a technique using TiO2 NPs for resilient pollutants in the context of wastewater treatment has become popular in recent years. Up to date, TiO2 NPs have drawn attention over other photocatalysts in every field of life. Over the last few decades, TiO2 NPs with high photocatalytic efficacy has been tested to reduce the pollution load from various industrial units. The conventional wastewater treatment methods mostly come up with high costs as well as lower efficiencies. However, the advantages of the use of TiO2 NPs (non-toxic, inexpensive, stable, and reusable NPs) appeared as a promising strategy to save the environment from pollution.
3. Impacts of TiO2 NPs in the Agro-Environment
In agro-environment, soil is the main and complex matrix in which analyzing the fate of TiO2 NPs is a challenging task. Furthermore, the impacts of TiO2 NPs are difficult to measure in the soil due to the high geogenic background of Ti (≈0.6% of the terrestrial crust). Up until now, modeling studies had helped to estimate the approximate amount of TiO2 NPs that is accumulating in the environment. According to recent forecasts, TiO2 NPs sludge treated soils (with 45,000 tons) were observed to be the largest sink for NPs release among different environmental compartments [4]. The crop plants served as an entry route for NPs’ uptake into the food chain. Presently, there are limited data available about these NPs interactions within the soil matrix. As nanotechnology is emerging in the field of agriculture sector in terms of growing global food production, nutritional contents, quality, food safety, and security [17]. Besides all these aspects, there are several other applications of NPs in agro-environments such as food processing and production, nano-fertilizer, nano-pesticides, etc. but the important concern arises here is the fate of these NPs.
Scientists have investigated the effects of TiO2 NPs on the soil–plant continuum and have observed diverse impacts based on different characteristics of NPs, plant species, experimental conditions, and exposure period. For example, Figure 3, shows the TiO2 NPs effects on plants with respect to different stages, concentration range, and exposure time. In a recent study, experiments were conducted on growth-promoting rhizobacteria (PGPR) inoculation with and without TiO2 NPs in peat soil under the three stress situations. TiO2 NPs were reported to enhance the performance of growth-promoting rhizobacteria which further promotes the solubilization of insoluble phosphates [18]. A grassland soil was treated with TiO2 NPs at the rate of 0, 500, 1000, and 2000 mg kg−1 of soil. These NPs were observed to negatively affect the soil bacterial communities after 60 days of exposure [19]. TiO2 NPs effects on several bacterial taxa were also studied using incubated soil microcosms having concentrations range of TiO2 NPs 0, 0.5, 1.0, and 2.0 mg g−1 soil. Of the identified taxa that exist in all samples, 9 taxa were found to be positively correlated with TiO2 NPs, 25 taxa were negatively correlated whereas 135 taxa were not affected by TiO2 NPs [20]. In another study, TiO2 NPs effects were investigated at concentrations ranging from 0.05 to 500 mg kg−1 dry soil on different bacterial communities. The abundance of ammonia-oxidizing archaea was reported to decrease by 40% in response to TiO2 NPs whereas Nitrospira was not affected at all. Furthermore, the abundance of ammonia-oxidizing bacteria and Nitrobacter were also reported to reduce due to TiO2 NPs treatments [21].
Figure 3. Effects of TiO2 NPs on plants with respect to different stages, concentration range, and exposure time. (a) represents the effects of TiO2 NPs on germination % of fennel seeds after short term exposure in a petri dish, the lowercase letters show the level of significance such as ‘a’ represent significant increase in germination percentage at Nano 60 treatment compared to control group. Adapted with permission from [22], published by ELSEVIER, 2013, (b) shows the effects of these NPs on lettuce plants after long term exposure of 90 days in soil, Adapted with permission from [24], published by American Chemical Society, 2015.
TiO2 NPs (0, 5, 20, 40, 60, and 80 mg/kg) were used to study phytotoxicity and stimulatory impacts on fennel after 14 days of exposure. The mean germination percentage was increased by 76% at 60 mg L−1, while the mean germination time was decreased by 31% at 40 mg L−1 [22]. Similarly, in another study, plant shoot-root length was increased by 49% and 62%, respectively at 100 mg kg−1 of NPs treatment in lettuce after 14 days exposure in soil medium [23]. Another study was performed using TiO2 NPs treatments (0, 50–250 mg kg−1) in soil medium for a period of 90 days. The total dry biomass was observed to increase 1.4-fold and phyto-available phosphorus (P) in soil by 2.2-fold, respectively [24]. Table 1 enlists the recent studies conducted for the investigation of TiO2 NPs effects on different plants.
