Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Dae Sik Jang and Version 3 by Catherine Yang.
Diospyros kaki (persimmon) leaves have long been utilized as traditional medicine for the treatment of ischemic stroke, angina, and hypertension and as a healthy beverage and cosmetic for anti-aging. This study aimed to isolate as many compounds as possible from an ethanol extract of the persimmon leaves to identify the biologically active compounds. The antioxidative effect of the ethyl acetate layer from the ethanol extract of the persimmon leaves was demonstrated by 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay and online high-performance liquid chromatography-2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (HPLC-ABTS) analysis.
- Diospyros kaki
- persimmon leaves
- flavonoid
- antioxidant
1. Introduction
Diospyros kaki Thunb. (persimmon) belongs to the family of Ebenaceae and is widely distributed in Korea, China, and Japan. Its fruit is eaten fresh or dry, while the leaves have long been used as a traditional medicine to treat ischemic stroke, angina, hypertension, atherosclerosis, and infectious diseases [1]. Furthermore, its leaves have been utilized as healthy beverages and cosmetics due to their anti-aging properties and abilities to help prevent cholesterol and melanin accumulation [1]. Recent research has suggested that the extracts of the persimmon leaves possess a wide range of biological properties, including radical scavenging, neuroprotection, thrombosis inhibition, anti-atherosclerosis, and anti-allergy [2][3][4][5][6][2,3,4,5,6]. A previous phytochemical investigation suggested that various types of flavonoids and terpenoids are the main constituents [7], and several tannins, naphthoquinones, coumarins, ionones, and fatty acids were also reported [8][9][10][11][12][8,9,10,11,12].
Reactive oxygen species (ROS) are reactive molecules produced in biological systems, and the balance between the generation and elimination of ROS is well controlled in normal cellular physiology [13]. However, excessive generation of ROS causes oxidative damage, and in turn, aging and age-related diseases including cancer, diabetes, and Parkinson’s disease [14]. Hence, discovering antioxidants such as flavonoids and phenolic compounds could be a promising strategy to treat these diseases.
As part of our continuous project to find biologically active compounds [15], the antioxidative effect of the ethanol (EtOH) extract and solvent partitions from the persimmon leaves was evaluated using 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay and online high-performance liquid chromatography-2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (HPLC-ABTS) analysis. A phytochemical study on the persimmon leaves led to the isolation of one new flavonoid (1) and one new natural compound (3), along with 25 previously known compounds. The structures were characterized by the application of spectroscopic and spectrometric methods. All isolated compounds were rapidly screened for their antioxidative effects using online HPLC-ABTS. Furthermore, the quantitative analysis of all isolated compounds was performed in the present study.
2. Antioxidative Effect of the Persimmon Leaves
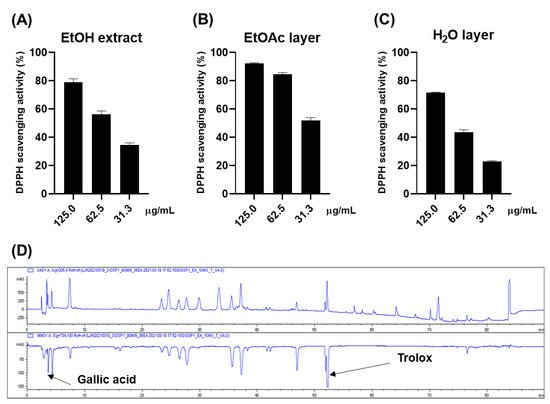
The antioxidative effect of the ethanol (EtOH) extract of persimmon leaves was evaluated for a preliminary screening through DPPH (Figure 1A). The 0.125 mg/mL of the extract scavenged approximately 80% of the DPPH radical, while 0.025 mg/mL of ascorbic acid made up 94% of the radical. The online HPLC-ABTS assay was carried out to rapidly ensure the reliability of these results (Figure 1D). Gallic acid and Trolox were used as internal standards. The chromatogram at 734 nm (negative peak) suggested that approximately nine constituents could have antioxidative activities. Gallic acid and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were used as internal standards to ensure the reliability of the results. It was inferred that most of these peaks were flavonoid derivatives such as flavonoid glycoside and flavanol, based on the dereplication analysis performed by comparing ultraviolet (UV) and mass spectra of the compounds with the published data. Bioassay-guided fractionation suggested that these antioxidative compounds were abundant in the ethyl acetate (EtOAc) layer, while the water (H2O) layer showed weak activity (Figure 1B,C).