Table 1. TiO2 NPs applications since 2010 on different plants and their impacts.
| Experimental Conditions | Plants | Impacts of TiO2 | Ref. |
|---|---|---|---|
| TiO2 NPs Size: 20–30 nm Treatments: 0, 50, 100 and 200 mg L−1) in the growth medium of cocopite and perlite. Period: 60 days |
Moldavian balm | Plants cultivated in salt stress conditions were observed to have improved physical traits and increased antioxidant enzyme activity in response to TiO2 NPs treatment compared to control. | [25] |
| TiO2 NPs Size: 50 and 68 nm Treatments: 100 mg nTiO2/kg on 10 mg kg−1 of Cd-spiked soils Period: 14 days |
Cowpea | No change in chlorophylls occurred. In leaves and roots, both ascorbate peroxidase and catalase activities were improved by NPs. TiO2 NPs have the potential for soil nano-remediation and could be an environmentally friendly option to tolerate soil Cd toxicity in cowpea plants. |
[26] |
| TiO2 NPs Size: 30 nm Treatments: 0, 30, 50 and 100 mg kg−1 Period: 60 days |
Wheat | TiO2 NPs without P fertilizer increased Ca (316%), Cu (296%), Al (171%), and Mg (187%) contents in shoots at 50 mg kg−1 TiO2 NPs treatment which shows improved grain quality and crop growth. | [27] |
| TiO2 NPs Treatments: 0, 5, 10, 15, and 20 mg L−1 (foliar spray) Medium: Soil Period: 55 days |
Rice (Oryza sativa) | The foliar spray of TiO2 NPs reduced the soil bioavailable Cd by 10, 14, 28, and 32% in response to 5, 10, 20, and 30 mg/L NPs treatments compared to their control values. These NPs also significantly decreased the Cd concentration in the shoot as well. | [28] |
| TiO2 NPs Size: <40 nm Treatments: 0, 50, and 100/mg kg−1 Medium: Soil Period: 40 days |
Wheat (Triticum aestivum) | Shoots and root lengths of wheat plants increased by16% and 4%, respectively. Phosphorus in shoots and roots was increased by 23.4% and 17.9% at 50/mg kg−1 of soil compare to control. |
[29] |
| TiO2 NPs Size: <40 nm Treatments: 0, 25, 50, 150, 250, 500, 750 and 1000 mg L−1 Medium: Soil |
Wheat (Triticum aestivum) | TiO2 NPs at the highest treatment level of 1000 mg kg−1, plant growth, biomass. Phosphorus content along with other tested parameters did not shown any improvement in the testing soils. |
[30] |
| TiO2 NPs Treatments: 0, 100 and 500 mg kg−1 Medium: soil Period: 60 days |
Wheat (Triticum aestivum) | No effect of phytotoxicity was observed in plant growth, chlorophyll content, and biomass. | [31] |
| TiO2 NPs Treatments: 0–750 mg kg−1 Medium: Soil Period: 90 days |
Rice (Oryza sativa) | Phosphorus concentration was increased in roots by 2.6-fold, shoots 2.4-fold, and grains 1.3-fold upon 750 mg kg−1 of NPs treatment. Metabolomics study revealed that levels of amino acids, glycerol content, and palmitic acid were also improved in grains. |
[32] |
| TiO2 NPs Treatments: 0, 100, 150, 200, 400, 600, and 1000 mg L−1 Medium: Hydroponics Period: 7 days |
Barley (Hordeum vulgare L.) | No adverse effect on shoot growth. Root growth inhibited as the concentration of TiO2 NPs increases. No effect on chlorophyll a and b. No significant effect on biomass. |
[33] |
| TiO2 NPs Treatments: 0–100 mg kg−1 Medium: Soil Period: 60 days |
Wheat (Triticum aestivum) | NPs treatment at the rate of 20, 40, and 60 mg kg−1 increased plant growth and phosphorus uptake. 32.3% of chlorophyll content increased at 60 mg kg−1 while 11.1% decrease at 100 mg kg−1. |
[34] |
| TiO2 NPs Size: >20 nm Treatments: 0, 100, 250, 500 and 1000 mg L−1 Medium: Soil Period: 5 weeks |
Arabidopsis thaliana (L.) | Plant biomass and chlorophyll content decreased as the NPs treatment increase. Higher concentrations of NPs improved root growth. NPs treatments from 100 to 1000 µg mL−1 affect vitamin E content in plants. Decrease in plant biomass by 3-fold in response to 500 and 1000 mg/ml NPs treatment, whereas, at 100 mg/mL, the biomass decreases to half relative to control. |
[35] |
| TiO2 NPs Treatments: 250 and 500 µg/mL |
Cabbage, Cucumber, Onion | The germination of cabbage significantly increased. In cucumber and onion, significant root elongation was observed. |
[36] |
| TiO2 NPs P25: 29 ± 9 nm, E171: 92 ± 31nm, Non-nanomaterial TiO2: 145 ± 46 nm Treatments: 1, 10, 100, 1000 mg kg−1 Period: 12 weeks |
Wheat, Red clover | TiO2 NPs showed restricted mobility from soil to leachate. No significant translocation of Ti was observed in both plant species, while average Ti content increased from 4 to 8 mg kg−1 at the highest treatments. |
[37] |
| TiO2 NPs Size: 22 and 25 nm Period: 6 weeks |
Soya bean | Plant growth significantly decreased which corresponds to the reduced carbon content in leaves. | [38] |
| TiO2 NPs Treatments: 0, 10, 20, 40 and 80 mg L−1 Medium: Petri dish Period: 10 days |
Alyssum homolocarpum, Salvia mirzayanii, Carum copticum, Sinapis alba, and Nigella sativa | TiO2 NPs affected the germination and seedling vigor of 5 medicinal plants. Appropriate concentration levels had improved the germination as well as the vigor index of the subjected plant. |
[39] |
| TiO2 NPs Treatments: 0, 10, 20, 30, and 40 mg mL−1 |
Parsley | Significant increase in seedlings germination percentage, germination rate index, shoot-root length, fresh biomass, vigor index, and chlorophyll content. 30 mg mL−1 was observed to be the optimum concentration of NPs. Increased germination percentage (92.46%) was observed at 40 mg mL−1 treatment, relative to the lowest one (44.97%) at control. |
[40] |
| TiO2 NPs Treatments: 0, 0.01%, 0.02%, and 0.03% Medium: Soil Period: 14 days |
Wheat (Triticum aestivum) | Under the water-stressed conditions, the plant’s length, biomass, and seed number along with the other tested traits like gluten and starch content were increased at 0.02% of NPs treatment. | [41] |
| TiO2 NPs Size: 14–655 nm |
Wheat (Triticum aestivum) | NPs treatment improved root length. NPs above 140 nm diameter are not accumulated in wheat roots. NPs above 36 nm threshold diameter, can be accumulated (at concentration 109 mg Ti/kg dry weight) in wheat root parenchyma cells but are unable to translocate to the shoot. Enhanced wheat root elongation was observed when exposed to 14 and 22 nm TiO2 NPs. |
[42] |
| TiO2 NPs Size: 5 nm Treatments: 0.25% NPs Medium: Hoagland nutritive fluid Period: 35 days |
Arabidopsis thaliana | Improved photosynthesis and growth in plants were reported. Generally, the absorption of light in chloroplast and light-harvesting complex II was supposed to be stimulated by TiO2 NPs; thus, enhancing the transformation of light energy to electronic energy, the evolution of oxygen, and water photolysis. | [43] |
| TiO2 NPs (43%) with sucrose coating Size: >5 nm |
Arabidopsis thaliana | Results revealed that small NPs entered plant cells and got accumulated in distinct subcellular locations. | [44] |
| TiO2 NPs Size: <100 nm Treatments: 0, 5, 10 and 20 mg L−1 Period: 20 days |
Zea mays L. | TiO2 NPs treatment significantly reduced the shoot, root biomass, and chlorophyll contents of leaves in a dose-dependent manner. Whereas positive effects were reported on the N, P, K, Zn Mn, and Cu contents except for Fe. | [45] |
| TiO2 NPs Size: <100 nm Treatments: 15, 30, 60, 120 and 240 mg L−1 Period: at different time intervals up to a maximum of 82 days |
Vicia faba | TiO2 NPs were reported to induce variations in a meiotic activity which results in an increased number of chromosomal abnormalities in the plant’s reproductive parts. | [46] |
| TiO2 NPs Size: <100 nm (tetragonal crystals), <10 nm (spherical shape) Treatments: 50 mg L−1 Period: 3 days |
Vicia faba | Based on the characteristics of size and shape, TiO2 NPs can induce different levels of toxicity in terms of seed vigor index, aberration index and oxidative stress in plants. | [47] |