Figure 1. DPPH scavenging effects of the EtOH extract (A), EtOAc layer (B), and H2O layer (C) H2O layer of the persimmon leaves; (D) online ABTS-HPLC chromatogram of the EtOH extract.
3. Phytochemical Investigation
To identify these antioxidative compounds, various chromatographic and spectroscopic methods were carried out for the isolation and structural characterization of the compounds. A new flavonoid (1) and a new natural compound (3) were obtained from the ethyl acetate layer of the ethanol extract, together with 25 previously reported compounds, namely kaempferol-3-O-β-2″-coumaroylglucoside (2) [16], (+)-catechin (4) [17], hyperoside (5) [17], isoquercitrin (6) [18], quercetin-3-O-β-2″-galloylgalactoside (7) [19], quercetin-3-O-β-2″-galloylglucoside (8) [20], trifolin (9) [18], astragalin (10) [18], kaempferol-3-O-β-2″-galloylgalactoside (11) [21], kaempferol-3-O-α-arabinoside (12) [22], kaempferol-3-O-β-2″-galloylglucoside (13) [23], quercetin-3-O-β-2″-coumaroylglucoside (14) [24], quercetin (15) [15], kaempferol (16) [25], (6S,9S)-roseoside (17) [26], scopoletin (18) [27], umbelliferone (19) [28], 1-(2,4-dihydroxy-6-methylphenyl)ethanone (20) [29], barbinervic acid (21) [30], diospyric acid B (22) [7], rotungenic acid (23) [31], pomolic acid (24) [32], siaresinolic acid (25) [33], oleanolic acid (26) [25], and ursolic acid (27) [25] by using spectroscopic and spectrometric and physical data in comparison with the published data and also with thin layer chromatography (TLC) analysis (Figure 2 and Figure 3). Among these, compounds 2, 16, 17, 19, 20, and 24 were firstly isolated from D. kaki.
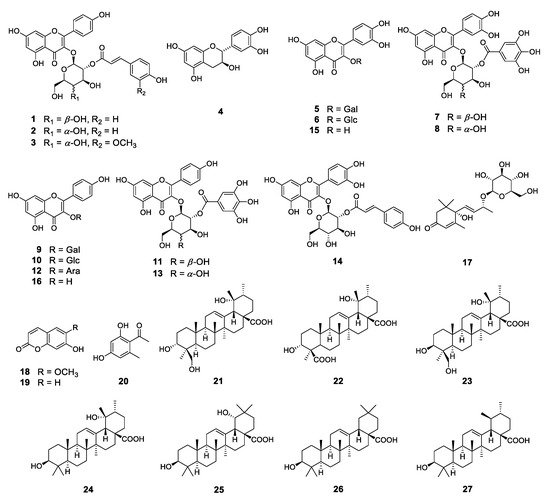

Figure 2. Structures of the compounds isolated from D. kaki leaves.
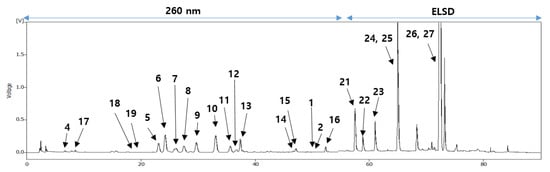

Figure 3. HPLC chromatogram of the EtOH extract from the persimmon leaves.
Compound 1 was obtained as a yellow powder, in which the molecular formula was established as C30H26O13 based on high-resolution mass spectrometry (HRMS) data. The UV spectrum exhibited absorption bands at 207 and 315 nm, indicating that compound 1 had a flavonol backbone. The 1H nuclear magnetic resonance (NMR) data (Table 1, Figure S1) showed a typical pattern of coumaroylated flavonol glycoside, showing two sets of AA′BB′-type signals (δH 8.00 (2H, d, J = 8.5 Hz, H-2′ and H-6′), 6.87 (2H, d, J = 8.5 Hz, H-3′ and H-5′)) in the B ring of kaempferol as well as the signals (δH 7.45 (2H, d, J = 8.5 Hz, H-2′′′ and H-6′′′), 6.81 (2H, d, J = 8.5 Hz, H-3′′′ and H-5′′′)) in the aromatic ring of the coumaroyl group. Two doublet signals (δH 7.65 (1H, d, J = 15.5 Hz, H-7′′′)) and δH 6.35 (1H, d, J = 16.0 Hz, H-8′′′) were observed, indicating trans-olefinic protons of the coumaroyl group. Additionaly, an anomeric proton signal (δH 5.57 (1H, d, J = 8.0 Hz, H-1′′)) was observed in the sugar region, suggesting the presence of the β-configurated cyclic sugar group. The 13C and distortionless enhancement by polarization transfer NMR data (Table 1, Figure S2) showed 30 resonances comprising two trans-olefinic carbons, ten aromatic carbons, and six glucosyl moiety carbons, and ten non-protonated carbons including two carbonyl carbons. In particular, the chemical shifts at C-2, C-3, and C-4 (δC 158.1, 134.9, and 179.2) were characteristic signals of flavonol 3-O-glycoside. Additionally, the carbonyl carbon signal at C-1′′′ (δC 168.7) and two trans-olefinic carbon signals at C-2′′′ and C-3′′′ (δC 146.9, 115.2) were typical chemical shifts of the coumaroyl group. The anomeric carbon signal at C-1′′(δC 100.4) and other signals for the glycosyl moiety from C-2′′ to C-6′′ (δC 74.3, 73.4, 70.5, 77.4, and 62.0) were observed. These one-dimensional (1D) NMR data were superimposable to those of kaempferol-3-O-β-2′′-coumaroylglucoside (2) [16]. However, the careful comparison of the 13C NMR data between the two compounds suggested that compound 1 had a galactose moiety, which was further demonstrated by the nuclear Overhauser enhancement spectroscopy (NOESY) NMR data (Figure S6). While the NOESY correlation between H-2′′ and H-4′′ was observed in compound 2, there was no correlation between these protons in compound 1. In general, interpreting 13C NMR and NOESY NMR data is an effective method to determine the type of glycosyl moiety. The location of the galactose moiety was deduced to be at C-3 according to the downfield shift of C-2 and C-4, as further evidenced by the heteronuclear multiple bond correlation (HMBC) between H-1′′ and C-3 (Figure S5). The position of the coumaroyl group was demonstrated to be at C-2′′ based on the downfield shift (δH 5.36 (1H, dd, J = 10.0, 8.0 Hz, H-2′′)) and the HMBC correlation between H-2′′ and C-1′′′. As a result, the structure of compound 1 was determined as kaempferol-3-O-β-2″-coumaroylgalactoside. Although compound 2 was previously isolated from various sources, including Quercus suber [16] and Allium porrum [34], compound 1 was isolated and structurally characterized for the first time.
Table 1. 1H and 13C NMR data of compounds 1 and 3 in methanol-d4
| Number of Carbon |
1 | 3 | ||
|---|---|---|---|---|
| δH Multi (J in Hz) | δC | δH Multi (J in Hz) | δC | |
| 2 | 158.1 | 158.6 | ||
| 134.9 | 134.8 | |||
| 4 | 179.2 | 179.0 | ||
| 5 | 163.1 | 163.0 | ||
| 6 | 6.16 d (1.5) | 101.2 | 6.11 d (2.0) | 100.6 |
| 7 | 167.7 | 168.3 | ||
| 8 | 6.34 s | 95.1 | 6.29 d (2.0) | 95.2 |
| 9 | 158.5 | 158.1 | ||
| 10 | 105.3 | 105.2 | ||
| 1′ | 122.7 | 122.8 | ||
| 2′,6′ | 8.00 d (8.5) | 132.1 | 7.98 d (9.0) | 132.1 |
| 3′,5′ | 6.87 d (8.5) | 116.3 | 6.88 d (9.0) | 116.2 |
| 4′ | 161.6 | 161.6 | ||
| 1′′ | 5.57 d (8.0) | 100.4 | 5.64 d (8.0) | 100.7 |
| 2′′ | 5.36 dd (10.0, 8.0) | 74.3 | 5.03 dd (9.0, 8.0) | 75.8 |