Studies have shown the positive effects of TiO2 NPs on the physiology of red bean plants, leaving no negative biochemical impacts in plants [48]. Low concentrations of TiO2 NPs were reported with their positive effects on chickpea cells especially when they were exposed to cold stress. However, TiO2 NPs especially at 5 mg kg−1 concentration level was reported to reduce cold-induced damages in sensitive and resistant chickpea genotypes. Such domino effects raised key questions regarding the potential mechanisms. It was supposed that the activation of the defensive mechanisms in chickpea seedlings after the absorption of TiO2 NPs support the plants in cold stress. These results are quite interesting for further practice in cases of environmentally stressed conditions. These new findings could pave the way to increase the use of NPs especially to improve the cold stress tolerance in major crops [49]. Furthermore, in future studies, TiO2 NPs application in combination with fertilizers could be an effective option to search out a way for better application of these agrochemicals in a sustainable way. We further need to explore the potential of nanotechnology by upscaling the present studies by investigating the effects of NPs at different stages in the life cycle of plant species and understand their mechanism of environmental exposure.
4. Conclusions and Future Perspectives
Based on the recent developmental facts related to TiO2 NPs in wastewater treatment technologies and the agro-environment, the use of TiO2 NPs will further increase for promising applications in the near future. In wastewater treatment technologies, downstream separation of these NPs after photocatalytic degradation is still a matter of concern which can be minimized by using TiO2 in photocatalytic reactors either in slurry form or immobilized on a solid substrate. Immobilization might result in loss of potential active sites which could be minimized by adding NMs into the polymeric substrate. The polymer can provide firm anchoring to TiO2 NPs, however, there is still a chance of NPs leaching into the treated water and reaching the agricultural soils via irrigation. Since the agriculture sector is the backbone of the economy in most countries, studies based on crop improvement using TiO2 NPs could help to overcome the burden of nutrient deficit in soils providing better crop yield. Apart from the potential benefits of TiO2 NPs there are also some limitations that we could not ignore. At this stage, we could not claim with surety that the use of NPs is fully safe for human health and the environment or if it is harmful. Risks associated with chronic exposure of these NPs, interaction with flora and fauna, and their possible bioaccumulation effects have not been fully considered yet. The other limitations include the lack of information about a safe range of NPs’ concentration, scalability of research and development for prototypes, industrial production, and public concern about health and safety issues. Detailed investigations are necessarily required to resolve these concerns and provide conclusive statements. We need to optimize the useful concentration levels of TiO2 NPs for various applications and limit their usage for environmental safety.
References
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X.; Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019, 75, 40–53.
- Kalpana, S.R.; Rashmi, H.B.; Rao, N.H.; Nanotechnology Patents as R&D Indicators for Disease Management Strategies in Agriculture. J. Intellect. Prop. Rights 2010, 15, 197–205.
- Gogos, A.; Knauer, K.; Bucheli, T.D.; Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. J. Agric. Food Chem. 2012, 60, 9781–9792.
- Kaegi, R.; Ulrich, A.; Sinnet, B.; Vonbank, R.; Wichser, A.; Zuleeg, S.; Simmler, H.; Brunner, S.; Vonmont, H.; Burkhardt, M.; et al.et al. Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment.. Environ. Pollut. 2008, 156, 233–239.
- Westerhoff, P.; Song, G.; Hristovski, K.; Kiser, M.A.; Occurrence and removal of titanium at full scale wastewater treatment plants: Implications for TiO2 nanomaterials. J. Environ. Monit. 2011, 13, 1195–1203.
- Mackevica, A.; Foss Hansen, S.; Release of nanomaterials from solid nanocomposites and consumer exposure assessment—A forward-looking review. Nanotoxicology 2016, 10, 641–653.
- Windler, L.; Lorenz, C.; von Goetz, N.; Hungerbühler, K.; Amberg, M.; Heuberger, M.; Nowack, B.; Release of Titanium Dioxide from Textiles during Washing. Environ. Sci. Technol. 2012, 46, 8181–8188.
- Sun, T.Y.; Bornhöft, N.A.; Hungerbühler, K.; Nowack, B.; Dynamic Probabilistic Modeling of Environmental Emissions of Engineered Nanomaterials. Environ. Sci. Technol. 2016, 50, 4701–4711.
- Gottschalk, F.; Sun, T.; Nowack, B.; Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 2013, 181, 287–300.
- Kim, B.; Murayama, M.; Colman, B.P.; Hochella, M.F.; Characterization and environmental implications of nano- and larger TiO2 particles in sewage sludge, and soils amended with sewage sludge. J. Environ. Monit. 2012, 14, 1129.
- Sharma, B.; Sarkar, A.; Singh, P.; Singh, R.P.; Agricultural utilization of biosolids: A review on potential effects on soil and plant grown. Waste Manag. 2017, 64, 117–132.
- Wu, M.; Deng, J.; Li, J.; Li, Y.; Li, J.; Xu, H.; Simultaneous biological-photocatalytic treatment with strain CDS-8 and TiO2 for chlorothalonil removal from liquid and soil. J. Hazard. Mater. 2016, 320, 612–619.
- Zimbone, M.; Cacciato, G.; Boutinguiza, M.; Privitera, V.; Grimaldi, M.G.; Laser irradiation in water for the novel, scalable synthesis of black TiOx photocatalyst for environmental remediation. Beilstein J. Nanotechnol. 2017, 8, 196–202.
- Addams, L.; Boccaletti, G.; Kerlin, M.; Stuchtey, M. Charting Our Water Future: Economic Frameworks to Inform Decision-Making: 2030 Water Resources Group; McKinsey & Company: New York, NY, USA, 2009
- Analouei, R.; Taheriyoun, M.; Safavi, H.R.; Risk assessment of an industrial wastewater treatment and reclamation plant using the bow-tie method . Environ. Monit. Assess. 2020, 192, 1–16.
- Cao, H.; Chen, J.; Zhang, J.; Zhang, H.; Qiao, L.; Men, Y.; Heavy metals in rice and garden vegetables and their potential health risks to inhabitants in the vicinity of an industrial zone in Jiangsu, China. J. Environ. Sci. 2010, 22, 1792–1799.
- Singh Sekhon, B.; Nanotechnology in agri-food production: An overview. Nanotechnol. Sci. Appl. 2014, 7, 31–53.
- Timmusk, S.; Seisenbaeva, G.; Behers, L.; Titania (TiO2) nanoparticles enhance the performance of growth-promoting rhizobacteria . 2018 Sci. Rep., 8, 1–13.
- Ge, Y.; Schimel, J.P.; Holden, P.A.; Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ. Sci. Technol. 2011, 45, 1659–1664.
- Ge, Y.; Schimel, J.P.; Holdena, P.A.; Identification of soil bacteria susceptible to TiO2 and ZnO nanoparticles. Appl. Environ. Microbiol. 2012, 78, 6749–6758.
- Simonin, M.; Martins, J.M.F.; Le Roux, X.; Uzu, G.; Calas, A.; Richaume, A.; Toxicity of TiO2 nanoparticles on soil nitrification at environmentally relevant concentrations: Lack of classical dose–response relationships. Nanotoxicology 2017, 11, 247–255.