| 3′′ | 3.75 dd (10.5, 3.5) | 73.4 | 3.64 t (9.0) | 76.3 |
| 4′′ | 3.89 d (3.5) | 70.5 | 3.41 t (10.0) | 71.5 |
| 5′′ | 3.55 t (6.0) | 77.4 | 3.29 m | 78.8 |
| 6′′ | 3.67 m | 62.0 | 3.78 dd (12.0, 2.0) | 62.5 |
| 3.61 m | ||||
| 1′′′ | 127.2 | 127.8 | ||
| 2′′′ | 7.45 d (8.5) | 131.2 | 7.18 d (1.5) | 111.7 |
| 3′′′ | 6.81 d (8.5) | 116.8 | 149.4 | |
| 4′′′ | 161.3 | 150.7 | ||
| 5′′′ | 6.81 d (8.5) | 116.8 | 6.81 d (8.5) | 116.5 |
| 6′′′ | 7.45 d (8.5) | 131.2 | 7.07 dd (8.5, 1.5) | 124.1 |
| 7′′′ | 7.65 d (15.5) | 146.9 | 7.66 d (16.0) | 147.2 |
| 8′′′ | 6.35 d (16.0) | 115.2 | 6.37 d (16.0) | 115.5 |
| 9′′′ | 168.7 | 168.4 | ||
| 3′′′-OCH3 | 3.91 s | 56.4 | ||
Compound 3 was isolated as a yellow powder, and the molecular formula was established as C31H28O14 by analyzing HRMS data. The UV spectrum showed the UV absorption at 210 and 327 nm due to the same aglycone with compounds 1 and 2. The 1H NMR data (Table 1, Figure S10) were similar to those of compound 2, but compound 3 had a feruloyl group instead of the coumaroyl group, as evidenced by the presence of an additional methoxy group (δH 3.91 (3H, s, 3′′′-OCH3)). Additionally, an anomeric proton signal (δH 5.64 (1H, d, J = 8.0 Hz, H-1′′)) was observed, indicating that the glycosyl linkage was a β-configuration, and the downfield-shifted signal (5.03 (1H, t, J = 8.5 Hz, H-2′′)) was shown in the sugar region, as with compound 1. The 13C NMR data (Table 1, Figure S11) showed 31 resonances comprising two trans-olefinic carbons, ten quarternary carbons, ten aromatic carbons, six glucosyl moiety carbons, and one methoxy carbon, and ten non-protonated carbons, including two carbonyl carbons corresponding to kaempferol, feruloyl, and glucose groups. In particular, carbon signals from C-2′′ to C-6′′ (δC 75.8, 76.3, 71.5, 78.8 and 62.5) suggested the presence of a glucose moiety. The locations of the glucose moiety and feruloyl group were assigned by the long-range HMBC correlations between H-1′′ and C-3 (δC 134.8) and H-2′′ and C-1′′′ (δC 168.4) (Figure S14). The position of an additional methoxy group was determined by the key correlation between 3′′′-OCH3 and C-3′′′ (δC 149.4). The above results suggested the structure of compound 3 as kaempferol-3-O-β-2”-feruloylglucoside. To the best of our knowledge, compound 3 was only reported as a product of the hydrolysis of 3-O-β-(2-O-feruloyl)-glucosyl-7,4′-di-O-β-glucosylkaempferol, isolated from Allium tuberosum [35]. Therefore, the structure of 3 was elucidated as a new natural compound.
Compound 11 was isolated as a yellow powder. The 1H NMR data (Figure S19) displayed a set of AA′BB′-type signals (δH 8.06 (2H, d, J = 9.0 Hz, H-2′, H-6′), 6.87 (2H, d, J = 9.0 Hz, H-3′, H-5′)) in the B ring of kaempferol and a singlet signal at δH 7.02 (2H, s, H-3′′′, H-7′′′) of a galloyl moiety in aromatic region, which is a characteristic signal of galloylated flavonol. An anomeric proton signal (δH 5.78 (1H, d, J = 8.0 Hz, H-1′′)) indicated that the glycosyl linkage was a β-configuration. Furthermore, a downfield shifted proton signal (5.27 (1H, t, J = 9.5 Hz, H-2′′)) suggested that the galloyl group was attached at the hydroxyl group of C-2′′ because this shift could be attributed to the anisotropic influence of the O-galloyl moiety [21]. The 13C NMR data (Figure S20) exhibited 26 resonances, indicating galloylated flavonol glycoside. The carbon signals from C-2′′ to C-6′′ (δC 71.1, 72.7, 68.2, 76.0, and 60.1) suggested the presence of a galactose moiety. Therefore, the structure of compound 11 was confirmed as kaempferol-3-O-β-2′′-galloylgalactoside. Although compound 11 was previously isolated from various sources, including D. kaki [21][36][21,36], only the 1H NMR and MS data were previously reported. Thus, the 13C NMR data was reported for the first time in this study.