- Feizi, H.; Kamali, M.; Jafari, L.; Rezvani Moghaddam, P.; Phytotoxicity and stimulatory impacts of nanosized and bulk titanium dioxide on fennel (Foeniculum vulgare Mill). Chemosphere 2013, 91, 506–511.
- Zahra, Z.; Arshad, M.; Rafique, R.; Mahmood, A.; Habib, A.; Qazi, I.A.; Khan, S.A.; Metallic Nanoparticle (TiO2 and Fe3O4) Application Modifies Rhizosphere Phosphorus Availability and Uptake by Lactuca sativa. J. Agric. Food Chem. 2015, 63, 6876–6882.
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S.; Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 1–14.
- Ogunkunle, C.O.; Gambari, H.; Agbaje, F.; Okoro, H.K.; Asogwa, N.T.; Vishwakarma, V.; Fatoba, P.O.; Effect of Low-Dose Nano Titanium Dioxide Intervention on Cd Uptake and Stress Enzymes Activity in Cd-Stressed Cowpea [Vigna unguiculata (L.) Walp] Plants. Bull. Environ. Contam. Toxicol. 2020, 104, 619–626.
- Ullah, S.; Adeel, M.; Zain, M.; Rizwan, M.; Irshad, M.K.; Jilani, G.; Hameed, A.; Khan, A.; Arshad, M.; Raza, A.; et al.et al. Physiological and biochemical response of wheat (Triticum aestivum) to TiO2 nanoparticles in phosphorous amended soil: A full life cycle study. J. Environ. Manag. 2020, 263, 110365.
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Malik, S.; Adrees, M.; Qayyum, M.F.; Alamri, S.A.; Alyemeni, M.N.; Ahmad, P.; Effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa). Acta Physiol. Plant. 2019, 41, 1–12.
- Zahra, Z.; Maqbool, T.; Arshad, M.; Badshah, M.A.; Choi, H.K.; Hur, J.; Changes in fluorescent dissolved organic matter and their association with phytoavailable phosphorus in soil amended with TiO2 nanoparticles. Chemosphere 2019, 227, 17–25.
- Zahra, Z.; Ali, M.A.; Parveen, A.; Kim, E.; Khokhar, M.F.; Baig, S.; Hina, K.; Choi, H.-K.; Arshad, M.; Exposure–Response of Wheat Cultivars to TiO2 Nanoparticles in Contrasted Soils . Soil Sediment Contam. Int. J. 2019, 28, 184–199.
- Larue, C.; Baratange, C.; Vantelon, D.; Khodja, H.; Surblé, S.; Elger, A.; Carrière, M.; Influence of soil type on TiO2 nanoparticle fate in an agro-ecosystem. Sci. Total Environ. 2018, 630, 609–617.
- Zahra, Z.; Waseem, N.; Zahra, R.; Lee, H.; Badshah, M.A.; Mehmood, A.; Choi, H.-K.; Arshad, M.; Growth and Metabolic Responses of Rice (Oryza sativa L.) Cultivated in Phosphorus-Deficient Soil Amended with TiO2 Nanoparticles. J. Agric. Food Chem. 2017, 65, 5598–5606.
- Kořenková, L.; Šebesta, M.; Urík, M.; Kolenčík, M.; Kratošová, G.; Bujdoš, M.; Vávra, I.; Dobročka, E.; Physiological response of culture media-grown barley (Hordeum vulgare L.) to titanium oxide nanoparticles.. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 67, 285–291.
- Rafique, R.; Zahra, Z.; Virk, N.; Shahid, M.; Pinelli, E.; Park, T.J.; Kallerhoff, J.; Arshad, M.; Dose-dependent physiological responses of Triticum aestivum L. to soil applied TiO2 nanoparticles: Alterations in chlorophyll content, H2O2 production, and genotoxicity . Agric. Ecosyst. Environ. 2018, 255, 95–101.
- Szymańska, R.; Kołodziej, K.; Ślesak, I.; Zimak-Piekarczyk, P.; Orzechowska, A.; Gabruk, M.; Zadło, A.; Habina, I.; Knap, W.; Burda, K.; et al.et al. Titanium dioxide nanoparticles (100–1000 mg/l) can affect vitamin E response in Arabidopsis thaliana.. Environ. Pollut. 2016, 213, 957–965.
- Andersen, C.P.; King, G.; Plocher, M.; Storm, M.; Pokhrel, L.R.; Johnson, M.G.; Rygiewicz, P.T.; Germination and early plant development of ten plant species exposed to titanium dioxide and cerium oxide nanoparticles. Environ. Toxicol. Chem. 2016, 35, 2223–2229.
- Gogos, A.; Moll, J.; Klingenfuss, F.; Heijden, M.; Irin, F.; Green, M.J.; Zenobi, R.; Bucheli, T.D.; Vertical transport and plant uptake of nanoparticles in a soil mesocosm experiment. J. Nanobiotechnol. 2016, 14, 40.
- Burke, D.; Pietrasiak, N.; Situ, S.; Abenojar, E.; Porche, M.; Kraj, P.; Lakliang, Y.; Samia, A.; Iron Oxide and titanium dioxide nanoparticle effects on plant performance and root associated microbes. Int. J. Mol. Sci. 2015, 16, 23630–23650.
- Hatami, M.; Ghorbanpour, M.; Salehiarjomand, H.; Nano-anatase TiO2 modulates the germination behavior and seedling vigority of some commercially important medicinal and aromatic plants. J. Biol. Environ. Sci. 2014, 8, 53–59.
- Dehkourdi, E.H.; Mosavi, M.; Effect of anatase nanoparticles (TiO2) on parsley seed germination (petroselinum crispum) in vitro. Biol. Trace Elem. Res. 2013, 155, 283–286.
- Jaberzadeh, A.; Moaveni, P.; Tohidi Moghadam, H.R.; Zahedi, H.; Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress. Not. Bot. Horti Agrobot. Cluj Napoca 2013, 41, 201–207.
- Larue, C.; Veronesi, G.; Flank, A.M.; Surble, S.; Herlin-Boime, N.; Carrière, M.; Comparative uptake and impact of TiO2 nanoparticles in wheat and rapeseed. J. Toxicol. Environ. Health Part A Curr. Issues 2012, 75, 722–734.
- Ze, Y.; Liu, C.; Wang, L.; Hong, M.; Hong, F.; The Regulation of TiO2 Nanoparticles on the Expression of Light-Harvesting Complex II and Photosynthesis of Chloroplasts of Arabidopsis thaliana. Biol. Trace Elem. Res. 2011, 143, 1131–1141.
- Kurepa, J.; Paunesku, T.; Vogt, S.; Arora, H.; Rabatic, B.M.; Lu, J.; Wanzer, M.B.; Woloschak, G.E.; Smalle, J.A.; Uptake and distribution of ultrasmall anatase TiO2 alizarin reds nanoconjugates in Arabidopsis thaliana. Nano Lett. 2010, 10, 2296–2302.
- Dağhan, H.; Effects of TiO2 nanoparticles on maize (Zea mays L.) growth, chlorophyll content and nutrient uptake. Appl. Ecol. Environ. Res. 2018, 16, 6873–6883.
- Kushwah, K.S.; Patel, S.; Effect of Titanium Dioxide Nanoparticles (TiO2 NPs) on Faba bean (Vicia faba L.) and Induced Asynaptic Mutation: A Meiotic Study. J. Plant Growth Regul. 2019, ., 1–12.
- Ruffini Castiglione, M.; Giorgetti, L.; Bellani, L.; Muccifora, S.; Bottega, S.; Spanò, C.; Root responses to different types of TiO2 nanoparticles and bulk counterpart in plant model system Vicia faba L.. Environ. Exp. Bot. 2016, 130, 11–21.
- Jahan, S.; Alias, Y.B.; Bakar, A.F.B.A.; Yusoff, I.; Bin Toxicity evaluation of ZnO and TiO2 nanomaterials in hydroponic red bean (Vigna angularis) plant: Physiology, biochemistry and kinetic transport. J. Environ. Sci. 2018, 72, 140–152.
- Mohammadi, R.; Maali-Amiri, R.; Abbasi, A.; Effect of TiO2 Nanoparticles on Chickpea Response to Cold Stress. Biol. Trace Elem. Res. 2013, 152, 403–410.
